Preparation and Characterization of Pt@Au/Al2O3 Core-Shell Nanoparticles for Toluene Oxidation Reaction
2020-08-23ChaoZhangSihanLiChenliangWuXiaoqingLiXinhuanYan
Chao Zhang, Sihan Li, Chenliang Wu, Xiaoqing Li, Xinhuan Yan
State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, Zhejiang University of Technology, Hangzhou 310014, P. R. China.
Abstract: Customizing core-shell nanostructures is considered to be an efficient approach to improve the catalytic activity of metal nanoparticles. Various physiochemical and green methods have been developed for the synthesis of core-shell structures.In this study, a novel liquid-phase hydrogen reduction method was employed to form core-shell Pt@Au nanoparticles with intimate contact between the Pt and Au particles, without the use of any protective or structure-directing agents. The Pt@Au core-shell nanoparticles were prepared by depositing Au metal onto the Pt core;AuCl4- was reduced to Au(0)by H2 in the presence of Pt nanoparticles. The obtained Pt@Au core-shell structured nanoparticles were characterized by transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy(EDX), high-resolution TEM, fast Fourier transform, powder X-ray diffraction (PXRD), X-ray photoelectron spectroscopy(XPS), Fourier transform infrared spectroscopy (FTIR), and H2-temperature programmed reduction (H2-TPR)analyses.The EDX mapping results for the nanoparticles, as obtained from their scanning transmission electron microscopy images in the high-angle annular dark- field mode, revealed a Pt core with Au particles grown on its surface. Fourier transform measurements were carried out on the high-resolution structure to characterize the Pt@Au nanoparticles. The lattice plane at the center of the nanoparticles corresponded to Pt, while the edge of the particles corresponded to Au. With an increase in the Au content, the intensity of the peak corresponding to Pt in the FTIR spectrum decreased slowly, indicating that the Pt nanoparticles were surrounded by Au nanoparticles, and thus confirming the core-shell structure of the nanoparticles.The XRD results showed that the peak corresponding to Pt shifted gradually toward the Au peak with an increase in the Au content, indicating that the Au particles grew on the Pt seeds; this trend was consistent with the FTIR results. Hence, it can be stated that the Pt@Au core-shell structure was successfully prepared using the liquid-phase hydrogen reduction method. The catalytic activity of the nanoparticles for the oxidation of toluene was evaluated using a fixed-bed reactor under atmospheric pressure. The XPS and H2-TPR results showed that the Pt1@Au1/Al2O3 catalyst had the best toluene oxidation activity owing to its lowest reduction temperature, lowest Au 4d & 4f and Pt 4d & 4f binding energies, and highest Au0/Auδ+ and Pt0/Pt2+ proportions. The Pt1@Au2Al2O3 catalyst showed high stability under dry and humid conditions. The good catalytic performance and high selectivity of Pt@Au/Al2O3 for toluene oxidation could be attributed to the high concentration of adsorbed oxygen species, good low-temperature reducibility, and strong interaction.
Key Words: Catalyst; Synergy; Nanomaterials; Core-shell structure; VOCs
1 Introduction
In the last few years, there have been comprehensive studies on nano new materials with many innovative advances in the precise manipulation of their structure and morphology by scientific research workers, especially for the study of bimetallic nanomaterials1-4. Bimetallic noble metal nanostructures,including hierarchical heterostructures, alloy nanostructures,and especially core-shell structure have been receiving increasing research attentions, which have broad application prospects in catalytic, optics, electronic, sensing, energy conversion, storage, biomedicine and other fields5-10. Bimetallic core-shell nanostructures possess superb chemical and physical properties due to the synergies included “ensemble effect” and“ligand effect” as well as the lattice strain created between the core and shell region compared to their single-component counterparts11-13.
