MUTYH:Not just polyposis
2020-09-14
Maria Cristina Curia,Gitana Maria Aceto,Department of Medical,Oral and Biotechnological Sciences,“G.d'Annunzio” University of Chieti-Pescara,Chieti,Via dei Vestini 66100,Italy
Teresa Catalano,Department of Clinical and Experimental Medicine,University of Messina,Messina,Via Consolare Valeria 98125,Italy
Abstract
Key words:MUTYH;Polyposis;Colorectal cancer;Base excision repair;Mutations;Single nucleotide polymorphism
INTRODUCTION
Biallelic germlineMUTYH(MutY homologEscherichia coli,homolog ofMYH,hMYH)gene variants were historically identified in a fraction ofAdenomatous Polyposis Coli(APC)mutation-negative cases with a phenotype overlapping with attenuated familial adenomatous polyposis (AFAP) or classical familial adenomatous polyposis (FAP)(MIM# 604933)[1,2].In humans,MUTYH protein is considered a cell protective factor able to counteract oxidative damage.It is a DNA glycosylase involved in the restoration of post-replicative mispairs in double-stranded DNA,where MUTYH recognizes and specifically removes adenine or 2-hydroxyadenines misincorporated in 7,8-dihydro-8-oxoguanine (8-oxoG) pairs with adenine or cytosine inducing G:C to T:A transversions as a consequence of DNA replication errors or DNA recombination[3-5].
In 1996E.coli mutYgene (mutY) was cloned and characterized for the first time[6].It encodes a protein involved in the bacterial repair system together with mutM,homologous of human 8-Oxoguanine glycosylase 1 (OGG1),and mutT that hydrolyzes 8-oxo-dGTP[7-9].InE.coliMutY,MutM and MutT compose the GO (formally known as Guanine Oxidation) system responsible for removing and correcting mutations due to adducts[7].Human MUTYH and OGG1 act like theirE.colihomologs MutT and MutM to avoid the mutagenic consequences induced by 8-oxoG.Moreover,MUTYH shows 41% of homology to theE.colicorresponding protein[6].In 1998,the sequence ofSpMYHgene,themutYhomolog of the yeastSchizosaccharomyces pombe,was identified[10].The SpMYH protein,that removes misincorporated G from 8-oxoG,shows 28% and 31% identity toE.coliMutY and human MUTYH,respectively[10,11].In 2002,the role of theMUTYHgene in inherited predisposition to colorectal cancer(CRC) was identified in a British family,suggesting that an inefficient MUTYH protein and a defective base excision repair (BER) increase the incidence of somatic G >T transversions inAPCgene[1].Subsequent studies described germline biallelic mutations involved in MUTYH associated polyposis (MAP),a recessive heritable colorectal polyposis with an increased risk of CRC[12,13].Further research exploredMUTYHmodifications associated with a hypermutable phenotype involving different organs and the development of various diseases,including cancers,through the accumulation of unrepaired 8-oxoG under conditions of oxidative stress.
In this review we evaluate biochemical and functional aspects as well as the role of MUTYH in BER.Moreover,we focus on current and updated studies on human diseases induced by alteration of MUTYH through research that associate it with neoplastic and non-neoplastic pathologies different to colon polyposis and CRC.This will produce novel insights into the understanding of the molecular basis underlyingMUTYH-related pathogenesis.Furthermore,we describe the association betweenMUTYHsingle nucleotide polymorphisms (SNPs) and different cancer and non-cancer diseases.
We address the utility to increase our knowledge regardingMUTYHin light of recent advances in the literature with the aim of a better understanding of the potential for identifying new therapeutic targets.
MUTYH STRUCTURES AND FUNCTIONS
In mammals,oxidized bases are continuously induced by endogenous reactive oxygen species (ROS) and more abundantly after oxidative stress.In the genome the oxidized bases are mutagenic due to their incorrect coupling property[14,15].Moreover,to prevent the establishment of a new mutated base,the error must be repaired before DNA replication.In fact,the oxidized bases present in the model filament do not impede the replicative polymerases to prevent mutation[16].BER is one of the most important DNA repair pathways,which improves intrinsic DNA damage due to metabolic processes[17-19]and plays a very important role in maintaining the integrity of the genomic structure.Therefore,the functions of its main components are highly conserved during evolution[20].The repair mechanisms of DNA damage are sustained by proteins belonging to different signaling pathways.Among these pathways,the BER plays a crucial role in the correction of DNA errors due to oxidation,deamination and alkylation[5].The BER pathway consists of a series of coordinated enzymatic events:Initially the specific DNA glycosylases recognize and exclude damaged bases or abasic sites and then,in a subsequent step,different sequential proteins correct the DNA through the template-direct insertion of one or more nucleotides,starting from the damaged site.
In mammals,MUTYH is functionally associated with OGG1 to eliminate and replace the oxidized guanine with a guanine[5,9,21,22].The incorrect incorporation of 8-oxoG into DNA is initially recognized by the bifunctional glycosylase OGG1,that recognizes 8-oxoG coupled to the C base.This glycosylase breaks down 8-oxoG and subsequently catalyzes its elimination from which an abasic site is formed at 5’ of the nascent DNA[23].If the excision due to the OGG1 action does not occur correctly,the persistence of 8-oxoG in the nascent DNA leads to the formation of damaged pairs of 8oxoG:A,which is fairly stable and can easily escape the correction activity by DNA polymerase.At this point the role of MUTYH is fundamental because the elimination of the correct base A induces the formation of an 8-oxoG:C base pair,offering OGG1 another opportunity to eliminate the 8-oxoG lesion before the mutation becomes permanent in the nascent DNA[23].Finally,Nudix hydrolase (NUDT1,alias MutT human homolog 1 or MTH1) intervenes in the GO repair pathway to hydrolyze the nucleoside triphosphates 8-oxoG (8-oxoGTP) into monophosphates (8-oxoGMP) in order to prevent their incorporation into the genome.In this way NUDT1 also prevents that the removal of base A,from the DNA strand model,becoming promutagenic after the action of MUTYH[23].
MUTYH is a bifunctional A/G-specific adenine DNA glycosylase[24],and is one of five BER-initiating enzymes in mammalian cells.Its glycosylase function has been known for at least thirty years and consists,as already mentioned,of eliminating the adenines or the 2-hydroxy-adenines (2-OH-A) paired with respect to 8-oxoG[25].8-oxoG is an oxidized form of the guanine base derived from ROS,is a stable product on DNA and has a lower oxidation potential than guanine.Traditionally it is considered one of the main mutagenic lesions of DNA with evolutionary and adaptive meaning[26].In fact,8-oxoG manages to couple with both adenine and cytosine during DNA replication;in this way it has the potential ability to cause a high transversion rate from G:C to T:A and A:T to G:C[27,28].
