有机小分子纳米粒子的光学诊疗应用
2020-11-13LEEJiyoung李方园凌代舜
邵 伟,LEE Jiyoung,李方园,凌代舜
(浙江大学药学院,药物制剂研究所,杭州310058)
1 Introduction
Cancer is the leading cause of human death worldwide due to the high mortality rate[1].It is imperative to exploit sensitive diagnostics and effective therapeutic methods to treat cancer.Phototheranostics,which perform imaging and therapy simultaneously under near-infrared(NIR)laser irradiation,are promising strategies to combat cancer[2—12].NIR fluorescence imaging is a fundamental technique in cancer diagnosis with advantages of deep tissue penetration and minimal tissue autofluorescence.Fluorescence imaging in the second NIR window(NIR-Ⅱ,1000—1700 nm)is more appealing to researchers as compared with that in the first NIR window(NIR-Ⅰ,650—950 nm)due to the further reduced light scattering and absorption in this region[13—15].Additionally,photoacoustic imaging(PAI),which transforms NIR excitation energy into ultrasonic signals originated from the thermal expansion of the medium caused by the photothermal effect of the contrast agents,is an emerging noninvasive imaging modality,providing better spatial resolution and deeper tissue penetration than conventional ultrasound and optical imaging[16,17].
Similar to NIR-Ⅱ fluorescence imaging,NIR-Ⅱ PAI is also superior to that of NIR-Ⅰdue to the deeper penetration and lower background interference[18—20].Besides NIR laser excitation-based imaging techniques,NIR laser-initiated phototherapies,such as photothermal therapy(PTT)and photodynamic therapy(PDT),have been extensively studied in recent years owing to their noninvasiveness,minimal harm to normal tissues,and high spatial selectivity[21,22].PTT is achieved by the heat-induced cancer cell death caused by the photothermal effect of the photosensitizers(PSs)under NIR laser irradiation.While PDT utilizes reactive oxygen species(ROS)such as singlet oxygen(1O2)and hydroxyl radicals(·OH)generated by the PSs under NIR laser irradiation to kill cancer cells(Fig.1)[23].By combining diagnostic imaging and therapeutic methods,phototheranostics holds great potential in cancer treatment.

Fig.1 Jablonski diagram showing different photophysical and photochemical processes of photosensitizers under laser excitation employed in phototheranostics[23]
Generally,imaging and therapeutic agents are integrated into a single platform to afford theranostic agents[24,25].However,the agents prepared by such method usually suffer from several drawbacks including poor stability and tedious synthetic procedure[26—28].Alternatively,“all-in-one”theranostic agents composed of a single component capable of diagnosis and therapeutics,have recently been developed as promising agents in cancer treatment[29—31].Ideal phototheranostic agents should have intense absorption in the NIR region with high molar extinction coefficient,excellent water-solubility,colloidal stability and photostability in the physiological environment.Moreover,good biocompatibility,low dark toxicity and high phototoxicity(heat generation for PTT and ROS generation for PDT)are indispensable for phototheranostic agents.To improve the theranostic outcome,tumor-targeting capability should be incorporated to the agents.The active tumor-targeting can be realized by specific ligand modification,while the passive tumor-targeting takes advantage of the enhanced permeability and retention(EPR)effect of the nanoparticles(NPs)[32].
Currently available phototheranostic agents are mainly inorganic and organic materials.Representative inorganic materials are noble metal nanomaterials[33—35],transition metal oxide(sulfide/selenide)NPs[36—40],quantum dots(QDs)[41],two-dimensional(2D)materials[42—46]and carbon-based nanomaterials[47—49].Despite the satisfactory theranostic performance,these inorganic materials have several disadvantages such as poor biodegradability and potential long-termin vivotoxicity,which hinder their further clinical translation.Compared to their inorganic counterparts,chemically-defined organic small molecules(OSMs)exhibit good reproducibility,well property-tunability,excellent biocompatibility and easy metabolism in biological systems,showing great potential in clinical applications[50,51].
OSMs include conventional small molecule dyes,such as phorphyrin,phthalocyanine,borondipyrromethane(BODIPY),cyanine and squaraine,etc[52—56],donor-acceptor(D-A)conjugated small molecules[57—67]and small molecule fluorogens with aggregation-induced emission property(AIEgens)[9,68—74](Fig.2 and Fig.3).Conventional small molecule dyes are recognized as the first-generation PSs.D-A conjugated small molecules and AIEgens are emerged as the second-generation PSs due to their easily tunable optical properties.Withπ-electrons delocalized on the extended backbone,these OSMs possess strong NIR absorbance,which is beneficial for deep-tissue tumor diagnosis and phototherapy.However,free OSMs are hydrophobic and lack tumor-targeting capability.Thus,OSMs are fabricated into water-soluble colloidal organic small molecule NPs(OSMNs)assisted by amphiphilic copolymers,such as Pluronic F127,F68 and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethylene glycol)(DSPE-mPEG),etc,as encapsulating matrixesviathe mostly used nanoprecipitation method[75—79](Fig.2 and Fig.3).

Fig.2 Schematic illustration of OSMNs preparation and their applications in phototheranostics
This nanoparticle-forming strategy not only prevents these OSMs from being rapidly cleared by kidneys but also substantially extends the circulation time in blood so as to be better utilized[80].More importantly,OSMNs endow these OSMs passive tumor-targeting capabilityviaEPR effect[32],which is the prerequisite for further tumor phototheranostics.Multifunctional theranostics based on OSMNs can also be achieved by targeting ligand functionalization[81—83].With such fascinating features and advantages mentioned above,OSMNs have reshaped the concept of phototheranostics,and been extensively explored in cancer theranostics.There are several reviews of phototheranostics regarding different materials such as organic and inorganic nanoparticles[84],perylene diimide-and aza-BODIPY-based nanomaterials[85,86],and organic small molecule dye-based nanoparticles[87].
In this review,we comprehensively summarize the recent advances in OSMNs for phototheranostics(Fig.2 and Fig.3),and discuss their future challenges and perspectives in potential clinical applications.
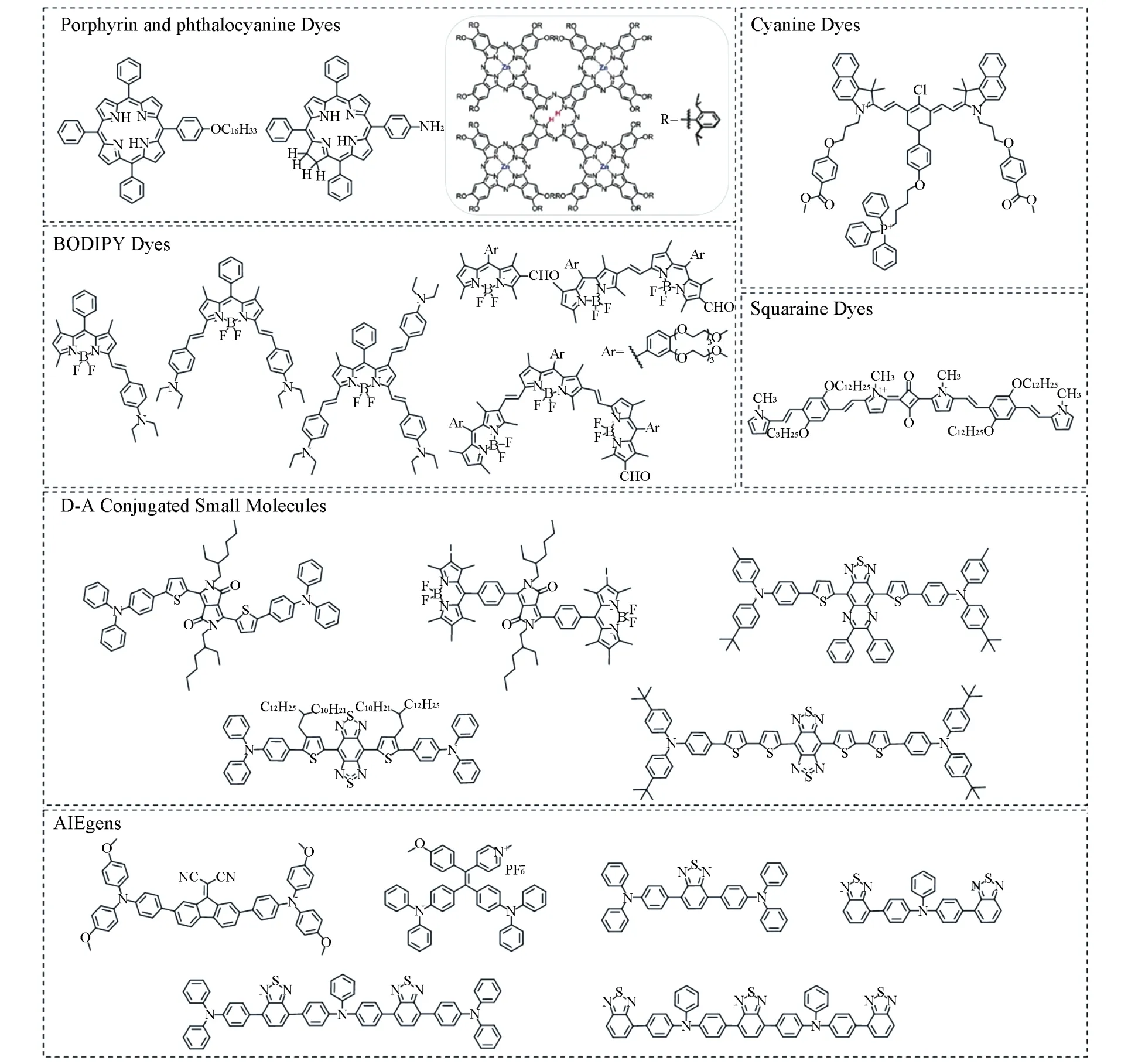
Fig.3 Representative OSMs used in phototheranostics
2 Conventional Small Molecule Dye-Based NPs
NIR-absorbing conventional small molecule dyes have attracted tremendous attention for various applications in phototheranostics.These dyes have several inherent advantages such as high molar extinction coefficient,bright fluorescence,and good photostability.These conventional small molecule dyes used for imaging and therapeutics are mainly porphyrin and phthalocyanine dyes,BODIPY dyes,cyanine dyes,and squarainedyes[51,53,56].Owing to the poor water-solubility of these organic dyes,under most circumstances,they are encapsulated into amphiphilic block copolymer matrix for colloidal NPs[76,77,79].Moreover,some other functionalities such as tumor-targeting,drug loading,and additional imaging capabilities can also be realizedviafurther NPs modification[81].Taking advantage of the merits of these conventional small molecule dyes,they have emerged as promising phototheranostic agents.