Recently, great attention has been paid on the control of volatile organic compounds (VOCs)emission in China, due to their negative influence on human health and the atmospheric environment by means of the generation of organic aerosols,ozone and smog14-17. Several techniques for eliminating VOCs have been investigated, such as thermal incineration, catalytic oxidation, condensation, absorption, bio-filtration, adsorption and membrane. Among these methods, catalytic oxidation has been an efficient, cost-effective, versatile and environmentally benign technology to eliminate the VOCs at low temperature. In the catalytic oxidation of VOCs, supported noble metals, such as Pt and Au, are recognized to be most efficient systems18-24.Many studies have focused on bimetallic catalyst, especially core-shell catalyst, due to its high activity and selectivity comparing to single metal catalysts.
In this study, a series of bimetallic Pt@Au core-shell structures nanoparticles were successfully synthesized by a facile hydrogen reduction. First, the Pt cores were synthesized by using hydrogen reduction. Au nanoparticles were then grown on the Pt colloids to obtain the Pt@Au core-shell nanoparticles in the absence of both surfactant agents and stubborn surfactant agents. This preparation method is simple to operate, and does not require any protective agent, structure guide agent and endsealing agent. Compared with the conventional method, it can effectively control the particle size and crystal structure of the metal25-28. The as-prepared Pt@Au/Al2O3have demonstrated catalytic activity towards the oxidation of toluene, which have been applied to eliminate toluene with high conversion,selectivity and stability.
2 Experimental and computational section
2.1 Materials
HAuCl4·4H2O and K2PtCl4(AR)were purchased from Shanghai Tuosi Chemical Co., Ltd. Propylene carbonate(AR)was purchase from Dongguan Youte environmental protection materials co. Ltd. γ-Al2O3(AR)was brought from Shanghai Lvqiang New Material Co., Ltd. China. Dba(dibenzylideneacetone)(AR)was purchased from Zhejaing Alpha Chemical Technology Co. Ltd.
2.2 Preparation of Pt nanoparticles
Pt2(dba)3(dba = dibenzylideneacetone)was synthesized according to the literature29. The solution of K2PtCl4(1.40 g,AR)in distilled water (12 mL)was added to a solution of sodium acetate (2.8 g, AR)and dibenzylideneacetone (2.36 g)in ethanol(60 mL, AR)at 50 °C. When the initial pale yellow suspension dissolved, the mixture was refluxed at 90 °C. After refluxing for 1 h, the dark violet precipitate formed. The mixture was filtrated,washed and settled overnight. Then the solid was washed three times with the deionized water, dried overnight under vacuum,further washed three times with n-pentane (AR), and finally dried under vacuum overnight.
The Pt nanoparticles were prepared by hydrogen reduction method. The measured amount of Pt2(dba)3was dissolved in 100 mL propylene carbonate (PC)and added to a steel autoclave,followed by pressurizing to 4 MPa with H2. The solution was vigorously stirred for 3 h at 40 °C to obtain a brown colloid of Pt. After that, γ-Al2O3was added to above solution with continuous adsorbing for 24 h. The sample was filtrated, washed,dried and eventually calcined in air at 400 °C for 4 h to obtain Pt/Al2O3.
2.3 Preparation of Pt@Au core-shell nanoparticles
The calculated amount of HAuCl4·4H2O with various molar ratios (Pt/Au = 1 : 1, 1 : 2, 1 : 3 and 1 : 4)was added in the above-prepared Pt nanoparticles solution. Then the mixed solution was added to the autoclave pre-equilibrated with H2(to exclude the inside air), followed by pressurizing to 4 MPa H2.The solution was vigorously stirred for 2 h at 40 °C to obtain Pt@Aux(x = 1, 2, 3, 4)core-shell nanoparticles. The supported catalyst was prepared using a method of directly adsorbing nanoparticles. A certain amount of the pretreated γ-Al2O3was added to the above nanoparticles solution. After impregnating 24 h, the sample was filtered, dried and then calcined in the muffle furnace at 400 °C for 4 h to obtain combined core-shell Pt@Aux(x = 1, 2, 3, 4)/Al2O3catalysts.