MUTYH,together with the other enzymes that repair the 8-oxo-G mis-pairing is a phylogenetically conserved protein (as MutY).Indeed,MUTYH homologous proteins have been identified both in prokaryotes and eukaryotes confirming the importance of its function in the protective mechanism from oxidative damage to DNA[15,29].
In mammals,the connection between C and N-terminal MUTYH domains,called the interdomain connector (IDC),is significantly longer than that of its bacterial counterpart[30].Furthermore,even among mammals the homology of the IDC sequence is variable (i.e.H.Sapiensshares 78% of MUTYH withM.musculus) and this region has probably evolved within eukaryotic homologs giving way to MUTYH to interact with other enzymes of the reparative response to DNA damage[3,28,31].Although the role of MUTYH glycosylase has been biochemically well defined for some time[32],new functional elements have recently been discovered:In fact,in addition to the Fe-S cluster,the need for a cofactor of the Zn2+ion for the recognition and repair of damage has been demonstrated[31].
To perform its functions,MUTYH associates with various partner proteins[33].This is necessary to ensure effective BER in coordination with other cellular processes.Figure 1 shows the known functional domains of MUTYH protein (data derived from the Ensembl genome database[34]and are representative ofMUTYHwell-known typical mutations).MUTYH consists of two globular domains linked by a flexible IDC,the Nterminal harbors the mitochondrial targeting signal (MTS),an adenine recognition domain,the helix-hairpin-helix-GPT (HhH-GPT) domain and a [4Fe-4S]+2cluster cofactor coordinated to 4 Cys residues[35];the switching between the reduced and oxidized form allows sensing of the DNA helical integrity damage.Peculiar characteristics of mammals in the IDC are 3 Cys coordinated with a Zn+2ion;this “zinc linchpin” region could play an important role in coordinating the N-terminal and Cterminal domains of MUTYH for the correct 8oxoG:A recognition/excision and subsequent repair[30,31].In fact,C-terminal domains are necessary for 8-oxoG recognition[36].Evidence suggests that post-translational modifications in MUTYH may be a mechanism to modulate the BER pathway[37].Indeed,phosphorylation of Ser524,on the PCNA binding site,modifies the affinity for the DNA substrate;recently it has been demonstrated that MUTYH protein levels may be regulated by momoubiquitination E3 ligase Mule (also called Huwe1,ArfBP1 and Lasu1),with target 5 Lys in the C-terminal[38].Poly [ADP-ribose] polymerase 1 (PARP1) and p53 may regulate MUTYH expression and participate in MUTYH-mediated cell death[22,39,40].MUTYH also displays crosstalk with the mismatch repair complex MSH2/MSH6[41,42],interacts with sirtuin (SIRT6) and may be modulated by acetylation[43].It directly associates with PCNA and co-localizes with PCNA to nucleus replication foci during G1/S phase[44];this improves polymerase processivity[45].
The protein interaction domains and other MUTYH motifs are shown in Figure 1;all reported information are available in the Entrez Gene NCBI database,the http//Ensembl.org database[46],and the ClinVar database [http://simpleclinvar.broadinstitute.org][47].Rad9-Hus1-Rad1 complex (9–1–1) facilitates ATRmediated Chk1 phosphorylation and activation,which in turn elicit cellular responses such as arresting cells in the G2/M phase[48].The N-terminal 32-aa peptide also contains a binding site for replication protein A,indicating that some isoforms of MUTYH may participate in replication-coupled repair[49].In the rat brain,specific mitochondrial isoforms of MUTYH protein have been observed:They are developmentally regulated and induced by respiratory hypoxia in the hippocampus[50].
Initially,MUTYHmutations have been linked to the MAP syndrome known as MUTYH associated polyposis[1],an autosomal recessive disorder characterized by multiple colorectal adenomas.In the intestinal epithelium,progenitor cells with loss of MUTYH function can escape from programmed cell death,accumulate Kras and APC,thereby promoting carcinogenesis[28](Figure 2);although later SNP variants that decreased the affinity and catalytic activity on 8oxoG:A[51]were also associated with other complex pathologies (see below).
MUTYHgene is located on chromosome 1 (p32.1-p34.3) (seq ref NM_001128425.1.)and consists of 16 exons[6,52].In human cells,three primary transcripts (α,β,γ) were experimentally identified;from these multiple transcripts were generated by alternative splicing and exon skipping[53].Transcription initiation and splicing of theMUTYHpre-mRNA produces at least thirteen mRNA isoforms and nine protein isoforms with different 5’-terminal mRNA and N-terminal protein sequences[53].Of the two major MUTYH isoforms,MUTYH 1 (p57) is the product of the α transcript and contains the mitochondrial targeting signal (MTS) at its N-terminus to establish it in mitochondria,whereas MUTYH 2 (p60) is produced from the β or γ transcript,lacks the MTS and is localized in the nucleus[54].Moreover,there is an overlapping gene,Target Early Growth Response 1 (EGR1) member 1 (TOE1) at the 5’ end ofMUTYH,and the transcription of these two genes proceeds in opposite directions[28].TOE1 inhibits cell growth rate and cell cycle,induces Cyclin Dependent Kinase Inhibitor 1A(CDKN1A) expression as well as Transforming Growth Factor-beta (TGF-beta)expression,mediates the inhibitory growth effect of EGR1 involved in the maturation of snRNAs and snRNA 3'-tail processing[55,56].The immediate early gene and transcription factor EGR1 is an important mediator and regulator of synaptic plasticity and neuronal activity in both physiological and pathological conditions[57].

Figure1 MUTYH functional domains and its binding partners (see UniProtKB - E5KP25).
Considering that a total of 3081 public variants and 352 unique variants are currently listed on the public databases,it can easily be assumed that much still needs to be defined on the functions of MUTYH,especially at the level of specific tissues,even if most of theMUTYHmutations have been detected in polyposis associated with MUTYH and in other types of gastrointestinal carcinoma.It remains to be clarified whether there are hormesis effects in the functions of all MUTYH isoforms and whether particular alternative splicings have a pathophysiological involvement in other diseases besides gastrointestinal diseases.
MUTYH IN POLYPOSIS AND COLORECTAL CANCER
Until 2002,mutations in a second gene,MUTYH,besidesAPC,were found in patients that presented with AFAP,in which noAPCgene mutation was identified.MUTYH,together withAPCgermline defects,are responsible for the majority of clinically wellcharacterized patients with FAP and AFAP phenotype,and with more than 30 colorectal adenomas[58,59].In particular mutations ofMUTYHare found in 7%-12% and 40% of FAP and AFAP patients,respectively,and in 26% of patients with multiple colorectal adenomas (MRCA)[59-61].In MRCA,MUTYHmutations differed according to the number of colorectal adenomas,being more frequent in patients with 30-99 adenomas than in those with 10-29 adenomas[59].
