植物TCP转录因子的研究进展
2020-09-06安新艳楼盼盼郝娟
安新艳 楼盼盼 郝娟

摘要TCPs是一类植物所特有的并普遍存在于植物中的转录因子家族。大多数 TCP蛋白具有保守的非典型螺旋-环-螺旋的结构域,研究表明,TCP转录因子与真核转录因子的bHLH类似,且该家族基因在植物体内发挥着重要作用,如植物生长发育、激素反应和逆境胁迫等。因而,TCP转录因子被越来越多的科研工作者所关注。迄今为止,科研工作者对TCP转录因子家族成员的生物学功能研究已经取得了重大进步。总结了TCP转录因子的研究进展,为植物的遗传改良、次生代谢产物合成和生物学功能提供了参考。
关键词TCP转录因子;结构特性;生长发育;生物学功能
中图分类号Q943.2文献标识码A文章编号0517-6611(2020)15-0020-04
doi:10.3969/j.issn.0517-6611.2020.15.006
开放科学(资源服务)标识码(OSID):
Research Progress on Plant TCP Transcription Factors
AN Xinyan, LOU Panpan, HAO Juan
(Hangzhou Normal University, Hangzhou, Zhejiang 310000)
AbstractCharacteristic of the TCPs is a kind of universal existence in the family of transcription factors in plants. Most TCP proteins have conservative atypical helixloophelix structure domain, studies show that TCP transcription factors is similar to the bHLH eukaryotic transcription factors, the family genes in plants play an important role in the body, such as plant growth and development, and adversity stress hormone responses. Therefore, TCP transcription factors are being paid more and more attention by more and more researchers. So far, researchers have made significant progress in the biological function research of TCP transcription factors family members. The latest research progress on TCP transcription factors was summarized, in order to provide reference for plant genetic improvement, synthesis of secondary metabolites and biological function.
Key wordsTCP transcription factors;Structural characteristics;Growth and development;Biological function
基金項目
浙江省新苗计划项目“CrSLS参与UV-B调控长春花生物碱合成的功能分析”(2025C5151910177);国家自然科学基金项目“棉花TCP转录因子家族基因的表达模式分析”(Y201533081),“陆地棉纤维发育相关TCP转录因子的挖掘和功能研究”(31601343)。
作者简介安新艳(1994—),女,河南焦作人,硕士研究生,研究方向:药用植物生物碱合成。*通信作者,讲师,博士,从事药用植物次生代谢调控研究。
收稿日期2019-10-11;修回日期2019-12-05