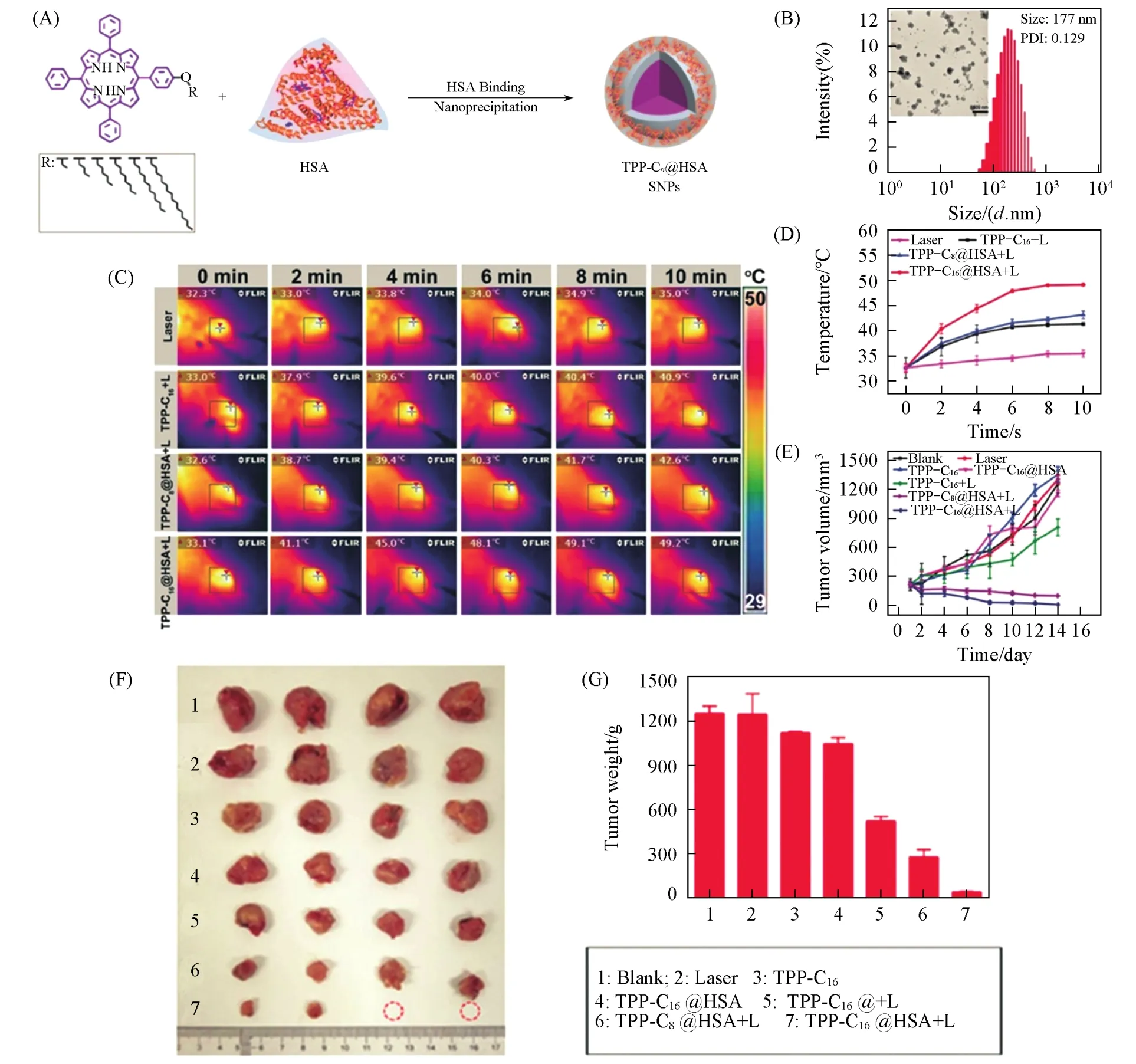
Fig.4 TPP⁃Cn@HSA SNPs for photothermal therapy[94]
2.1 Porphyrin and Phthalocyanine Dyes
The typical tetrapyrrole structures of both porphyrin and phthalocyanine dyes have diverse coordination chemistry properties that can form various complexes with almost all the transition metals[52,88].Well electron delocalization of these tetrapyrrole structures leads to their extended absorption wavelengths and high extinction coefficients,which is beneficial for phototheranostics.There are two types of PDT processes reported so far:typeⅠroute with hydroxyl radicals generation and typeⅡ route with1O2generation,of which typeⅡ route is believed to be more effective[23].Generally,tetrapyrrole photosensitizers,such as porphyrin and phthalocyanine derivatives,tend to produce1O2viathe TypeⅡ process for effective PDT.As the representative photosensitizer in phototheranostics,various porphyrin and phthalocyanine derivatives were reported[31,89—95](Fig.4).Zhenget al.[94]synthesized a series of triphenylporphyrin(TPP)derivatives attached with different lengths of alkoxyl side chains(TPP-C2,TPP-C4,TPP-C6,TPP-C8,TPP-C12and TPP-C16)and prepared their corresponding NPs(TPP-C2@HSA SNPs,TPP-C4@HSA SNPs,TPP-C6@HSA SNPs,TPP-C8@HSA SNPs,TPPC12@HSA SNPs and TPP-C16@HSA SNPs)viahuman serum albumin(HSA)-binding.They proved that TPPC16@HSA SNPs with the longest alkoxyl side chain exhibited the best photothermal effect with a photothermal conversion efficiency(η)of 45%under a 638 nm laser irradiation among these NPs.Effective PTT under NIR laser irradiation is demonstrated by intravenous(i.v.)injection of TPP-C16@HSA SNPs into U14 tumor-bearing mice.
Phototheranostic agents that can simultaneously perform PTT and PDT under NIR laser irradiation are appealing in cancer treatment due to the synergistic effect[96,97].However,currently existing agents with such functionalities are mainly dual wavelengths-excited multicomponent-based materials with sophisticated preparing procedure and unsatisfactory stability[98—101].Thus,single component-based phototheranostic agents used for synergistic PTT and PDT,such as porphyrin derivatives,are more desired.By partial reduction of 5-(4-aminophenyl)-10,15,20-triphenylporphyrin(TPP-NH2)with toluene sulfonhydrazide,Zhenget al.[31]synthesized a porphyrin derivative(TPC-NH2).TPP-NH2and TPC-NH2were then linked by 3,3-dithiodipropionic acidviaamidation reaction to afford their dimer of TPP-SS and TPC-SS,which were further self-assembled into nanoparticles.TPC-SS NPs exhibit much high absorbance than TPP-SS NPs,resulting in enhanced photodynamic and photothermal effects under a single NIR laser(635 nm)irradiation.Highly effective cancer cellular uptake and photo cytotoxicity of TPC-SS NPs can be observedin vitro(HeLa cells).Furthermore,PAI-guided synergistic PTT/PDT on U14 subcutaneous xenograft murine models is achieved by using TPC-SS NPs(Fig.5).