Single polyps undergo carcinogenesis but the exact route to carcinoma seems to differ between conditions.Patients with MAP can present with serrated adenomas,hyperplastic/sessile serrated polyps,and mixed (hyperplastic and adenomatous)polyps[62];duodenal adenomas are common,with an increased risk of duodenal cancer[63,64].In addition,gastric fundic gland polyps are common and although a higher risk for gastric cancer (GC) than in the general population was observed,the trend was not significant[64].Extraintestinal reported features include thyroid nodules and benign adrenal lesions[65,66].
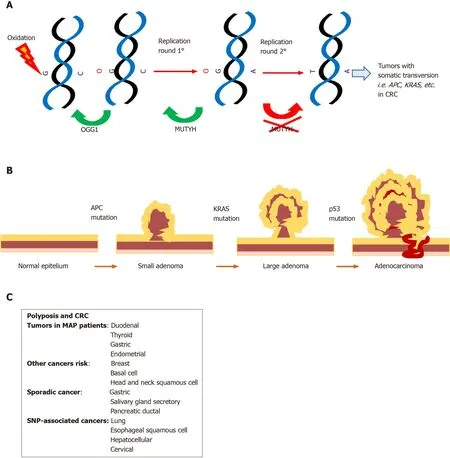
Figure2 Roles of MUTYH in cancer.
MAP patients are characterized by a greatly increased lifetime risk for CRC (43%-63% at age 60 years) that increases to 80%-90% in the absence of timely surveillance[67,68].In patients with MAP,colon cancer is right-sided in 29%-69% of cases and metachronous or synchronous colon cancers occurred in 23%-27%[69,70].
At first,the two non-conservativeMUTYHmutations c.536A >G,p.Tyr179Cys(Y179C) and c.1187G >A,p.Gly396Asp (G396D) were found to reduce the removal of A from G:A mismatches,suggesting that a defect in MUTYH activity leads to accumulated mutations in patients with FAP-like phenotype,referred to as MAP or FAP2;this is the unique mechanism to induce CRC with a recessive mode of inheritance[1].The polyposis of this syndrome tends to be less pronounced than FAP.However,the penetrance is very high,showing almost 100% penetrance for cancer by the age of 55 years[71,72].The prevalence of MAP is around 1% in all CRC cases[73].This estimate rises to 2% when young-onset CRC patients are considered and to 5%–25% in CRC patients with somaticKrasG12C substitution[67].
In contrast toAPC,no relationship has been observed between the location of the mutation and the phenotype of the disease.Mutations located throughout the entireMUTYHhave been described in MAP,but only two missense mutations,p.Tyr179Cys and p.Gly396Asp,are the most prevalent in Caucasians and account for approximately 75% of the reported mutations in MAP patients[74].Other population-specificMUTYHmutations have been found[34,52,69,75].
A large meta-analysis study defined the CRC risk associated with bi-allelic mutations in theMUTYHgene as 28-fold (95%CI:6.95–115) following logistic regression analysis[76].Bi-allelic carriers of the p.Gly396Asp variant and p.Tyr179Cys/p.Gly396Asp compound heterozygotes were also significantly associated with a similar increase in CRC risk [OR = 23.1 (95%CI:3.15–169) and 21.6 (95%CI:2.94–159),respectively].On the other hand,the risk associated with oneMUTYHmutant allele is controversial[67,73,77,78].Furthermore,the differences in the frequency of individualMUTYHvariants in the population have made it difficult to replicate study findings.In particular,with regard toMUTYHp.Tyr179Cys,because of the rarity of the variant(allele frequency 0.002),the evidence presented raises the possibility of a mono-allelic effect for this variant[76].Nevertheless,a two-fold increase in CRC risk was observed in mono-allelic carriers,thus providing evidence of a mono-allelic effect of theMUTYHgene,and was demonstrated in other studies[68,79-82].
ROLE OF MUTYH IN OTHER CANCERS
Mutations
For years it remained unclear whether variants in theMUTYHgene were associated with an increased risk of other cancers beyond that of the colorectal and the results were controversial.
Several studies have reported an increased risk of bladder,ovarian,skin,breast and endometrial cancer for biallelic mutation carriers[64,83-85]and a slightly increased cumulative risk inMUTYHheterozygotes for gastric,liver,endometrial and breast cancer[64,84,86,87].
Breast cancer:Previous studies on the association betweenMUTYHvariants and the risk of breast cancer (BC) produced controversial results[83,88,89]and it is equally unclear if BC is increased in women with MAP.The risk was found to be higher,with a median age at diagnosis of 53 years (range 45-76) in the study by Vogtet al[64].Six heterozygousMUTYHmutations,including p.Tyr179Cys,p.Gly396Asp and p.Pro405Leu (c.1214C >T,P405L),resulted in an association in families with both BC and CRC,but not polyposis[84]even if two years later an extensive case-control study did not confirm the association betweenMUTYHvariants and BC,but did not exclude BC susceptibility[89].An interesting result is the finding of one male with biallelic germlineMUTYHpathogenic variants in a large cohort of males with BC[90].In the same study,no biallelicMUTYHmutations been reported in women from large BC cohorts.In a case-control study,930 Jewish women with a high prevalence ofMUTYHmutations were investigated for the two variants p.Gly396Asp and p.Tyr179Cys,and patients with BC revealed a 6.7% prevalence of p.Gly396Asp[91].
Basal cell carcinoma:Is a relatively benign skin cancer caused by UV exposure and it is known that the DNA repair mechanisms in the skin are interconnected to protect against UV mutagenesis,involving,other than nucleotide excision repair (NER) also base excision and mismatch repairs[92].In a study by Choet al[93],saliva samples from patients affected by basal cell carcinoma (BCC) were collected,DNA was extracted and 29 genes were analyzed for germline mutations.Individuals with a high frequency of BCC (presence of metastatic prostate,primary ovarian and BC) harbored pathogenic mutations in DNA repair genes:APC,BARD1,BRCA1,BRCA2,CDH1,CHEK2,MLH1,MSH2,MSH6,MUTYH,NBN,andPALB2.Individuals with germline mutations in DNA repair genes had an increased risk of malignancy especially other skin cancers,such as melanoma and squamous cell carcinoma,possibly due to UV exposure.These results indicate that these patients might benefit from multigene cancer-susceptibility panel testing,as performed for gastrointestinal cancer[94].