2.4 Catalyst characterization
Transmission electron microscopy (TEM)and high-resolution transmission electron microscopy (HRTEM)were performed using a Tecnai G2 F30 S-Twin microscope (Philips-FEI,Netherlands)operated at 200 kV, high-angle annular dark-field scanning TEM (HAADF-STEM)and Elemental analysis was conducted on an energy dispersive X-ray (EDX)spectrometer.Powder X-ray diffraction (XRD)patterns of the samples were recorded on PANalytical X’Pert PRO X-ray diffractometer using Cu Kα radiation (λ = 0.1541 nm)at 40 kV and 40 mA. FT-IR spectra were acquired with Nicolet 6700 FTIR Spectrometer with Continuum Microscope. X-ray photoelectron spectroscopy(XPS)measurements were performed with a Kratos AXIS UItra DLD spectrometer using the Al X-ray source. The working voltage was 15 kV and the working current was 15 mA. The binding energies were calibrated using C 1s peak of contaminant carbon (284.8 eV)as standard. The spectra were performed with XPS PEAK software (Ver. 4.1). The different H2reduction temperature and hydrogen consumption of different oxidation state substances were measured using H2-TPR (Temperature programmed reduction)method with online TCD recorded the temperature dependence of H2concentration.
2.5 Catalytic activity measurements
The catalytic activity of the catalyst was evaluated in the selfmade atmospheric fixed bed catalytic reaction device. The 0.5 g catalyst was placed in the middle of the reaction tube. The saturated toluene vapor of 0 °C was introduced into the reaction tube by bubbling method. The concentration (volume fraction)and gas hourly space velocity (GHSV)of VOCs in the feed stream were 1000 × 10-6and 18000-54000 mL·g-1·h-1,respectively. The toluene and oxide content in the tail gas was monitored online by gas chromatography with a flame ionization detector (FID)and a thermal conductivity detector (TCD)to determine toluene conversion and CO2selectivity at different temperatures, and the temperature at which toluene achieved 98% conversion was recorded as T98.
The conversion of toluene is calculated as follows:

In the above equation, φ(C7H8)inlet and φ(C7H8)outlet represent the volume fraction of toluene before and after reaction,respectively; θ(CO2)and θ(CO)represent the volume fraction of CO2and CO.
3 Results and discussion
3.1 Characterization of the Pt@Aux/Al2O3 core-shell nanoparticle
To investigate the structure and surface morphology of the prepared Pt@Au core-shell NPs, the TEM images and particle size distributions of Pt nanoparticles and Pt@Au nanoparticles were showed in Fig. 1. The Pt NPs sizes were in the range of 1.6-2.9 nm and the average size was 2.1 nm (Fig. 1a). Whereas,the bimetallic Pt@Au core-shell NPs were significantly larger than the monometallic Pt and the NPs sizes existed in the range of 10-18 nm (the average size was 14 nm)(Fig. 1b). The size increment before and after Au deposition could be clearly seen,indicating that the Au grew on the surface of Pt, not forming its own nucleus.
To confirm the existence of Pt@Au core-shell, an energy dispersive X-ray spectrometer (EDS)and Elemental mapping images attached to the TEM equipment was used. The distributions of the metallic elements in the Pt@Au NPs were shown Fig. 2. The yellow and green colors represented the distribution of Pt and Au elements, respectively. The merged mapping patterns provided clear evidence for the elemental distribution of Pt and Au, which further indicated the core-shell structure of the Pt@Au. The EDX signal for Au was more diffused and the size of nanoparticles shaped by Au signal was larger than that suggested by the Pt signal. It was confirmed by the merged Pt + Au EDX map in which Au signal not only overlapped with that of Pt but also formed a “ring” around Pt core. It was clearly suggested that the Au element was deposited on the surface of Pt nanoparticles, which further confirmed the successful fabrication of core-shell Pt@Au nanoparticles structure. However, the Pt and Au signal were visible among Pt@Au nanoparticles. The Pt diffused signal might originate from small nanoparticles deposited separately and it could be thought that the face-centered cube structure of Pt enables it to have abundant (111)crystal planes with lower surface energy,which could be selectively matched with Au (111)crystal planes, while the other crystal planes could not be well matched and exist separately12,30. The weaker Au signal might be assigned to traces of non-reduced Au-precursor trapped on the support.