This work inspires researchers to exploit more efficient phototheranostic agents that integrate imaging and therapeutic capability in a single component-based material which is beneficial for future cancer treatment.Phototheranostics in the NIR-Ⅱ biowindow is superior to that in the NIR-Ⅰbiowindow in terms of both maximum permissible exposure(MPE)and deep penetration due to the reduced light scattering and absorption in this region[13—15,35,102].PAI with high spatial resolution and signal to noise(S/N)ratio can be obtained in the NIR-II region[103—106].Meanwhile,NIR-Ⅱ PTT and/or PDT can reach and ablate deep-buried tumors[102,107,108],which is of great interest in practical clinical application.
Unlike the series of inorganic materials used in NIR-Ⅱ phototheranostics,such as gold nanomaterials[35,109],copper sulfide nanocrystals[110],and some 2D materials[44,111],NIR-II OSMNs are rare for phototheranostics.Panet al.[93]synthesized a cruciform phthalocyanine pentad(Zn4-H2[Pc(OC12H17)24]) that was coprecipitated with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000](DSPE-PEG2000-OCH3)to afford the first small molecule-based NIR-Ⅱphotothermal agent(Zn4-H2Pc/DP NPs).With a high extinction coefficient of 52 L·g-1·cm-1,this nanoparticle exhibits a remarkableηof 58.3%.Such excellent photothermal effect endows Zn4-H2Pc/DP NPs efficient PAI performance.Therefore,the PAI-guided NIR-ⅡPTT on MCF-7 subcutaneous xenograft murine models is successfully realizedin vivo(Fig.6).This is a pioneering work in exploiting small molecule-based NIR-Ⅱ phototheranostic agents that opens a new avenue for the development of novel materials in cancer phototheranostics.
2.2 BODIPY Dyes
BODIPY dyes are featured with the chemical structure that two pyrrole rings are linked by a methine in a tricyclic fused ring and a six-membered ring with a boron atom flanked by two five-membered rings.Various methods have been developed for BODIPY synthesis[112,113]and BODIPY dyes have been extensively used in various applications,such as organic molecular wires[114],organic lasers[115],fluorescent probes[55,116],chemosensors[117—120]and photosensitizers[121—125].Unfortunately,most of the currently available BODIPY dyes have the disadvantages of short absorption wavelength and poor water-solubility,which hinder their further biomedical applications[118—121,123,125].

Fig.5 TPC⁃SS NPs for PAI⁃guided photothermal therapy[31]
Researchers have developed several approaches to endow BODIPY dyes with functionalities for biomedical applications.Specifically,the introduction of substituents such as aryl,alkynyl and styryl into a typical BODIPY core can effectively extend the absorption wavelength for deeper tissue penetration[122—126].In addition,the incorporation of heavy atoms(Br,I,etc)into the BODIPY core can facilitate the intersystem crossing(ISC)processviathe heavy atom effect,which will boost the PDT efficiency[127—129].Both attaching oligo polyethylene glycol(PEG)chains to BODIPY core[130—132]and coprecipitation of BODIPY dyes with amphiphilic copolymers(Pluronic F127,F68,and DSPE-mPEG,etc)[121,133,134]can overcome the water-solubility issue of BODIPY dyes.Zouet al.[125]designed and synthesized three phenyl-based BODIPY dyes(BDPmPh,BDPbiPh and BDPtriPh)with different numbers(1,2 and 3)of diethylaminophenyl groups attaching to the BODIPY core(BDP)(Fig.7).Such molecular design allows the absorption wavelength being easily tuned from 660 nm to 730 nm and finally 808 nm for BDPmPh,BDPbiPh and BDPtriPh NPs,respectively,allowing the penetration depth tunability.Moreover,due to the proton acceptor nature of the diethylamino groups in these BODIPY dye structures,these NPs exhibit pH-triggered enhanced PTT/PDT efficacy.Several other pH-responsive BODIPY dye-based NPs for PTT and/or PDT have also been developed based on a similar mechanism,showing great potential in phototheranostics[135].It is well established that PTT and PDT were related to the specific photophysical and photochemical processes of the PSs upon laser irradiation[23].Manipulating these processesviamolecular modification of the PSs can conveniently tailor their PTT and/or PDT functionalities[8,92,123].Yeet al.[123]rationally designed three BODIPY dyes of mono-BDP,di-BDP and tri-BDP.The di-BDP and tri-BDP were synthesized by coupling two and three molecules of mono-BDP(Fig.8).They found that the photoconversion could be shifted from fluorescence to singlet-to-triplet or nonradiative transitions for mono-BDP,di-BDP and tri-BDP,respectively.This molecular design strategy endows different PTT and PDT functionalities for mono-BDP-NPs,di-BDP-NPs and tri-BDP NPs.In particular,tri-BDP-NPs show preferable photothermal conversion and minor singlet oxygen generation under laser irradiation,achieving efficient tumor photothermal ablation.