Head and neck squamous cell carcinoma:The role of BER genes has also been investigated in squamous cell carcinomas of the head and neck[95].Development of this tumor is a multifactorial process associated with a variety of environmental risk factors such as tobacco and alcohol consumption,and,in this scenario,oxidative DNA damage and BER could be involved in the development of this tumor.MUTYH,OGG1andMTH1germline mutations or polymorphisms have been investigated using complete genomic sequencing in patients and controls and no pathogenic germline mutations were identified.However,common and rare new variants in the coding and adjacent intronic regions have been detected possibly indicating a minor role of the three BER genes in the tumorigenesis of sporadic head and neck squamous cell carcinoma (SCCHN)[95].
In sporadic cancers,which account for a large majority of human cancer,a multitude of genetic and environmental components cooperate.In contrast to germline mutations in DNA repair genes,which cause a strong deficiency in DNA repair activity in all cell types,the role of missense and SNPs in sporadic cancer is unclear because deficiencies in DNA repair are much milder.Furthermore,slow progress is due to a lack of functional and well-designed studies.
唐朝有和尚,号圆泽,与朋友李源善(一称李源)外出,见一孕妇在河边汲水,圆泽对李说:这妇人怀孕已三年,只待我去投胎,我一直避着,现在遇见没有办法了。三天之后,你到她家去看,如婴孩对你笑一笑,就是我了;再过13年(一称12年)中秋月夜,我将在杭州天竺寺等你。说罢那夜国泽圆寂,孕妇生一男孩,第三天李去探看,婴儿果然对地笑了一笑。13年后中秋夜,李源善到天竺寺,见一牧童坐在牛背上唱歌道:“三生石上旧精魂,赏月吟风不要论,惭愧情人远相访,此身虽异性常存。”李听了叹道国泽与这牧童真是有三生的缘分啊。
Gastric cancer:MUTYHmutations can contribute to the development of sporadic gastric cancer (GC) as documented by Kimet al[96].They found biallelic mutations,somatic missense in one allele and loss of the remaining allele,in GC patients withH.pylori-positive advanced intestinal-type GC and lymph node metastasis.It has been found that patients with GC exhibiting low MUTYH expression showed a poor outcome when compared to those expressing high levels of MUTYH.This finding may act as an independent predictor of poor survival in GC patients[97].
An increased accumulation of 8-oxoG in nuclei and reduced expression of MUTYH are also documented in the mucosa of patients with ulcerative colitis.In this mucosa mutations ofKrasbut notMUTYHwere found[98].Inflamed mucosa in these patients is known to be exposed to oxidative stress;therefore,accumulation of 8-oxoG is responsible forKrasmutations,thus indicating thatMUTYHplays a tumor suppressor role in ulcerative colitis as well as MAP patients[28].
Salivary gland secretory carcinoma:Pathogenic variants ofMUTYHwere also identified in salivary gland secretory carcinoma (SC),in particular acinic cell carcinoma,an aggressive phenotype with lymph node metastasis[99].This type of tumor also demonstrated mutations inMLH1andSerine/Threonine Kinase 11(STK11),genes involved in polyposis and cancer of the colon.Missense and splice site mutations in four genes were identified as pathogenic or likely pathogenic:Serine Protease 1(PRSS1) (c.47C >T;A16V),MLH1(c.1151T >A;V384D),MUTYH (c.934-2A >G;splice site),andSTK11 (c.842C >T;P281L).The most frequent mutations were inPRSS1gene and this is foreseeable considering that germline and somaticPRSS1mutations are associated with hereditary,chronic pancreatitis and pancreatic adenocarcinoma,and salivary glands are exocrine glands with a similar histology to the pancreas[100].No significant copy number alteration was shown in these genes.Secretory carcinoma displays a mutation spectrum well described in the cancer genome study by Vogelsteinet al[101];low-grade indolent tumors usually presented mutations inPRSS1 gene,while cases with aggressive clinicopathologic features such as lymph node metastasis and tumor recurrence also revealed mutations inMUTYHandMLH1genes.These findings may improve the diagnosis and treatment of different types of secretory carcinoma,taking into account the risk stratification of patients.
Pancreatic ductal adenocarcinoma:Pancreatic ductal adenocarcinoma (PDAC) is a highly metastatic and chemo-resistant disease which is best treated with surgery[102].It is also characterized by extensive fibrosis,which creates a hypoxic microenvironment and consequently leads to intracellular oxidative stress.Given the function of BER enzymes in protecting cells from oxidative DNA damage,they have been identified as important regulators of resistance to a variety of chemotherapeutics.The inhibition of their activity could represent a novel therapeutic approach for PDAC in reducing survival of cancerous cells[103].In this study,silencing ofMUTYHusing siRNA in a cultured PDAC cell line reduced proliferation,increased apoptosis and finally increased chemo-sensitivityin vitro,suggesting thatMUTYHis a novel therapeutic target for pancreatic cancer.PDAC has not or has rarely been reported in MAP patients[64,65,87].
SNPs
Genetic variations in DNA repair genes may modulate DNA repair ability and are thought to be related to cancer risk,but polymorphisms of DNA repair genes are little studied in genome-wide association studies[104,105].The majority of studies have provided inconsistent results due to very limited coverage of DNA repair-related genes,typically evaluating only a few repair genes that play a key role in the BER pathway,such as theX-Ray Repair Cross Complementing 1(XRCC1),OGG1,andApurinic/Apyrimidinic Endonuclease 1(APEX1) genes[106-108].These three genes are associated with human tumor susceptibility and radiation toxicity[109].In a study of nasopharyngeal carcinoma (NPC) patients,some polymorphisms were reported to have a significant correlation with the curative effect at the end of radiation therapy,but no influence on radiation toxicity has been reported[110].