TCP转录因子是一类高等植物所特有的转录家族,由TEOSINTE BRANCHED 1(TB1)、CYCLOIDEA(CYC)和PROLIFERATING CELL FACTORS(PCFs)这3个分离到的成员的首字母缩写而得名。鉴于TCP转录家族有着重要且广泛的调控作用,区分并说明TCP转录因子的作用机制对研究植物生物学功能具备重要的意义。随着不同物种基因组测序的完成,越来越多的TCP转录因子家族被相继挖掘和分析鉴定,如从番茄中分离到30个TCP转录因子[1],从西瓜中筛选出来27个TCP转录因子[2],从油菜中筛选到39个TCP转录因子[3],不同棉花品种中的TCP转录因子家族也随基因组测序的完成相继被分离鉴定出来并进行表达分析[4]。
许多报道表明TCP转录因子家族与植物成长发育的多个进程以及胁迫条件下的生存有关,因此TCP转录因子家族成员的功能研究受到科研工作者的普遍欢迎。笔者就植物TCP转录因子的最新研究进展进行介绍,并预测未来的研究方向。
1TCP转录因子的结构特点及分类
TCP转录因子家族参与植物生物学功能的多个进程,如植物分枝[5-6]、叶片发育[7]、激素途径[8-9]、种子萌发[10]、昼夜节律[11]。而这些转录因子之所以具有这些功能特征主要是因为它们拥有被称为TCP域的N末端非典型螺旋-环-螺旋(bHLH)结构域。但是,TCP 转录因子又与bHLH 转录因子几乎没有同源性,并和不同于bHLH 转录因子识别的DNA结构域结合[12]。 根据TCP蛋白保守结构域的序列同源性分析,可将TCP蛋白分为两类,即I类和Ⅱ类[13]。I类在TCP结构域中缺失4个保守的氨基酸,Ⅱ类在TCP结构域中插入4个保守的氨基酸,例如谷氨酸-半胱氨酸-谷氨酸(ECE)[14]延伸或富含精氨酸的R结构域[15-16]。Ⅱ类成员可以根据TCP结构域中的序列差异进一步细分为CIN和CYC两个分支[17]。CIN子代以Antirrhinum CINCINNATA(CIN)为代表[18],而CYC子代以CYC/TB1为代表[14]。
已知Ⅱ类TCP蛋白调节许多植物生长过程,最显著的是植物发育(如枝叶分化[5]、腋生分生组织发育[9,19]、激素信号传导[20]和胁迫[21]等)。例如,拟南芥中的AtBRC1[5]、水稻中的OsFC1[6]和郁金香中的TgTB1[22]参与腋芽的发育和分枝控制。另外,I类TCP成员参与植物子叶发育、生长和增殖[23-24]。如在拟南芥中,AtTCP15在雌蕊发育过程中调节内部遗传物质复制以及细胞分裂素和生长素的刺激[8],AtTCP14和AtTCP15共同调节植物节间长度[7]和种子发芽[10],而AtTCP23参与植物开花节律和植物发育[25-26]。然而,由于它们结合的顺式调控竞争位点相似,使得这两类基因共同参与调控植物生长和发育[24,27]。
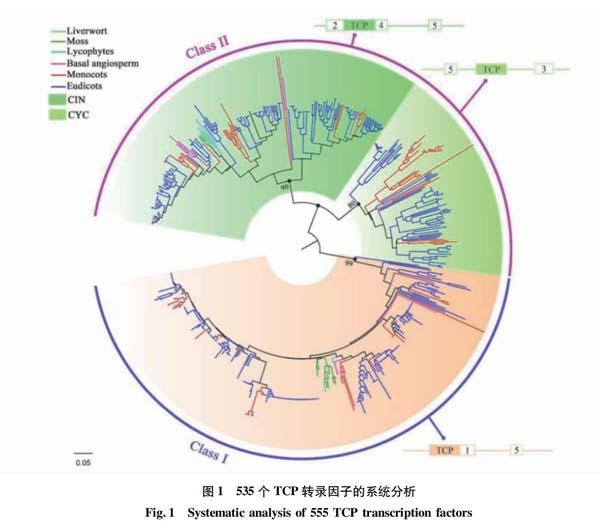
Liu等[28]利用系统发育分析表明TCP家族中的每个基因类别在其进化过程中均保守地分布在陆地植物中,并且Ⅱ类基因的CYC和CIN基因在陆地植物建立之初就出现了(图1)。
2TCP转录因子的生物学功能
最初的报道显示TCP转录因子家族与细胞增殖和植物生长的调控有关,此后又有许多研究表明TCP家族不同成员的详细作用,这些研究揭示了TCPs参与调控了多个生物学功能的进程并以不同的机制来发挥作用。
2.1植物生长发育功能
据报道TCP转录因子普遍参与了植物生长发育的生物学进程。研究表明,TCP转录因子的Ⅱ类(CIN)成员主要参与叶片发育。之前的研究已经报道了包括TCP2、TCP3、TCP4、TCP10和TCP24在内的拟南芥TCP基因具有miR319结合位点。而miR319已经证实了对这些AtTCP转录物具有切割活性,TCP转录因子的Ⅱ类(CIN)成员起着抑制叶子中细胞增殖的作用。具有高水平的miR319或低水平的miR319结合位点的TCP蛋白可能会导致过量的细胞增殖,从而导致拟南芥、金鱼草和番茄中的皱叶或单子叶植物(大米和蔓生的草皮)中的叶变大[29];AtTCP3间接调节边界特异性基因CUC和LOB的表达[30];在番茄LA中,这类基因主要参与调节叶片的形成[31]。