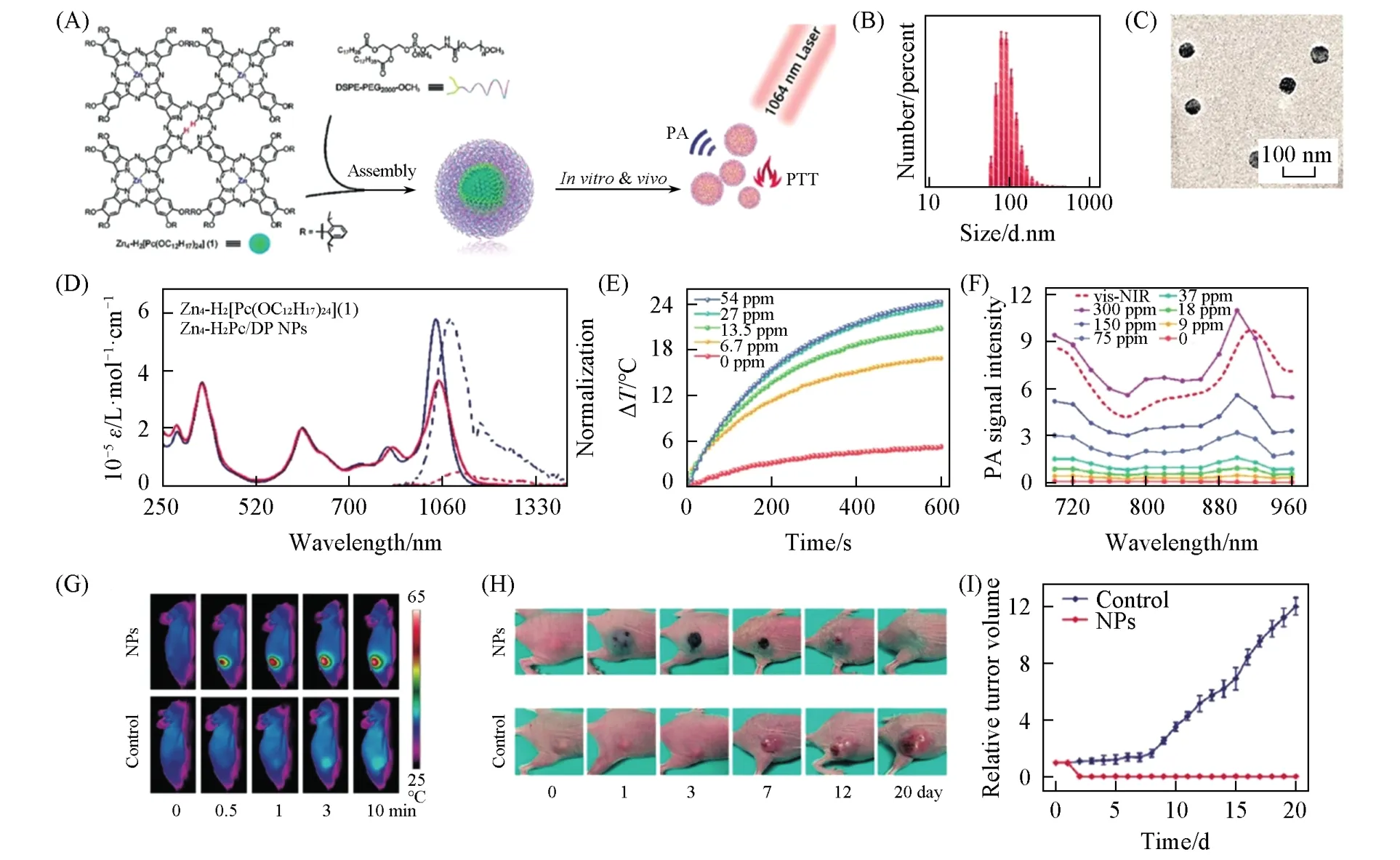
Fig.6 Zn4⁃H2Pc/DP NP for PAI⁃guided NIR⁃Ⅱ photothermal therapy[93]
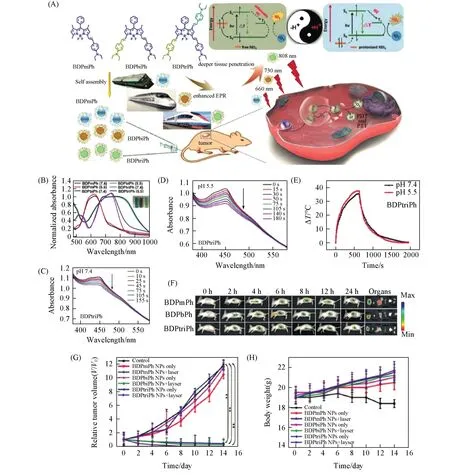
Fig.7 Penetration depth⁃tunable BODIPY⁃based nanoparticles for phototherapy[125]
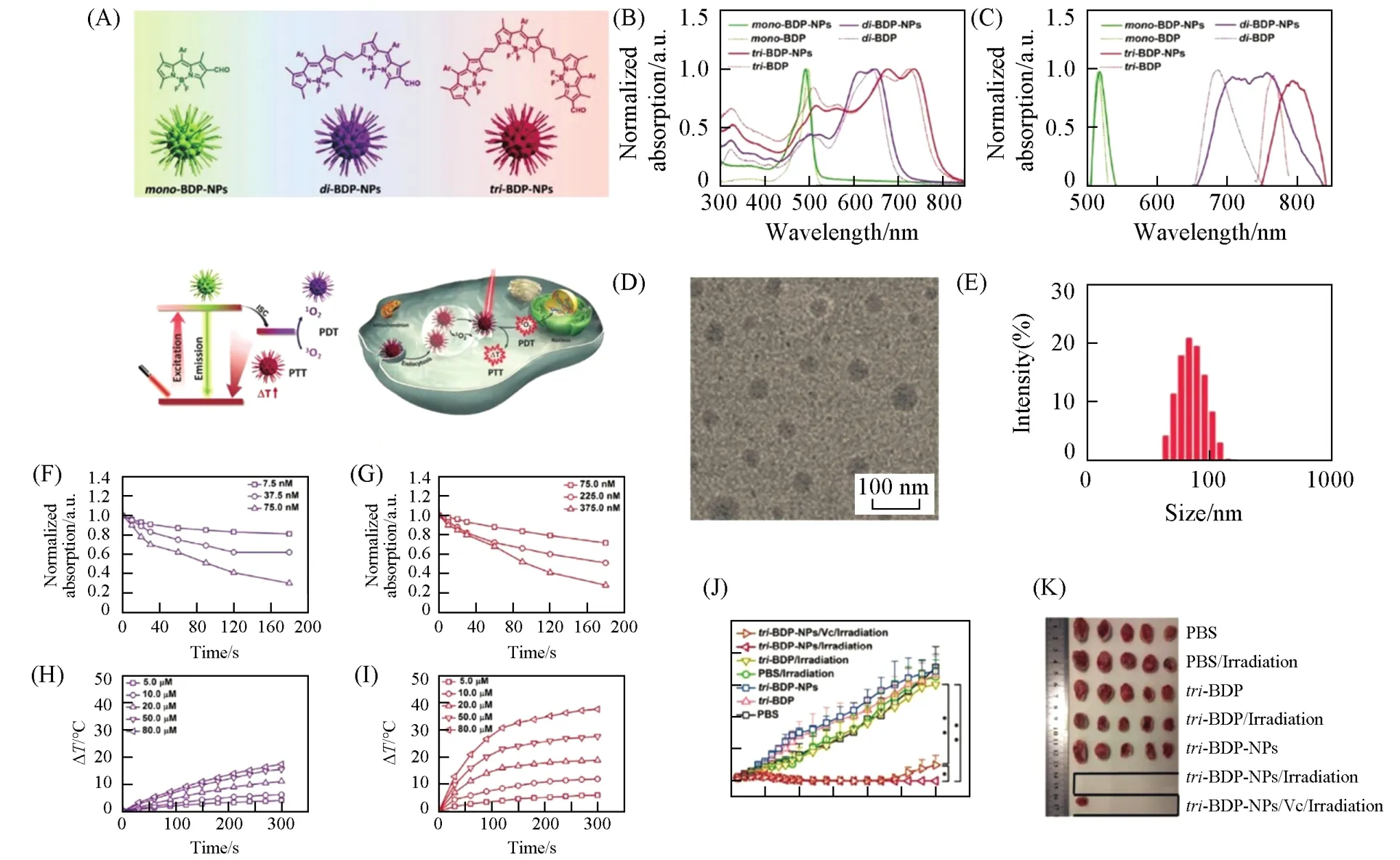
Fig.8 BODIPY⁃based nanoparticles with tunable phototherapeutic effect[123]
2.3 Cyanine Dyes
Cyanine dyes,which are also called polymethine cyanine dyes,are small organic molecules with two aromatic nitrogen-containing heterocycles linked by a polymethine bridge[54,136,137].As the simplest cyanine dyes,monomethine and trimethine cyanine(Cy3)exhibit absorption wavelengths in the visible region.However,the absorption wavelength can be easily bathochromic shifted ofca.100 nm by simply inserting one vinylene moiety into the linker bridge[138].As a result,pentamethinecyanine(Cy5)derivatives can subsequently achievea NIR(>700 nm)absorption,and the absorption wavelengths of heptamethinecyanines(Cy7)may beyond 1000 nm.Cy5,Cy5.5,Cy7,and their derivatives are representative NIR fluorescent dyes with high molar extinction coefficients and fluorescence quantum yields[139].Cyanine dyes have foundnumerous applications since their discovery,including nonlinearoptical materials[140,141],organic photovoltaics[142],chemosensors[143],and fluorescent probes[144—146].In recent years,the cyanine dyes have attracted increasing attention in phototheranostics due to their outstanding optical properties.Specifically,indocyanine green(ICG),which has beenapproved by U.S.food and drug administration(FDA)for almost over 50 years,has been applied for evaluating blood flow,drug clearance,and phototheranostics[147—150].However,ideal materials based on cyanine dyes for satisfactory phototheranostics are still lacking,because many cyanine dyes suffer from the inherent disadvantages of poor photostability and low fluorescence quantum yield.The photostability and fluorescence quantum yield of cyanine dyes play vital roles in their biomedical applications.Previous studies have revealed that enhanced photostability and fluorescence quantum yield can be achieved by introducing a rigid cyclohexenyl substituent to the middle of polymethine linker[151].In addition,functional groups can also be attached to the backbone of cyanine dyes for targeting.For instance,Huanget al.[152]developed a cyanine dye-based fluorophore with a triphenylphosphonio-modified side chain of triphenylphosphonio benzo cyanine(Cy-HPT)and co-assembled it with human serum albumin(HSA)to obtain colloidal HSA@Cy-HPT in aqueous solution(Fig.9).HSA@Cy-HPT was demonstrated to possess more stable optical properties,enhanced PTT/PDT efficiencies,and more satisfactoryin vivometabolism.Moreover,HSA@Cy-HPT exhibits outstanding tumor targeting in subcutaneous tumor xenograft models.Finally,HSA@Cy-HPT was successfully utilized in HepG2 tumor xenograft models and tumor growth was completely inhibited without any regrowth.The electron-donating(-withdrawing)property of the substituents also contributes the photostability of cyanine dyes.Chenet al.[153]found that the electron-withdrawing capability of theN-substituents of 3H-indolenine strongly affects the photostability of the cyanine dyes and the electron-donating groups are favorable for the improved photochemically stable cyanine dyes.They also observed that once substituted by the electron-donating group on the central chlorine atom atcyclohexene ring,the photostability of the cyanine dyes could be enhanced.Another study also suggests that the fluorescence quantum yield of cyanine dyes is significantly increased upon binding with nucleic acids or proteins as a result of the rigidization of the fluorophores[154].