Lung cancer:At first it seemed that the NER pathway had a stronger influence on lung cancer (LC) than the BER pathway[111].Tobacco smoke accounts for a huge generation of ROS species and thereby oxidative damage.Studies on the association with LC risk focused mostly on three key genes in the BER pathwayOGG1,APE1/APEX1andXRCC1[112,113].No association was found between cancer risk and theAPE1/APEX1p.Asp148Glu (D148E,rs3136820) andXRCC1p.Arg280His polymorphisms,while a positive association between two gene polymorphisms,OGG1p.Ser326Cys (S326C,rs1052133) andMUTYHc.972G >C,p.Gln324His (Q324H,rs3219489) orOGG1p.Ser326Cys alone and the risk of LC,has been reported[112,113].This is consistent with experimental evidence that these isoforms exhibit decreased enzyme activity.The two polymorphic variants have been extensively studied for their roles in cancer susceptibility and prognosis.The combined effect of bothOGG1p.Ser326Cys andMUTYHp.Gln324His on the risk of lung adenocarcinoma has been confirmed in a recent study performed on DNA from 326 LC cases and 330 controls by genotyping making use of polymerase chain reaction-restriction length fragment polymorphism[114].In the presence of both variants,the risk of LC was found to be independent of tobacco smoke.In particular,it was observed that heterozygotes forMUTYHp.Gln324His exhibited a 2-fold increased risk of LC (OR = 2.35,C.I.= 1.59-3.4,P<0.0001) in smokers and similarly in non-smokers (OR = 3.37,C.I.= 1.62-7.02,P=0.001).Despite these findings,the association betweenMUTYHpolymorphisms and the risk of LC remains controversial and requires further verification in a larger study population to facilitate the evaluation of multigenic effects of environmental exposure.Anin vitroanalysis ofMUTYHp.Gln324His showed that it has reduced enzyme activity similar to that of the known cancer variant p.Gly396Asp,providing evidence that this common variant may lead to increased colorectal and LC risk[115].
Esophageal squamous cell carcinoma and GC:In a pathway-based analysis,Liet al[116]identified a significant association between several DNA repair pathway genes and the risk of esophageal squamous cell carcinoma and GC.The most significant genes wereCHEK2,SMUG1(uracil-DNA glycosylase),TP53,butMUTYH(c.504+35A >G;rs3219487) was associated with the risk of cancer.To date,of the DNA repair related genes,CHEK2remains the only one associated with cancer to be identified by genomewide-associated studies (GWAS).
Hepatocellular carcinoma:The involvement of MUTYH in hepatocellular carcinoma(HCC) has been investigated by Sakuradaet al[117].The authors found a significant association between the intronicMUTYHSNP rs3219487 and the risk of developing HCC.Patients with A/A or G/A genotypes had reduced mRNA levels in peripheral mononuclear cells and a higher risk of developing HCC than those with the G/G genotype (OR = 9.27,95%CI = 2.39−32.1,P= 0.0005).Reduced enzyme activity was also confirmed in MUTYH-null mice that did not develop any tumors after an antioxidant-rich diet[117].
ROLE OF MUTYH IN NON-CANCER DISEASES
Mutations
Some diseases not associated with polyposis but characterized byMUTYHmutations or SNPs have recently been described.These genetic events can lead to dysregulation of MUTYH glycosylase activity with a lack of its protective antioxidant role.As organ functions reflect the various biochemical reactions of the products of gene transcription,changes inMUTYHexpression may be detected in distinct parts of the body and be responsible for different diseases.
Neurodegenerative diseases:Alterations inMUTYHgene or functional involvement of the MUTYH protein in severe stress conditions can predispose to different neurodegenerative disorders.The extreme variability of phenotypes and progression displayed in these pathologies is probably linked to the type and location of the cell populations in nervous tissue,and the relationships occurring among the cells involved in the pathology.
Parkinson’s disease:Is characterized by motor deficits,mainly related to an early loss of 40%-60% of dopamine-containing neurons in the substantia nigra.The occurrence of 8-oxoG is associated with elevated expression of MTH1,OGG1,and MUTYH proteins in nuclear and mitochondrial DNA or cytoplasmic RNA in nigrostriatal dopaminergic neurons that additionally show intense and diffuse MUTYH immunostaining only in the cytoplasm[119].The major MUTYH isoform in Parkinson’s disease brain is represented by a mitochondrial 47-kDa molecule,that might be derived from the MUTYH type α4 mRNA containing the MTS[119].This molecule could play a role in protection of the remaining neurons against possible oxidative stress factors,including excessive oxidized lipids,proteins,and DNA[119,120].In contrast,Nakabeppuet al[119]also suggested that the 47-kDa molecule could have no glycosylase activity in the BER mechanism as it lacks the DNA minor groove-reading motif.
Alzheimer’s disease:Results in a progressive loss of brain cells with a decline in memory and cognition.Its multifactorial etiology is probably associated with a reduction of BER efficacy,or excessive neuronal or microglial production of ROS,including 8-oxoG generated in mitochondria during dysregulated insulin-glucose metabolism[121-123].Single-strand DNA breaks (SSBs) and 8-oxoG activate the two distinct mitochondrial DNA (mtDNA) caspase-independent/calpain and nuclear DNA (nDNA) PARP-1-dependent death signaling pathways[124].At an early stage of Alzheimer’s disease (AD),accumulation of the amyloid β (Aβ) protein is associated with ROS production[125],that induces neurodegeneration through calcium-dependent inactivation of calpain[28,122,123].In the later stages,the Aβ and neurofibrillary deposition blocks the repair process[125]and induces apoptosis by the PARP-1-dependent pathway.Thus,damaged neurons stimulate a delayed inflammatory response,microgliosis,which involves the adjacent neurons[28,122-124].Shenget al[123]demonstrated that MTH1 and OGG1 can play a preventive role to avoid harmful MUTYH-mediated accumulation of 8-oxoG in a chemical AD model of neurodegeneration.Significant down-regulation ofAPE1,OGG1,MUTYH,PARP1andNeiLikeDNAGlycosylase1/Nei EndonucleaseVIII-Like 1(NEIL1)genes in AD peripheral blood lymphocytes,as compared to healthy subjects,was independent of the methylation status of gene promoters,and probably reflected other changes occurring in the AD brain[121].
Friedreich's ataxia:Is an autosomal recessive disease of the peripheral and central nervous systems and is caused by defects in the iron-sulfur cluster biogenesis consequent to a GAA trinucleotide repeat expansion within the first intron of theFXNgene or point mutations truncating the protein[126,127].This results in decreased levels of the small mitochondrial protein frataxin,with consequent Fe-S cluster deregulation associated with iron accumulation,mitochondria dysfunction and oxidative DNA damage due to increased 8-oxo-G in microglia[126,128,129].This induces high levels of MUTYH and PARP-1,microglia activation,neuroinflammation and neurodegeneration,elevated levels of arachidonic acid metabolites,such as prostaglandins and thromboxane B2,maintained by cyclooxygenase (COX2)overexpression induced by ROS[126,128,130].