除此之外,还有研究表明几个CIN同源基因在细胞增殖中起到明显作用[32];另外还有一些CYC家族成员参与调节枝条分化,如豌豆TCP转录因子PsBRC1作用于Strigolactones的下游以抑制芽的生长和控制枝条的分枝[32];有些基因在花的生长发育过程中起着重要作用[33-34]。总之,这些结果表明CIN和CYC两类基因主要参与植物发育,此外,虽然I类成员也参与调节植物的发育,但与Ⅱ类成员的作用相反。其中最显著的功能是叶片发育的调节:I类成员促进细胞增殖,而Ⅱ类(CIN)成员则负调控此过程[35-36]。除此之外,I类成员还可以在许多其他发育功能过程中发挥作用。如拟南芥中的AtTCP14和AtTCP15基因在节间、叶片和花组织的细胞增殖中起作用,并影响节间长度和叶片形状[7];菊花CmTCP14基因调节器官大小[37],蝴蝶兰PePCF10基因影响胚珠发育[3];陆地棉GhTCP14基因参与植物生长素介导的棉纤维发育[38]。
2.2非生物胁迫功能
Mukhopadhyay等[39]证实了过表达OsTCP19可以诱导一些反应通路如JA、ABA、IAA、CK和ET通路,并能促进脂肪滴合成、降低水分损失和減少氧离子,进而提高转基因株系的甘露醇处理和高盐的耐受力。
Guan等[40]、Almeida等[41]研究表明,使用一个杂化酵母(Y1H)系统,可以确定5个TCP转录因子,使OSPCF2、OSCPP5、AtTCP20这3个转录因子去结合OsNHX1启动子。研究发现这些转录因子编码的基因在OsNHX1作用下可以参与调节不同的非生物胁迫反应,并证实OsPCF2调节参与盐胁迫。
通过使用硝酸盐增强剂筛选酵母单杂交系统中拟南芥转录因子的文库,鉴定了转录因子基因TEOSINTE BRANCHED1 / CYCLOIDEA / PROLIFERATING的细胞因子1-20(TCP20)。TCP20属于古老植物特有的基因家族,可调控芽、花和胚的发育。该植物具有结合100多个受硝酸盐调节的基因能力,从而参与了硝酸盐信号传导。根据TCP20插入突变体的分析表明,它们在均质硝酸盐培养基上具有正常的初生和侧根生长,但在分裂根中的异质培养基上,其优先侧根生长(根觅食)受到损害。即使铵盐均匀地存在于培养基中,突变体仍优先抑制侧根生长,这表明TCP20对硝酸盐的刺激具有一定的响应。比较TCP20突变体与NLP7突变体,发现TCP20突变体在根生长的局部控制方面有缺陷,但在根觅食反应中没有缺陷,这表明TCP20功能独立于NLP7功能且与NLP7功能不同。进一步的分析表明,无论局部硝酸盐浓度如何,TCP20突变体均缺乏对根生长的系统控制。总之,TCP20在介导拟南芥根硝酸盐觅食的系统信号传导途径中起着关键作用[42]。
2.3生物胁迫功能
研究表明,在植物免疫中TCP基因有着显著的作用。如拟南芥中的一些TCP基因,如Ⅱ类(CIN)成员中的AtTCP13和I类成员中的AtTCP14,15,19和21是作为病原体的效应目标[43]。在对旱稻(O.sativa)报道中,Ⅱ类(CIN)成员中的OsTCP21参与病原体防御[44]。在对西红柿(S.lycopersicum)报道中,AtTCP14-2(AtTCP14的直系同源物)有助于增强对辣椒疫霉菌的免疫力[45]。同时,许多Ⅱ类TCP基因已被证明是植原体效应[46]。如在拟南芥中,SAP11作用在Ⅱ类不稳定的不同成员中,因此导致严重的叶起皱和茉莉酸水平下调[44,46]。最近,一些Ⅱ类(CYC)成员也被证明是SAP11效应子(如小麦蓝矮植原体效应子SWP1[47-48])。研究表明TCP13、TCP14和TCP19 这3种免疫互作因子是2种病原体效应物的直接靶标[49]。进一步的功能研究也表明了tcp13、tcp14和tcp19单突变体对2种不同的无毒性Hyalop-eronosporaarabidopsidis(Hpa)分离株(Emwa1和Emoy2)更敏感,因此这3种TCP转录因子中的每一种都是完整免疫系统功能所必需的;此外,TCP15突变体对毒性Hpa分离株Noco2的抗病性在增强[50]。
[6] TAKEDA T,SUWA Y,SUZUKI M,et al.The OsTB1 gene negatively regulates lateral branching in rice[J].The plant journal,2010,33(3):513-520.