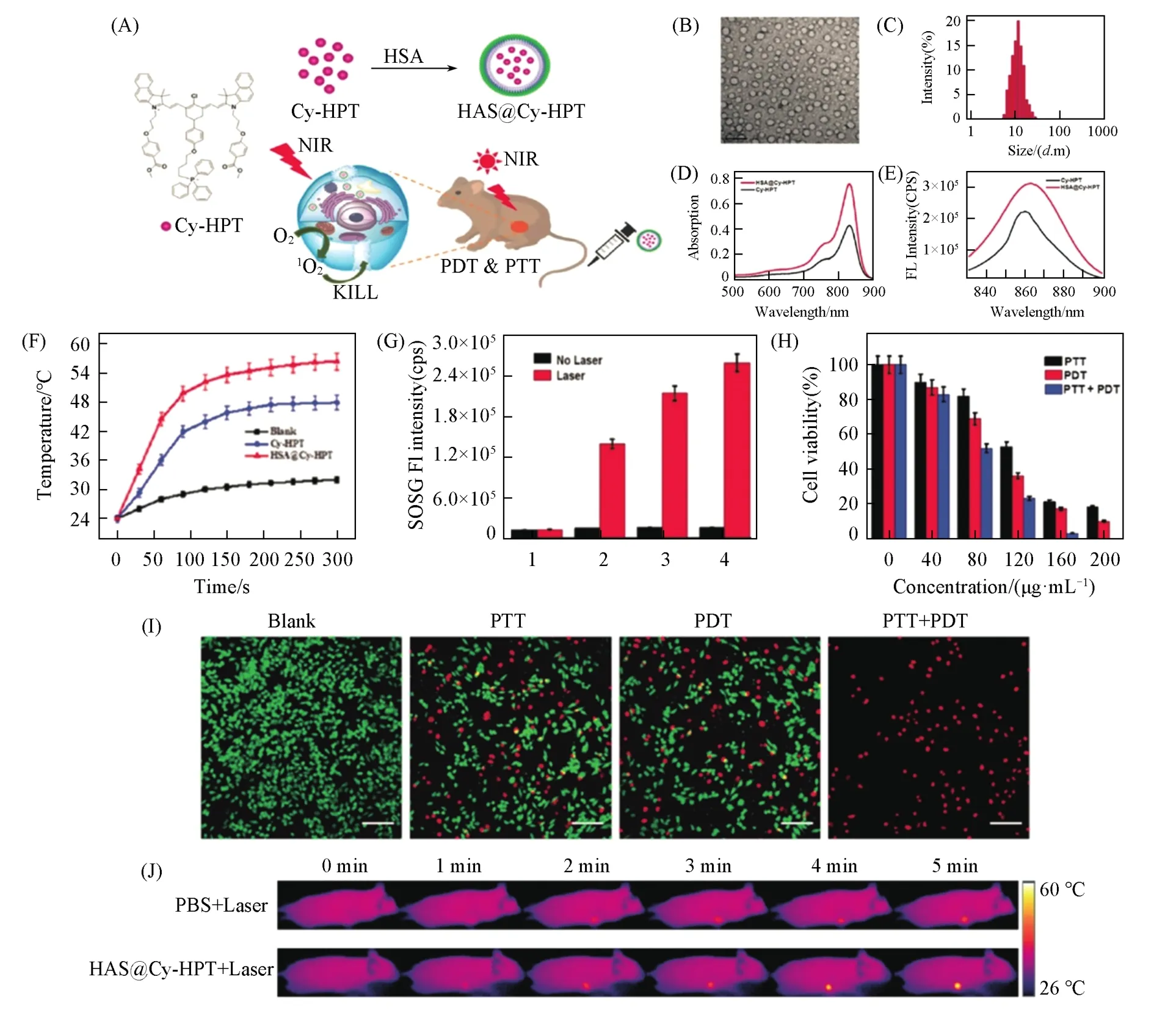
Fig.9 HSA@Cy⁃HPT for PTT and PDT[152]
2.4 Squaraine Dyes
Featured with a unique chemical structure of an oxocyclobutenolate core with aromatic or heterocyclic moieties at both ends of the molecules,squaraine dyes and their derivatives are also called squaryliumdyes[155].Squaraine dyes are generally synthesizedviaa condensation reaction between squaric acid derivatives,methylene bases,and electron-rich aromatics.Squaraine dyes possess excellent physicochemical properties including sharp absorption peak accompanied by intense NIR emission,high molar extinction coefficients,and good photoconductivity[156].With such unique characteristics,squaraine dyes are among the various promising functional dyes being used in a wide range of applications,such as dye-sensitized solar cells(DSSCs)[157],protein detection[158],fluorescence probes[159],and contrast agents forin vivoimaging[160,161].However,the large and hydrophobic nature of theπ-conjugated structures makes the biomedical applications of squaraine dyes challenging.Moreover,there are only few squaraine dyes that absorbing in NIR region.Numerous efforts have been made to obtain squaraine dyes with improved hydrophilicity and NIR-absorbing wavelengths.Umezawaet al.[162]synthesized a squaraine dye namely KSQ-4-H,with four water-soluble sulfonate groups attaching to a squaraine backbone.KSQ-4-H can be completely dissolved in PBS buffer with a sharp absorption peak at 775 nm.In addition,the bovine serum albumin(BSA)conjugates containing KSQ-4-H in phosphate buffer saline(PBS)solution exhibit absorption,excitation,and emission maxima of 787,760 and 812 nm,respectively,which are all in NIR region.Thanks to the elegant optical properties of this squaraine dye,it is believed to be promising for protein detection andin vivoimaging.Alternatively,Nakazumiet al.[163]conjugated two squaraines by a thiophene or pyrene unit to synthesize bis-squaraine dyes and these bis-squaraine dyes can readily bind to BSA as noncovalent labeling probes,which exhibit enhanced fluorescence in NIR region.Encapsulating the squaraine dyes into macrocycles is an alternative way to improve the stability and water-solubility of the dyes.For example,Gassensmithet al.[164]reported a squaraine dye with two rotaxanes:one rotaxane with four tri(ethyleneoxy)chains on the squaraine for water-solubility enhancement and the other rotaxane that has an encapsulating macrocycle for photostability improvement.This squaraine dye exhibits better stability in different solvents,such as water and serum,making it a potential versatile fluorescent scaffold for constructing various kinds of highly stable NIR imaging probes.Nevertheless,the development and application of squaraine dye-based NPs in phototheranostics fall behind as compared with aforementioned counterparts,such as porphyrin and phthalocyanine dyes,BODIPY dyes,and cyanine dyes.Sunet al.[165]synthesized a squaraine dye of SQP and prepared the J-and H-aggregate NPs of bispyrrole-sq-bispyrrole NPs[SQPNPs(J)]and bispyrrole-sq-bispyrrole NPs[SQP-NPs(H)]based on this dye(Fig.10).They found that SQPNPs(J)showed an emission maximum near 1100 nm with a 4.8 folds-enhanced emission than that of SQPNPs(H).Furthermore,SQP-NPs(J)can be used for NIR-Ⅱ fluorescence imaging-guided PTT against MCF-7 tumor due to the highly effective photothermal properties of SQP-NPs(J),which make significant contributions to precise tumor theranostics.This work presents a novel strategy to enhance the fluorescence emission of squaraine dye-based NPs in NIR-Ⅱ regionviamanipulating the molecular packing style in NPs.However,more efforts should be devoted to exploring efficient squaraine dye-based NPs for cancer phototheranostics.
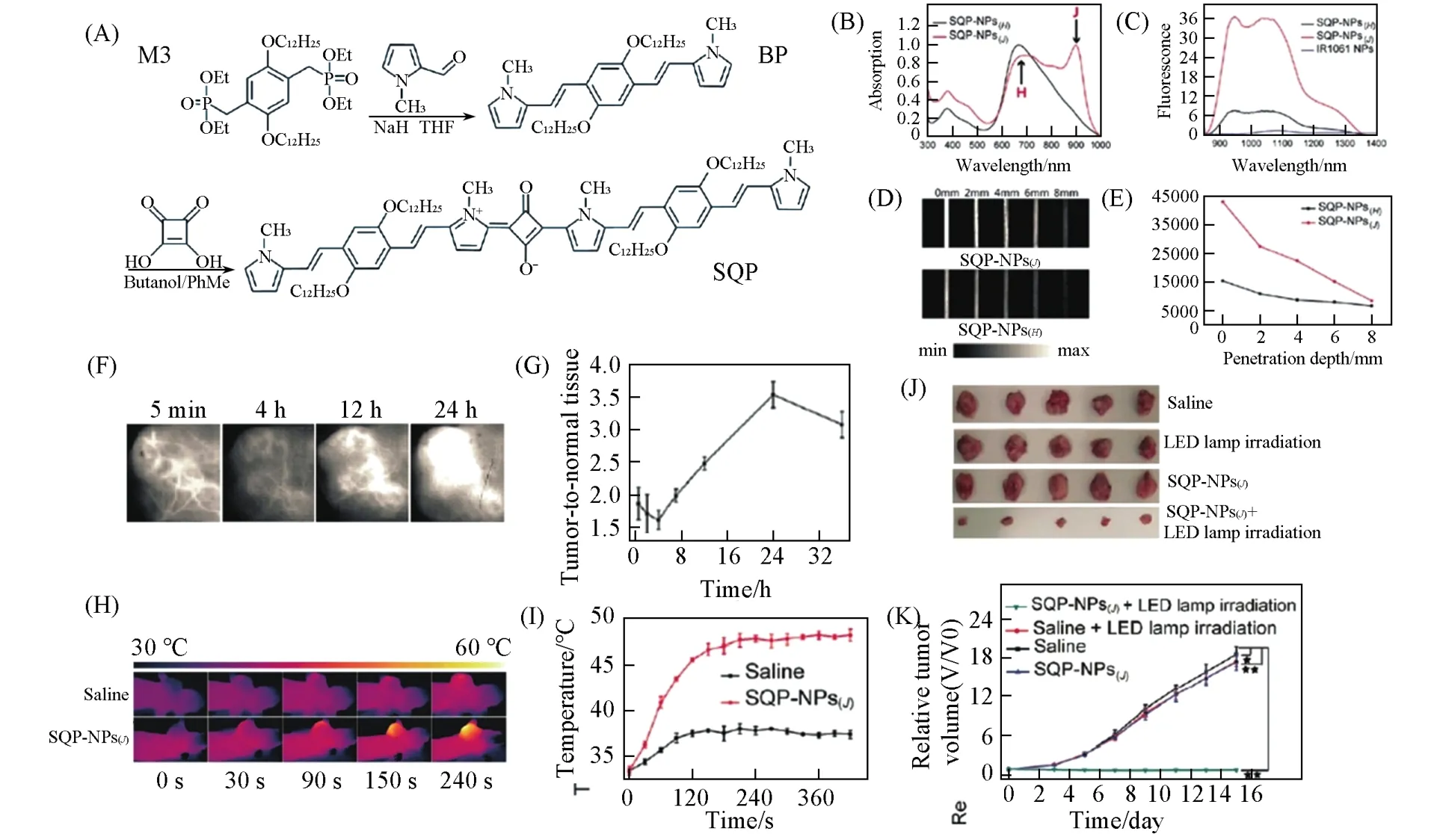
Fig.10 SQP⁃NPs(J)for NIR⁃II fluorescence imaging⁃guided PTT[165]
3 D-A Conjugated Small Molecule-Based NPs
D-A conjugated small molecules are organic small molecules consisting of electron donors and electron acceptors withπ-conjugated structures.On one side,orbital hybridization of the D-A structures leads to a narrow bandgap of the D-A conjugated small molecules,thus affording a long absorption wavelength of these molecules[166].On the other side,the extension of theπ-conjugated structures in D-A conjugated small molecules contributes to their high molar extinction coefficients[166].Taking these advantages together,D-A conjugated small molecules have found their wide applications in diverse fields,such as organic solar cells(OSCs)[167—172],organic light-emitting diodes(OLEDs)[173—175],organic field-effect transistors(OFETs)[176—178]and organic photodetectors(OPDs)[179—181].