Huntington’s disease:Is a hereditary neurodegenerative syndrome causing uncontrolled body movements,changes in behavior and a decline in cognitive abilities.It is determined by a somatic expansion of CAG repeats ranging between 35–121 trinucleotide repeats (TNRs) in the coding region of theHuntingtin(HTT) gene.Age onset and progression of Huntington’s disease depend on the length of the CAG repeat expansion in germ and brain cells[131,132].Mutant HTT protein shows an expanded glutamine tract and is correlated with increased ROS production.Kovtunet al[133]suggested a 'toxic oxidation' model using R6/1 transgenic mice in which DNA oxidized by ROS was subjected to an oxidation-excision cycle that was iterative with age,and caused a progressive CAG repeat expansion.Jaramet al[131,132,134]observed an aberrant BER pathway in which OGG1 slowly removes 8-oxoG that accumulates in the hairpins derived from TNR region self-anneal,resulting in expanded CAG tracts.In the presence of excessive oxidative damage,MUTYH DNA glycosylase,similar to OGG1,may modulate the TNR expansion[134].
Retinitis pigmentosa:Is an inherited disease characterized by the death of photoreceptor cells induced by oxidative DNA damage in microglia[135].In the early phases of retinal degeneration,increased levels of 8-oxoG,SSB formation,and PARP activation stimulate microglia during BER in the rd10 mouse model.Thus,under oxidative DNA damage,MUTYH mediated microglial activation inducing retina inflammation and photoreceptor degeneration[135].
Neurofibromatosis:Is an inherited dominant disease characterized by modifications in skin pigmentation and growth of tumors along nerves near the spinal cord,or in other parts of the body.It is associated with the development of different cancers.Neurofibromatosis includes neurofibromatosis type 1 (NF1) caused by heterozygous mutation ofNeurofibromin 1(NF1) gene,and neurofibromatosis type 2 (NF2),caused by mutations in theNeurofibromin 2(NF2) gene.Liet al[136]described a clinical case of NF2 in a 12-year-old patient carryingMUTYHc.53C >T,p.Pro18Leu (P18L)/c.74G >A,p.Gly25Asp (G25D) andAtaxia Telangiectasia And Rad3-Related(ATR) mutations inherited from her father,as well asMLH1andCheckpoint Kinase 2(CHEK2) mutations from her mother.As a consequence of the aberrant DNA repair mechanism,a newNF2germline mutation occurred in the young patient.
NovelMUTYHmutations were detected in an analysis of 39 candidate genes by array and confirmatory Sanger sequencing[137].The study involved 27 cases affected by rare mitochondrial disorders and 13 controls.At cDNA position 1307 ofMUTYH,Wanget al[137]identified a heterozygous deletion (A–) regarding a single base with amino acid substitution at position 364 (GAA >GAC),frameshift and premature termination (TGA) at position 393 (p.E364Dfs393X),in a region involving the DNAglycosylase-C domain.Another sample carried both a benignMUTYHc.1535C >T,p.Ser512Phe (S512F) variant and p.Gln324His that is considered a probable damaging SNP.
SNPs
Different authors have focused their attention on the genetic correlation betweenMUTYHSNPs and an increased individual predisposition to some non-cancer diseases.Although most of these clinical studies should be performed on a larger population to confirm the results,the association betweenMUTYHp.Gln324His polymorphism and susceptibility to different pathologies has been specifically suggested.The glutamine to histidine amino acid change determines a small local modification in the electron cloud,associated with a slight reduction in DNA binding activity,that might contribute to diminished efficacy of the BER,as the p.Gln324His variant was considered 34% less active than the corresponding wild-type[138].
AD:Kwiatkowskiet al[138]examined SNPs inPARP1,XRCC1,andMUTYHBER-related genes as well as the distribution of their genotypes in 110 patients with AD and 120 healthy controls from Poland.This study detected an increased risk for AD in the genotype combinationsMUTYHp.Gln324His andPARP1c.2285T >C,p.Val762Ala(V762A,rs1136410),orXRCC1c.580C >T,p.Arg194Trp (R194W,rs1799782),orXRCC1c.1196A >G,p.Gln399Arg (Q399R,rs25487)[138].Lilleneset al[139]investigated the presence of SNPs inMUTYHand the other BER component genesOGG1,APE1,polymerase β(PolB) andpolymerase γ(PolG),as well as theTranscriptionFactorA Mitochondrial(TFAM) gene in a cross-sectional case-control study with a total of 449 individuals from Norway,including AD affected and non-affected patients and healthy controls.TheMUTYHc.64G >A,p.Val22Met (V22M,rs3219484) SNP was rarely detected,whileMUTYHrs3219489 had the highest minor allele frequencies.To date,noMUTYHpolymorphism has been strongly associated with AD.
Keratoconus:No association was found betweenOGG1p.Ser326Cys,andMUTYHp.Gln324His polymorphisms or their combined genotypes and the occurrence of keratoconus (KC),a non-inflammatory disease of the cornea,in an investigation involving 205 patients with KC and 220 controls from Poland.The authors indicated that the small sample size was the main limitation in this research[140].
Primary open angle glaucoma:Another study aimed to evaluate the association betweenXRCC1p.Arg399Gln,XRCC1p.Arg194Trp,OGG1p.Ser326Cys,MUTYHp.Gln324His,ADP-ribosyltransferase(ADPRT),also known asPARP1,p.Ala762Val andAPE1p.Asp148Glu SNPs and the risk of primary open angle glaucoma (POAG) in 412 Polish patients and 454 healthy subjects[141].MUTYHp.Gln324His andADPRTp.Ala762Val genotypes had a protective role in the progression of POAG,while the combined genotypesXRCC1p.Arg399Gln andMUTYHp.Gln324His were associated with an increase in the risk of this pathology[141].These results confirmed the data from a previous investigation by the same research team in 170 patients with POAG and 193 healthy controls,with the aim of defining the role of theXRCC1,OGG1andMUTYHgene polymorphisms in the development of POAG[142].
Age-related macular degeneration:Synowiecet al[143]explored the relationship between the polymorphismsMUTYHp.Gln324His,OGG1p.Ser326Cys and agerelated macular degeneration (AMD),a disease involving the macula cells,with loss of photoreceptors and retinal pigment epithelium,and probably related to oxidative DNA damage associated with aging.The study was performed using DNA from the blood of 271 patients,comprising 101 subjects with wet and 170 subjects with dry AMD,and 105 healthy individuals.AMD was positively correlated with the C allele ofMUTYHp.Gln324His and the wet form of the disease.Nevertheless,this association was considered weak and medically not significant[143].