[7] KIEFFER M,MASTER V,WAITES R,et al.TCP14 and TCP15 affect internode length and leaf shape in Arabidopsis[J].Plant J,2011,68(1):147-158.
[8] LUCERO L E,UBERTIMANASSERO N G,ARCE A L,et al.TCP15 modulates cytokinin and auxin responses during gynoecium development in Arabidopsis[J].The plant journal,2015,84(2):267-282.
[9] NICOLAS M,CUBAS P.TCP factors:New kids on the signaling block[J].Current opinion in plant biology,2016,33:33-41.
[10] TATEMATSU K,NAKABAYASHI K,KAMIYA Y,et al.Transcription factor AtTCP14 regulates embryonic growth potential during seed germination in Arabidopsis thaliana[J].The plant journal,2010,53(1):42-52.
[11] PRUNEDAPAZ J L,BRETON G,PARA A,et al.A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock[J].Science,2009,323(5920):1481-1485.
[12] KOSUGI S,OHASHI Y.PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene[J].The plant cell,1997,9(9):1607-1619.
[13] NAVAUD O,DABOS P,CARNUS E,et al.TCP transcription factors predate the emergence of land plants[J].Journal of molecular evolution,2007,65(1):23-33.
[14] HOWARTH D G,DONOGHUE M J.Phylogenetic analysis of the “ECE”(CYC/TB1)clade reveals duplications predating the core eudicots[J].Proceedings of the national academy of sciences of the United States of America,2006,103(24):9101-9106.
[15] CUBAS P,LAUTER N,DOEBLEY J,et al.The TCP domain:A motif found in proteins regulating plant growth and development[J].The plant journal,2010,18(2):215-222.
[16] NAVAUD O,DABOS P E,CARNUS E,et al.TCP transcription factors predate the emergence of land plants[J].Journal of molecular evolution,2007,65(1):23-33.
[17] MARTNTRILLO M,CUBAS P.TCP genes:A family snapshot ten years later[J].Trends in plant science,2009,15(1):31-39.
[18] CRAWFORD B C W,NATH U,CARPENTER R,et al.CINCINNATA controls both cell differentiation and growth in petal lobes and leaves of Antirrhinum[J].Plant Physiol,2004,135(1):244-253.
[19] DOEBLEY J,STEC A,HUBBARD L.The evolution of apical dominance in maize[J].Nature,1997,386(6624):485-488.
[20] LUO D,CARPENTER R,VINCENT C,et al.Origin of floral asymmetry in Antirrhinum[J].Nature,1996,383(6603):794-799.
[21] LI S T.The Arabidopsis thaliana TCP transcription factors:A broadening horizon beyond development[J].Journal plant signaling & behavior,2015,10(7):1-12.