In recent years,D-A conjugated small molecules,especially their NPs,have further been applied in biomedical applications ranging from NIR fluorescence imaging[182,183]and PAI[182]to imaging-guided phototherapy(PTT and/or PDT)[58,61—64,66,67].Considering the diversity of the donor and acceptor units,one can obtain ideal D-A conjugated small molecules and their NPs with satisfactory phototheranostic performance by combining different donor and acceptor units and the D-A conjugated small molecule NPs may play an important role in future cancer phototheranostics.
3.1 Diketopyrrolopyrrole(DPP)-Based D-A Conjugated Small Molecules
Diketopyrrolopyrrole(DPP)is the most popular one among the various electron acceptors for constructing D-A conjugated small molecules[184].Owing to their NIR absorption,high molar extinction coefficients,and excellent photostability,DPP-based D-A conjugated small molecules have been used extensively as chromophores in the field of phototheranostics.Molecularly isolated DPP-based D-A conjugated small molecules in solution generally tend todisplay relatively high fluorescence quantum yields.However,this fluorescence can be efficiently quenched in aggregates.Therefore,DPP-based D-A conjugated small molecule NPs in their aggregation state are excellent PTT(PAI)-active agents with high photothermal conversion efficiency and satisfactory PAI performance.There are several reports on DPP-based D-A conjugated small molecule NPs for imaging(fluorescence,photoacoustic,and/or photothermal)-guided phototherapy(PTT,PDT and/or synergistic PTT/PDT)[58,61,64,67].A representative example among them is the work by Caiet al.[58],they reported a strong NIR-absorbing D-A-D conjugated small molecule(DPP-TPA)constructed by using ofN,N-diphenyl-4-(2-thienyl)aniline(TPA)substituents as the electron donors and DPP as the electron acceptor(Fig.11).Monodispersed DPP-TPA NPs with a size of 76 nm were obtained by reprecipitation of a THF solution of DPP-TPA into water.Under laser irradiation(660 nm,1 W/cm2,10 min),a PBS solution containing 80 μg/mL of DPP-TPA NPs gave rise to a rapid temperature increase ofca.35℃,whereas the control PBS solution has no significant increase.The photothermal conversion efficiency of DPP-TPA NPs was calculated to be 34.5%.In addition to the remarkable photothermal effect,DPP-TPA NPs were also proved to possess good singlet oxygen generation capability with a singlet oxygen quantum yield of 33.6%.A significant tumor temperature increase was observed in a murine cancer xenograft model under laser irradiation(660 nm,1 W/cm2)after 2 h(guided by PAI)of intravenous injection of DPP-TPA NPs,demonstrating efficient PAI-guided synergistic PTT/PDT of DPP-TPA NPs.The development of PSs with both enhanced fluorescence and singlet oxygen quantum yields is of great urgency and significance for cancer phototheranostics.By a photosensitizer synergistic effect,Zouet al.[67]developed a D-A-D conjugated small molecule(DPPBDPI)linking DPP and BODIPY with a benzene ring as the bridge(Fig.12).Interestingly,the combination of DPP and BODIPY can significantly increase the fluorescence quantum yield(5.0%)and the1O2quantum yield(80%)simultaneously by the synergistic effect of these two photosensitizers.Obtained by a nanoprecipitation method,DPPBDPI NPs not only exhibit high phototoxicity,but also present negligible dark toxicity towards HeLa cells,revealing their excellent PDT efficacyin vitro.In vivofluorescence imaging reveals that DPPBDPI NPs can rapidly accumulate in the tumor siteviathe EPR effect and can efficiently inhibit tumor growth by PDT.The enhanced fluorescence imaging and PDT performance of DPPBDPI NPs suggest that the synergistic effect of DPP and BODIPY presents a novel molecular design strategy for developing versatile D-A conjugated small molecule NPs-based phototheranostics.It should also be noted that except for the nanoprecipitation method for DPP-based conjugated small molecule NPs preparation,several other methods have also been reported for their preparation.For example,Heet al.[185]synthesized DPP-based carbon dots through a facile one-pot hydrothermal reaction in the presence of chitosan.The as-prepared carbon dots were successfully utilized as an efficient phototheranostic agent for fluorescence imaging-guided PDT.

Fig.11 DPP⁃TPA NPs for PAI⁃guided synergistic PTT/PDT[58]

Fig.12 DPPBDPI NPs for enhanced PDT[67]
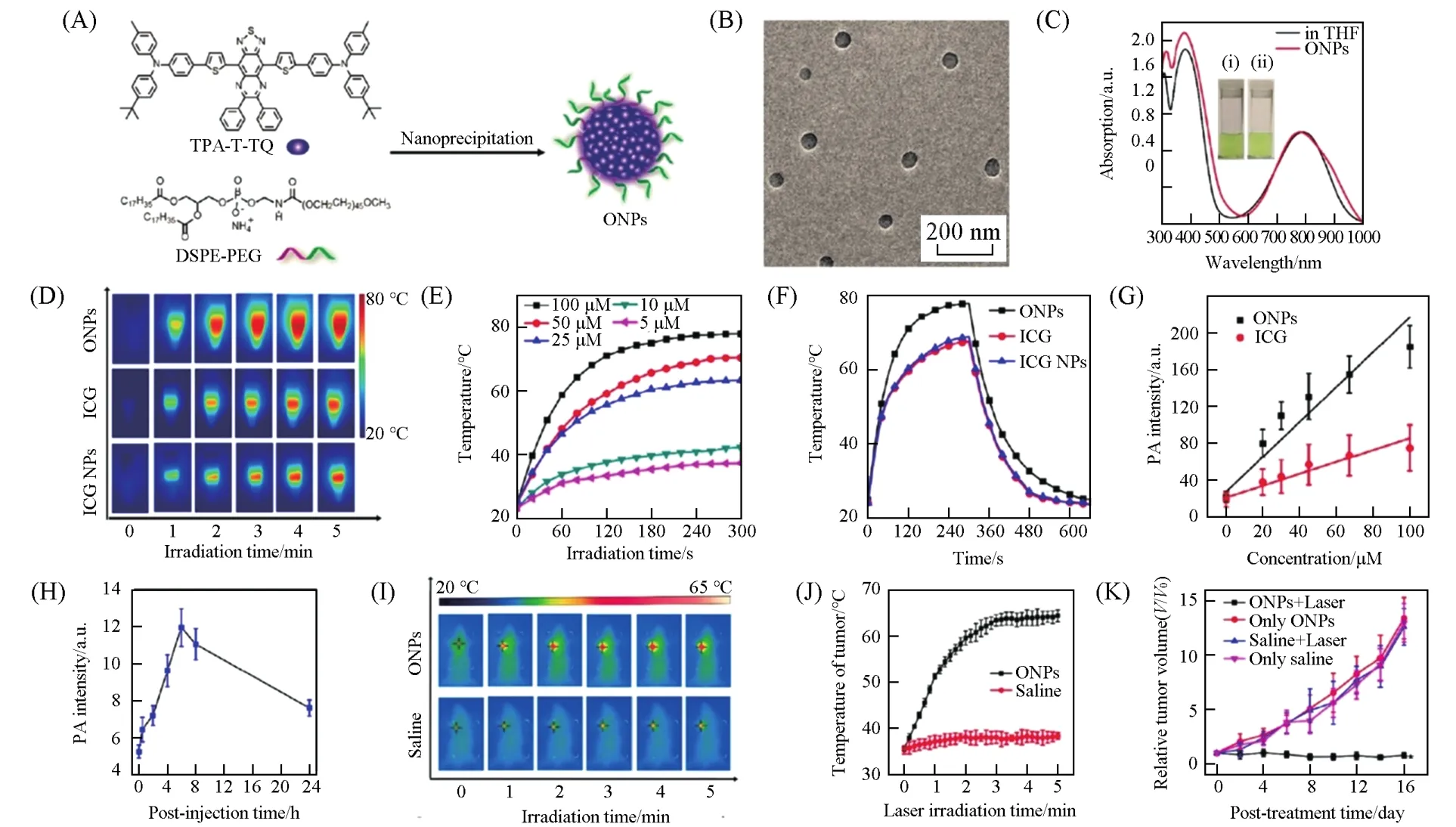
Fig.13 ONPs for PAI⁃guided PTT[63]
3.2 Other Acceptors-based D-A Conjugated Small Molecules