Recurrent depressive disorder:Czarnyet al[144]examined the link betweenOGG1p.Ser326Cys,MUTYHp.Gln324His,NEIL1c.*589G >C (rs4462560) SNPs and the occurrence of depression in 555 Polish individuals,including 257 patients and 298 healthy controls.MUTYHc.972G/G genotype was associated with late-onset depression,while the G allele ofNEIL1was positively related to early and late-onset disease[144].In a small number of patients,the combination ofMUTYHp.Gln324His andNEIL1c.*589G >C enhanced depression susceptibility[144].In another investigation on recurrent depressive disorder,Czarnyet al[145]analyzed 12 SNPs located inNEIL1,OGG1,MUTYH,PARP1,XRCC1,Flap Endonuclease 1(FEN1),APEX1,DNA Ligase 1(LIG1),andDNA Ligase 3(LIG3) genes in 43 patients and 59 healthy controls to evaluate the presence of variants in depression onset.OnlyAPEX1c.-468T >G (rs1760944)significantly increased the risk of depression,whileNEIL1 rs4462560 reduced the risk.Moreover,some SNPs influenced the efficiency of DNA damage repair (DRE) in depressed individuals,such asMUTYHc.972G >C,while the variantsMUTYHC/G and G/G did not influence DRE.This suggested that depression is associated with DNA damage caused by increased oxidative stress related to low DRE,in part attributed to definite SNP variants.Finally,the authors acknowledged some limitations in this study,such as the use of peripheral blood mononuclear cells instead of central nervous system cells in the experiments[145].
Osteoarthritis:Is a chronic disease that involves articular joints and leads to cartilage degeneration,with consequent functional impairment.The contribution ofMUTYHrs3219463 in osteoarthritis (OA) onset was evaluated in association with 5 SNPs of theCalcium Release-Activated Calcium Channel Protein 1(ORAI1) gene in a case-control study including 350 OA patients and 350 age- and gender-matched healthy controls from the Chinese Han population[146].Carriers ofMUTYHGG or GA rs3219463 genotypes andORAI1T allele rs7135617 showed a higher risk of developing OA.Moreover,patients withMUTYHGG or GA rs3219463 showed increased serum MUTYH levels than subjects with AA wild-type genotype.Dysfunction of the cartilage Ca2+signaling pathway in carriers ofMUTYHG allele rs3219463 andORAl1T allele rs7135617 was also found[146].Although these results suggested an association between both SNPs and OA susceptibility,the authors described some limitations in the study,such as the need forin vitroanalysis to determine the mechanisms influencing OA risk factors and the small sample size not representative of the population.
Rheumatoid arthritis:Kunget al[147]evaluated the association between genotypic and allelic distributions of the fourMUTYHpolymorphisms rs3219463,rs3219476,rs3219489,and rs3219493 in a Taiwan Chinese population by comparing 92 individuals with rheumatoid arthritis (RA),a long-term and progressive autoimmune disorder primarily affecting the articular joint,and 192 healthy controls.The study revealed significant differences in genotypic and allelic frequencies regardingMUTYHG/A andMUTYHG/G rs3219463,as well asMUTYHT/G andMUTYHT/T rs3219476 in RA and control groups.Moreover,the genotype frequency of G/− rs3219463 showed a significant increase in RA patients expressing the rheumatoid factor.The authors concluded that rs3219463 and rs3219476 polymorphisms were significantly associated with RA susceptibility,although they highlighted the relatively small number of subjects as a limitation in their study[145].Subsequently,another investigation found a statistically significant low risk of developing RA inMUTYHrs3219463 G carriers during the analysis of 368 Chinese Han RA patients from Taiwan and 364 healthy controls[148].In addition,individuals with RA showed 8.8% higher serum MUTYH protein levels compared to healthy controls,suggesting an association between RA andMUTYHrs3219463 polymorphism[148].To date,the first investigation on reverse insertion in the commonAluYb8MUTYH(rs10527342) insertion/deletion SNP was conducted in a selected healthy Chinese population.Homozygous status decreased from 20 to 59 years,suggesting that this condition was related to the onset of agerelated or chronic diseases or death.Moreover,the homozygosity was associated with increased levels of 8-oxoG in leukocytes,probably due to impaired DNA repair,as well as a significant increase in plasma interleukin-1[149].Subsequent studies assessed the link betweenAluYb8MUTYHand different pathologic conditions.
Type 2 diabetes mellitus:The role and the incidence ofAluYb8MUTYHand the other SNPs of BER geneshMTH1c.247G >A p.Val83Met (V83M,rs4866),and c.-53G >C(rs56387615),c.-23A >G (rs1801129),and c.-18G >T (rs1801126) in the 5′-UTR ofOGG1were evaluated in type 2 diabetes mellitus (T2DM) during a case–control study in a Chinese population[150].In the analysis by Caoet al[150]AluYb8MUTYH“A/P+P/P”genotype frequency was significantly higher in diabetic patients than in healthy controls.Moreover,the genetic combinations between theAluYb8MUTYH“A/P+P/P”and thehMTH1G/A variant or the three heterozygousOGG1genotypes had a synergistic effect that prejudiced the repair of DNA damage induced by oxidative stress and determined an increased risk of T2DM.A previous case-control study of 565 T2DM patients and 565 healthy controls from China found a slight increase in the percentage ofAluYb8MUTYHalleleP(44.7%vs40.3%) in T2DM patients compared to controls[151].
Chronic kidney disease:A recent study of a Spanish population,including 548 patients and 174 controls,aimed to identify 38 SNPs from 31 candidate genes associated with chronic kidney disease (CKD) and other related diseases,such as hypertension,diabetes and inflammation[152].CKD shows a progressive and irreversible loss of renal function and is associated with high genomic instability and the presence of positive markers of inflammation.Of the examined variants in BER genes,the genetic changeMUTYHc.1014G >C,p.Gln338His (also known as c.972G>C,p.Gln324His),rs3219489,was not associated with CKD[152].In contrast,an association betweenMUTYHrs3219489 and genomic instability was revealed by a recent study on polymorphisms in genes of BER,nucleotide excision repair,phase II metabolism,and antioxidant enzymes in 415 CKD patients and 174 controls[153].
End-stage renal disease:Caiet al[154]reported a Chinese case-control study on endstage renal disease (ESRD) in whichOGG1Ser326Cys (c.977C >G),MTH1p.Val83Met,MUTYHp.Gln324His (c.972G>C),andAluYb8MUTYHwere examined in 337 patients on hemodialysis (HD) and 404 healthy controls.The frequency of theMUTYHc.972G/G and theAluYb8MUTYHinsertion (A/PorP/P) genotypes were statistically higher in HD patients than in controls.Moreover,the combination ofMUTYHc.972G/G andAluYb8MUTYH A/PorP/Pgenotypes orOGG1c.977G/G further increased the risk of ESRD and the leukocyte DNA 8-oxoG levels in HD patients[154].Another study by Caiet al[154]investigated the effects ofOGG1c.977C >G,MUTYHc.972G >C,andAluYb8MUTYHpolymorphisms on chronic inflammation due to oxidative stress in HD patients,and evaluated the plasma levels of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-6.The study included 167 patients and 66 healthy controls.HD patients showed significantly increased levels of IL-1β and IL-6.In addition,patients carrying the c.972G/G genotype had higher IL-1β levels than patients with the MUTYH c.972C/C variant and theAluYb8MUTYHgenotype was strongly related to increased levels of both IL-1β and IL-6 in HD subjects.The combination ofAluYb8MUTYHwithOGG1c.977C >G orMUTYHc.972G >C genotypes was significantly associated with IL-1β and IL-6 levels in HD patients[154].Patients with the combination ofMUTYHc.972 C/G or G/G genotypes andAluYb8MUTYHA/P or P/P genotypes showed significantly higher IL-1β and IL-6 levels compared to those with c.972 C/C and A/A genotypes[154].