[22] MORENOPACHON N M,MUTIMAWURUGO M C,HEYNEN E,et al.Role of Tulipa gesneriana TEOSINTE BRANCHED1(TgTB1 )in the control of axillary bud outgrowth in bulbs[J].Plant reproduction,2017,31(2):1-13.
[23] VIOLA I L,UBERTI MANASSERO N G,RIPOLL R,et al.The Arabidopsis class I TCP transcription factor AtTCP11 is a developmental regulator with distinct DNAbinding properties due to the presence of a threonine residue at position 15 of the TCP domain[J].Biochem J,2011,435(1):143-155.
[24] DANISMAN S,VAN DER WAL F,DHONDT S,et al.Arabidopsis class I and class Ⅱ TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically[J].Plant Physiol,2012,159(4):1511-1523.
[25] LI Z Y,LI B,DONG A W.The Arabidopsis transcription factor AtTCP15 regulates endoreduplication by modulating expression of key cellcycle genes[J].Molecular plant,2012,5(1):270-280.
[26] BALSEMOPIRES E,ANDRADE L R,SACHETTOMARTINS G,et al.Functional study of TCP23 in Arabidopsis thaliana during plant development[J].Plant physiology and biochemistry,2013,67(3):120-125.
[27] LI S T.The Arabidopsis thaliana TCP transcription Factors:A broadening horizon beyond development[J].Plant signaling &behavior,2015,10(7):1-12.
[28] LIU MM,WANGMM,YANG J,et al. Evolutionary and comparative expression analyses of TCP transcription factor gene family in land plants[J].Molecular scienes,2019,20(14):359.
[29] BRESSO E G,CHOROSTECKI U,RODRIGUEZ R E,et al.Spatial control of gene expression by miR319regulated TCP transcription factors in leaf development[J].Plant Physiol,2017,176(2):1694-1708.
[30] SZKLARCZYK D,MORRIS J H,COOK H,et al.The STRING database in 2017:Qualitycontrolled proteinprotein association networks,made broadly accessible[J].Nucleic acids research,2017,45:362-368.
[31] ZUO J H,ZHU B Z,FU D Q,et al.Sculpting the maturation,softening and ethylene pathway:The influences of microRNAs on tomato fruits[J].BMC Genomics,2012,13(1):1-12.
[32] VADDE B V L,CHALLA K R,NATH U.The TCP4 transcription factor regulates trichome cell differentiation by directly activating GLABROUS INFLORESCENCE STEMS in Arabidopsis thaliana[J].The plant journal,2018,93(2):259-269.
[33] BROHOLM S K,THTIHARJU S,LAITINEN R A E,et al.A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera(Asteraceae)inflorescence[J].PNAS,2008,105(26):9117-9122.
[34] YUAN Z,GAO S,XUE D W,et al.RETARDED PALEA1 controls palea development and floral zygomorphy in rice[J].Plant physiology,2009,149(1):235-244.
[35] LI C X,POTUSCHAK T,COLNCARMONA A,et al.Arabidopsis TCP20 links regulation of growth and cell division control pathways[J].PNAS,2005,102(36):12978-12983.
[36] AGUILARMARTNEZ J A,SINHA N J.Analysis of the role of Arabidopsis class I TCP genes AtTCP7,AtTCP8,AtTCP22,and AtTCP23 in leaf development[J].Front Plant Sci,2013,4(1):1-13.
[37] ZHANG T,QU Y X,WANG H B,et al.The heterologous expression of a chrysanthemum TCPP transcription factor CmTCP14 suppresses organ size and delays senescence in Arabidopsis thaliana[J].Plant physiology and biochemistry,2017,115:239-248.
[38] WANG M Y,ZHAO P M,CHENG H Q,et al.The cotton transcription factor TCP14 functions in auxinmediated epidermal cell differentiation and elongation[J].Plant physiology,2013,162(3):1669-1680.
[39] MUKHOPADHYAY P,TYAGI A K.OsTCP19 influences developmental and abiotic stress signaling by modulating ABI4mediated pathways[J].Scientific reports,2015,5:1-11.