In addition to DPP,several other acceptors,such as benzobisthiadiazole(BBT)[60,62,65],thiadiazoloquinoxaline(TQ)[57,63],pyrrolopyrrolecyanine(PPCy)[186],pyrrolopyrroleaza-BODIPY[187],2-methylene-[3-(1,1-dicyanomethylene)-indanone][59]and naphthalene diimide-fused 2-(1,3-dithiol-2-ylidene)acetonitriles(NDTA and 2NDTA)[66],also have been incorporated with various donors to afford D-A conjugated small molecules for phototheranostics.Qiet al.[63]developed highly stable and biocompatible phototheranostic organic nanoparticles(ONPs)based on a D-A conjugated small molecule of TPA-T-TQ,which consists of TQ as the acceptor,TPA as the donor,and thiophene ring(T)as the linking bridge(Fig.13).The TPA-T-TQ ONPs possess superior PAI,PTT performance,thermal/photothermal stability,and photo bleaching resistance to the clinically used dye of ICG.With such merits combined in a sole system,the TPA-T-TQ ONPs can serve as an efficient phototheranostic agent forin vivoPAI-guided PTT.Regulating the photochemical/photophysical processes(radiative relaxation,nonradiative relaxation,and ISC,etc)in the excited states of the D-A conjugated small molecule NPs can precisely control their PTT and/or PDT functionalities,thus providing opportunities for researchers to exploit more phototheranostics with enhanced performance.Based on a“molecular motion in aggregates”strategy,Liuet al.[62]takes advantage of the twisted intramolecular charge transfer(TICT)state to promote the photothermal effect.They synthesized a series of D-A conjugated small molecules(NIR6,NIRb6,NIRb10,and NIRb14)with the same backbone attached with different sized alkyl side chains(Fig.14).With long alkyl side chains branched at the second carbon,NIRb14 exhibits enhanced photothermal effect as compared with NIRb6,with short branched side chains,and much better than NIR6,with short linear side chains,and the commercial gold nanorods.NIRb14 NPs can be used for PAI-guided PTT bothin vitroandin vivo.In addition,charge reversal poly(β-amino ester)(PAE)modification makes NIRb14 NPs specifically accumulate at tumor sites,which further improves the phototheranostic efficacy.Through a similar strategy,by combining tetraphenylethylene(TPE)and 2NDTA,Zhaoet al.[66]synthesized D-A conjugated small molecule of 2TPE-2NDTA and prepared their NPsviaa nanoprecipitation method with the assistance of DSPE-PEG2000.The elevated photothermal effect of 2TPE-2NDTA NPs makes them ideal agents for high-contrast PAI and effective PTT of tumors.Recently,a molecular engineering strategy of“increasing the number of thiophene bridges”was adopted by Shaoet al.[188]to synthesize the first NIR-Ⅱ D-A-D conjugated small molecule for PAI-guided PTT.Three conjugated small molecules(CSM0—2)were synthesized and the photo-responsivity is demonstrated to undergo a transition from NIR-Ⅰof CSMN0 to NIR-Ⅱ of CSMN2 with the number of thiophene bridges increasing from 0 to 2.With excellent biocompatibility and high photothermal efficiency in NIR-Ⅱ,CSMN2 enables the PAI-guided deep-tissue NIR-ⅡPTT on HuH-7 subcutaneous xenograft murine modelsin vivo(Fig.15).

Fig.14 NIRb14 NPs for PAI⁃guided PTT[62]

Fig.15 Molecularly engineered CSMN2 for PAI⁃guided NIR⁃II PTT[188]
4 AIEgen-Based NPs
As mentioned above,under most circumstances,the OSMs are in aggregation state as OSMNs for biomedical applications.However,conventional OSMs suffer from aggregation-caused quenching(ACQ)effect when they are formulated into OSMNs,leading to reduced imaging and therapeutic efficacy.AIEgens are the unique fluorophores that exhibit exactly opposite behavior and the enhanced fluorescence quantum yield of AIEgen-based NPs is ascribed to the restriction of intramolecular motion(RIM)inside NPs that largely avoids nonradiative decay[189,190].This feature of AIEgen-based NPs makes them capable of long-term cell tracking[191],intravital vasculature imaging[192],and so on.Except for the predominant advantage of enhanced fluorescence,AIEgen-based NPs also possess other advantages favorable for phototheranostics,such as excellent photostability and biocompatibility.
Recent years have witnessed the potential applications of AIEgen-based NPs forin vivoimaging and ima-ging(fluorescence and/or photoacoustic)-guided phototherapy(PTT and/or PDT)and surgery[9,69,189,190,193].In the meantime,due to the deeper understanding of biological systems,the development of designed materials with versatile functionalities and high efficacy have achieved great progress.For instance,the imaging depth of AIEgen-based NPs can be substantially deepened by extending their absorption/emission wavelengths to NIR-Ⅱ,due to the minimized energy loss and reduced autofluorescence interferencein tissue in this region[13—15,20,194].Additionally,the utilization of NIR AIEgen-based NPs also benefits multi-photon imaging through more effective excitation.However,researchers should further dedicate to this field to explore more efficient AIEgen-based NPs for phototheranostics.