Idiopathic pulmonary fibrosis:Is an interstitial lung disease characterized by extracellular matrix deposition and a poor prognosis.By analyzing 277 patients and 810 healthy controls,Zhouet al[54,150]found that idiopathic pulmonary fibrosis was associated with mitochondrial reduced expression of MUTYH1 protein as well as the presence of the variantAluYb8MUTYH.Of the three genotypes observed and defined by the presence (P) or absence (A) of the AluYb8 insertion,theP/Pgenotype showed unstable mtDNA.Moreover,this was correlated with early age-associated accumulation of ROS-induced oxidative DNA damage in lung tissues and a reduced capacity of DNA repair leading to increased 8-oxoG in dysfunctional mitochondria[54,155].Indeed,AluYb8MUTYHdecreased the rate of mtDNA turnover,reduced the expression of type 1 MUTYH protein and affected MUTYH-induced BER in mitochondria[155].
CONCLUSION
This review highlights the alterations inMUTYHgene and/or deregulated activity of MUTYH glycosylase,which causes a lack of its anti-oxidant function against the onset of non-polyposis diseases.In order to increase our understanding of the involvement ofMUTYHin the pathogenesis of diseases different to colon polyposis and CRC,we analyzed and summarized the current literature concerning this topic.We evaluated the role ofMUTYHon susceptibility to developing cancers and diseases such as neurological and ocular degenerative disorders.As the mechanisms of DNA repair are very important in protecting humans from cancer and avoiding disease,we also evaluated the incidence of genetic variants and SNPs inMUTYHgene able to influence the onset of neoplastic and non-neoplastic disorders.
The risk of CRC in MAP patients was reported to range from 43% to 63% at age 60 years and up to 80%-90% in the absence of timely surveillance.It is less certain inMUTYHmono-allelic carriers even though a two-fold increase in CRC risk has been reported in some studies,which is small and not clinically relevant.This may be due to several factors,such as the rarity of the allele,and possible modifying effects such as increased risk in cases with early age of onset.MUTYHalterations have also been reported in other cancers.Mutations in this gene can contribute to the development of sporadic GC and may act as an independent predictor of poor survival in patients affected by this tumor.The same cannot be said for BC in which the association withMUTYHvariants has resulted in controversial findings.Pathogenic variants ofMUTYHwere also identified in SC,in particular acinic cell carcinoma,an aggressive phenotype with lymph node metastasis,and in individuals with a high frequency of basal cell carcinoma.Both tumors also had mutations in other genes.Increased knowledge of the mutation spectrum may improve the diagnosis and treatment of these types of carcinoma,taking into account the risk stratification of patients.Patients affected by these two cancers might benefit from multigene cancer-susceptibility panel testing,similar to that for gastrointestinal cancer.Interestingly,MUTYH may be a new therapeutic target for PDAC,as its inhibition has been shown to reduce the survival of cancer cells and increase chemo-sensitivityin vitro.
With regard to neurological degeneration in stress conditions,the MUTYH 47-kDa molecule could have a protective anti-oxidative stress function in the remaining healthy neurons in patients with Parkinson's disease.In AD,a correlation betweenMUTYHc.972G >C SNP and polymorphisms of other BER genes was observed.However,another study confirmed that noMUTYHpolymorphism was strongly associated with AD.In Friedreich's ataxia,high levels of MUTYH were observed as consequence of decreased levels of frataxin,responsible for Fe-S cluster deregulation and oxidative DNA damage due to increased 8-oxo-G in microglia.In Huntington's disease,the alteration of MUTYH with aberrant BER may influence the trinucleotide expansion in HTT protein correlated with increased ROS production.MUTYH might also have a role in RP,since in conditions of severe oxidative DNA damage it mediates microglial activation with retina inflammation and photoreceptor degeneration.Finally,an aberrant DNA repair mechanism resulting inMUTYHmutation has been reported in NF.SNPs of DNA repair genes are little studied in genome-wide association studies due to a very limited coverage of these genes and to small patient populations.However,recently a robust study reported a 2-fold increased risk of LC in heterozygotes forMUTYHp.Gln324His,independent of tobacco smoke.Other findings reported evidence of increased colorectal and LC risk in the presence of the same variant.The involvement of MUTYH in HCC has been well studied.A significant association between the intronicMUTYHSNP (rs3219487) has been found as well as reduced enzymatic activity in MUTYH-null mice that did not develop tumors after an antioxidant-rich diet.MUTYHp.Gln324His has been associated with significantly poor prognostic factors in cervical carcinoma,such as cell differentiation grade and lymph node metastasis.Interestingly,these findings support the use of this variant as an early indicator of prognosis for patients at risk of cervical carcinoma and HPV infection.MUTYHSNP (rs3219489) andAluYb8MUTYHinsertion/deletion polymorphisms have also been suggested to significantly increase susceptibility to the development of other diseases,such as T2DM,OA and AR.
Therefore,we conclude that the involvement ofMUTYHmutations and/or genetic variants or alterations in the corresponding proteins could have the potential to identify new predictive and diagnostic biomarkers for diseases other than MAP.Moreover,MUTYH modifications could represent therapeutic targets to develop more efficient treatment approaches and strategies for these diseases.
猜你喜欢
杂志排行
World Journal of Clinical Oncology的其它文章
- Role of imaging biomarkers in mutation-driven non-small cell lung cancer
- Circulating cell-free nucleic acids as prognostic and therapy predictive tools for metastatic castrate-resistant prostate cancer
- Why natural killer cells in triple negative breast cancer?
- Is there a role for treatment-oriented surgery in liver metastases from gastric cancer?
- Complete response in anaplastic lymphoma kinase–rearranged oncocytic thyroid cancer:A case report and review of literature
- Mechanisms and anatomical risk factors of pneumothorax after Bevacizumab use:A case report