[40] GUAN P Z,RIPOLL J J,WANG R H,et al.Interacting TCP and NLP transcription factors control plant responses to nitrate availability[J].PNAS,2017,114(9):2419-2424.
[41] ALMEIDA D M,GREGORIO G B,OLIVEIRA M M,et al.Five novel transcription factors as potential regulators of OsNHX1 gene expression in a salt tolerant rice genotype[J].Plant molecular biology,2016,93(1/2):61-77.
[42] GUAN P Z,WANG R C,NACRY P,et al.Nitrate foraging by Arabidopsis roots is mediated by the transcription factor TCP20 through the systemic signaling pathway[J].Proceedings of the national academy of sciences of the United States of America,2014,111(42):15267-15272.
[43] LOPEZ J A,SUN Y,BLAIR P B,et al.TCP threeway handshake:Linking developmental processes with plant immunity[J].Trends in plant science,2015,20(4):238-245.
[44] ZHANG C,DING Z M,WU K C,et al.Suppression of jasmonic acidmediated defense by viralinducible MicroRNA319 facilitates virus infection in rice[J].Molecular plant,2016,9(9):1302-1314.
[45] SONG T Q,MA Z C,SHEN D Y,et al.An oomycete CRN effector reprograms expression of plant HSP genes by targeting their promoters[J].PLOS Pathogens,2015,11(12):1-30.
[46] SUGIO A,KINGDOM H N,MACLEAN A M,et al.Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis[J].Proceedings of the national academy of sciences of the United States of America,2011,108(48):19111-19112.
[47] MINATO N,HIMENO M,HOSHI A,et al.The phytoplasmal virulence factor TENGU causes plant sterility by downregulating of the jasmonic acid and auxin pathways[J].Scientific reports,2014,4:1-17.
[48] FAN G Q,ZHANG S,ZHAI X Q,et al.Effects of antibiotics on the paulownia witches broom phytoplasmas and pathogenic protein related to witches broom symptom[J].Scientia silvae sinicae,2007,43(3):138-142.
[49] MASUDA H P,CABRAL L M,DE VEYLDER L,et al.ABAP1 is a novel plant Armadillo BTB protein involved in DNA replication and transcription[J].The EMBO Journal,2014,27(20):2746-2756.
[50] KEBROM T H,BURSON B L,FINLAYSON S A.Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals[J].Plant physiology,2006,140(3):1109-1117.
[51] SUGIO A,KINGDOM H N,MACLEAN A M,et al.Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis[J].PNAS,2011,108(48):19111-19112.
[52] ROBINSON F R,FUCHS A F.The role of the cerebellum in voluntary eye movements[J].Annual review of neuroscience,2001,24(1):981-1004.
[53] MARTNTRILLO M,CUBAS P.TCP genes:A family snapshot ten years later[J].Trends in plant science,2010,15(1):31-39.
[54] HAO J,TU L L,HU H Y,et al.GbTCP,a cotton TCP transcription factor,confers fibre elongation and root hair development by a complex regulating system[J].Journal of experimental botany,2012,63(17):6267-6281.
[55] KOYAMA T,MITSUDA N,SEKI M,et al.TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164,as well as the auxin response,during differentiation of leaves in Arabidopsis[J].The plant cell,2010,22(11):3574-3588.
[56] UBERTIMANASSERO N G,LUCERO L E,VIOLA I L,et al.The class I protein AtTCP15 modulates plant development through a pathway that overlaps with the one affected by CINlike TCP proteins[J].Journal of experimental botany,2012,63(2):809-823.
[57] KROUK G,LACOMBE B,BIELACH A,et al.Nitrateregulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants[J].Development cell,2010,18(6):927-937.
[58] LI S T,ZACHGO S.TCP3 interacts with R2R3MYB proteins,promotes flavonoid biosynthesis and negatively regulates the auxin response in Arabidopsis thaliana[J].The plant journal,2013,76(6):901-913.