In order to develop AIEgen-based NPs with enhanced performance,there are several strategies to modulate the energy conversion efficiency of the specific energy decay pathways related to fluorescence,photoacoustic imaging,PTT and PDT.(1)Enhanced photothermal and photoacoustic effect enabled by boosting the nonradiative decay.It is well known that the fluorescence quantum yield of AIEgens is predominantly dependent on the RIM effect of the rotor-like structure.On the other hand,the free motion of these rotor-like structures could also be well utilized through nonradiative decay of the excited state to release heat for photothermal and photoacoustic effect enhancement.Wanget al.[72]reported an AIEgen of TFM,which sheds light on the rational design of highly effective phototheranostic AIEgen-based NPs(Fig.16).Featuring a D-A-D structure with triphenylamine/methoxyl groups as the donors and 2-(9H-fluoren-9-ylidene)malononitrile as the acceptor,TFM exhibited a narrow band gap of 1.77 eV,thus leading to its NIR absorption.The large D—A conjugation and HOMO-LUMO separation also led to a small singlet-triplet energy gap(ΔEST=0.09eV)of TFM,which facilitated the ISC process and promote ROS generation.The fluorescence quantum yield of TFM NPs is as low as 0.17%,indicating its great potential for PAI and PTT through utilizing nonradiative decay pathways.Both X-ray diffraction(XRD)measurement and molecular dynamics(MD)simulation revealed that TFM molecules packed loosely and disorderly in an amorphous state in TFM NPs,which favored nonradiative decay pathways.The photothermal conversion efficiency of TFM NPs was calculated to be as high as 51.2%.Moreover,a 10 folds-amplified PA intensity can be observed at the tumor site compared to that of the background after injection of TFM NPs.With strong heat,ROS generation capability,and pronounced photoacoustic effect,this single component-based agent can be readily used as a highly efficient phototheranostics for PAI-guided synergistic PTT/PDT.(2)Enhanced fluorescence emission and1O2generation enabled by suppressing nonradiative decay.It is well known that suppressing nonradiative decay of the singlet excited state is an effective approach to afford enhanced fluorescence emission and1O2generation.Specifically,crystallization-induced restriction of intramolecular motions of AIEgens within the NPs leads to enhanced fluorescence emission.Previous report has revealed that AIEgen nanocrystals exhibit more than 4 folds-enhanced fluorescence emission as compared to that of amorphous AIEgen nanoaggregates[195].Alternatively,the encapsulating matrix used in nanoprecipitation also plays an important role in controlling the behavior of AIEgen NPs.A recent work by Guet al.[70]utilized rigid corannulene to suppress the nonradiative decay of AIEgens for fluorescence and ROS enhancement(Fig.17).Bowl-shaped corannulene is highly hydrophobic and expected to form a confined microenvironment in aqueous solution.An AIEgen of 4-{2,2-bis[4-(diphenylamino)phenyl]1-(4-methoxyphenyl)vinyl}-1-methyl pyridinium hexafluoro phosphate(TPP-TPA)was encapsulated by DSPE-PEG and corannulene-PEG(Cor-PEG),respectively,to demonstrate this proof-of-concept.Cor-AIE dots showed a 4.0 folds-enhanced fluorescence quantum yield and 5.4 folds-enhanced ROS generation capability as compared with that of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]-AIE(DSPE-AIE)dots.Both nuclear magnetic resonance(NMR)spectroscopy and density functional theory(DFT)calculation validated that positively charged TPP-TPA interacts strongly with corannulene by dipole-dipole and electrostatic interactions,resulting in a more confined and rigid microenvironment to restrict the intramolecular motion of the AIEgens.All the calculation and experimental results suggest that Cor-AIE dots exert more effective suppression of nonradiative decay,thus leading to the increased energy flow to both fluorescence emission and ROS generation for efficient cancer phototheranostics.(3)Enhanced1O2generation enabled by regulating spin-orbital coupling(SOC)and ΔEST.1O2generation can be tuned by manipulating the ISC process from a singlet to triplet state,whose rate is determined by SOC and ΔEST[196].Introducing of heavy atoms can effectively promote SOC,which is known as the“heavy atom effect”,and this strategy is generally used in conventional small molecule dyes,such as BODIPY dyes as mentioned above[128,129].Numerous attempts have been made to reduce the ΔESTof AIEgens for improved1O2generation capability.For example,an AIEgen of PPDC exhibits a high1O2quantum yield of 89%with a small ΔESTof 0.27 eV[197].A novel concept of“polymerizationenhanced photosensitization”has been proposed by Wuet al.[198].The polymerized AIEgen CP1 possesses a 5.06 folds-enhanced1O2quantum yield as compared with its monomer SM1,the high1O2and fluorescence quantum yields(6.2%)endow CP1 NPs excellent performance for fluorescence imaging-guided PDT.This“polymerization-enhanced photosensitization”originates from the increased channels available for the ISC processes.More recently,several well-designed AIEgens with triphenylamine(T)as donor and benzothiadiazole(B)as acceptor have been developed by Liuet al.[71].They studied the effects of various D-A conjugation fashion on the photosensitization and observed an interesting D-A even-odd effect on the photosensitization of the AIEgens,which provides deeper insights into the structure-property relationship of the AIEgens(Fig.18).

Fig.16 TFM NPs for PAI⁃guided PTT/PDT[72]
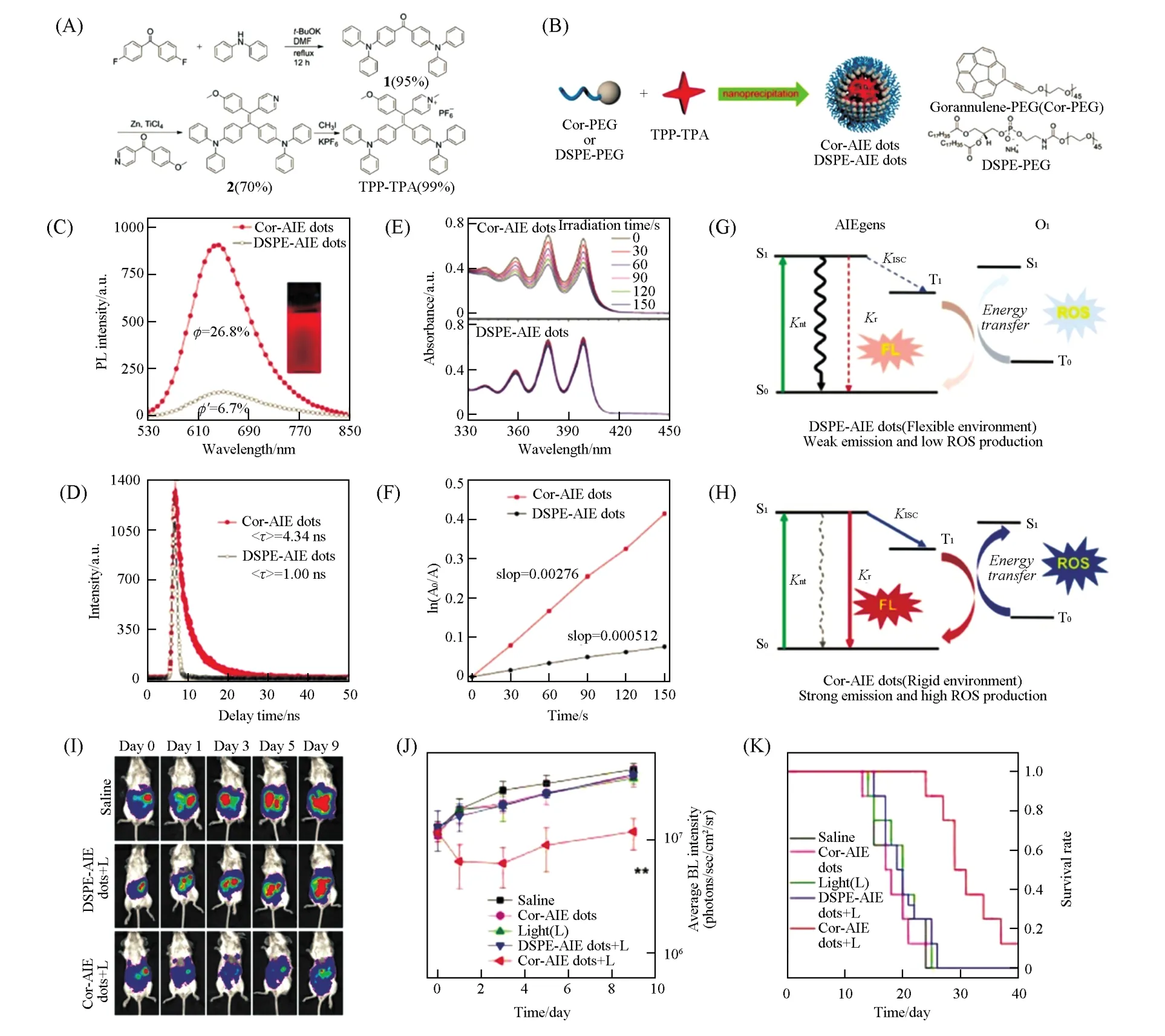
Fig.17 Cor⁃AIE dots for enhanced PDT[70]
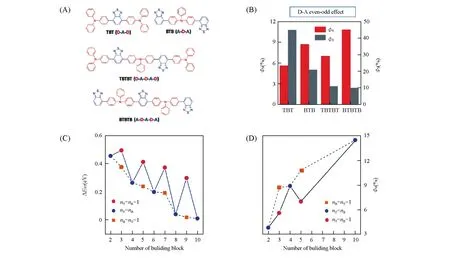
Fig.18 D⁃A even⁃odd effect on the photosensitization of the AIEgens[71]
5 Concluding Remarks and Perspectives
The past decades have witnessed the development of functional materials in cancer phototheranostics including inorganic nanomaterials,polymeric nanoparticles,and OSMNs as summarized in this review.Despite the satisfactory antitumor efficacy of OSMNs in animal models described in literature,it is still far from the final clinical applications of these agents.Researchers should devote more to further the progress of this field.
Since tumor accumulation of most of the phototheranostic agents takes advantage of the EPR effect,which is a passive targeting strategy with low efficacy.It is necessary to endow phototheranostic agents active targeting capability for the improved theranostic outcome.One can achieve this goal either by encapsulating OSMs by targeting ligand-bearing matrix or by targeting ligand post-modification of OSMNs.On the other hand,the inherent tumor microenvironment such as low pH,high ROS level can be used to construct stimuli-responsive phototheranostic OSMNs,which is also believed to be an effective approach for high theranostic efficacy.
From a more basic point of view,researchers should gain more insights into the structure-property relationship of OSMs and OSMNs.For example,the diversity of D and A units provides opportunities for researchers to construct various D-A conjugated small molecules and more information on the structure-property relationship of these materials may be obtained.Therefore,more efficient phototheranostic agents may be explored,for instance,those can be used in NIR-Ⅱ.It is also of great significance to study the property of OSMNs with different stacking fashions of OSMs.Different stacking fashions of OSMs lead to different optical properties of OSMNs,which may contribute to their different phototheranostic functionalities.
This paper is supported by the National Key Research and Development Project-Nanotechnology Funding,China(No.2016YFA0203600).