Fig. 1 TEM images of (a)Pt nanoparticles, (b)Pt@Au nanoparticles and the corresponding particle size distributions.

Fig. 2 HAADF-STEM image, elemental mapping images and EDS spectrum of Pt@Au.

Fig. 3 HRTEM image of Pt@Au core-shell and (A-G)the corresponding FFT patterns of the each area.
To further characterize the Pt@Au NPs nanostructures,HRTEM images of Pt@Au NPs were obtained at zoom-in images (Fig. 3). The interface between the core and shell was not clearly visible, which might be due to the same imaging contrast,the similar electron density and the same crystal structure31,32.We performed Fourier transform on the high-resolution structures to obtain the corresponding diffraction patterns. From the analysis of HRTEM image and FFT patterns, it was found that the (111)lattice plane at the center position of the nanoparticles of Pt@Au NPs with d-spacing value of 0.226 nm corresponded to the fcc metallic Pt33, and the (111)lattice plane at the B-G region of the nanoparticles of Pt@Au NPs with dspacing value of 0.235 nm, 0.234 nm, 0.234 nm, 0.234 nm, 0.236 nm, 0.235 nm respectively fitted well with fcc metallic Au34.According to the literature35, the HRTEM image of the Pt-Au nanoparticles revealed that the d-spacings of adjacent fringes for metal cores and metal surrounding were (0.225 ± 0.002)nm and(0.235 ± 0.002)nm, respectively, corresponding to the (111)planes of face-centered cubic Pt and Au, indicating that bimetallic core-shell NPs had been obtained36-38. Therefore, the Pt and Au precursors were reduced to form two separate phases,and Pt served as a seed for the growth of Pt@Au NPs. The results indicated that the lattice spacing of A and B-G regions matched well respectively with the (111)planes of the Au and Pt.Therefore, it could be illustrated the Pt@Au nanoparticle could be core-shell structure, which accorded with elemental mapping of Pt@Au nanoparticles.
For further confirmation of the formation of the Pt@Au coreshell structure, FTIR spectra of Pt@Aux/Al2O3were measured and showed in Fig. 4. The peaks at 3445 cm-1, 1634 cm-1and 757 cm-1were observed, which were assigned to γ-Al2O339,40.The sample of Pt/Al2O3observed a peak at 1420 cm-1, which was assigned to Pt41. With the Au content increasing, the peak at 1420 cm-1slowly weakened to disappear, which indicated that Au grew on the surface of Pt to wrap it instead of forming an alloy or forming a new core. The FTIR spectra results were further demonstrated the core-shell structure.
The structural identification and phase composition of the prepared samples were further investigated by wide-angle XRD measurements (Fig. 5). According to the standard card of pure Pt (JCPDS-87-0642)and pure Au (JCPDS-89-3697), the peaks at two theta values of 39.6°, 46.1°, 67.2°, 80.9° and 85.7° could be indexed to the (111), (200), (220), (311)and (222)crystal planes of face-centered cubic Pt and the peaks at 38.2°, 44.4°,64.6°, 77.5°, and 81.7° were in good agreement with (111),(200), (220), (311)and (222)planes of ace-centred cubic (fcc)Au, respectively. For Pt/Al2O3NPs, the Pt peak only at 39.6°could be observed due to the overlap with Al2O3of the support.For Pt@Aux/Al2O3NPs, as the Au content continued to increase,the Pt peak of 39.6° slowly shifted to the Au peak of 38.2°, and its peak shape became sharp. The Pt peak of 67.2° also slowly moved to the Au peak of 64.6°. And the peak at 77.5° was more obvious as the Au content increasing which was indicated the increasing of Au coated on the Pt surface to form Pt@Au coreshell structure. Combined with the HR-TEM, Elemental mapping images and IR spectra, it could conclude that Pt@Au core-shell nanoparticle was successfully synthesized by a seed growth method in non-presence of protective and structure directing agent.
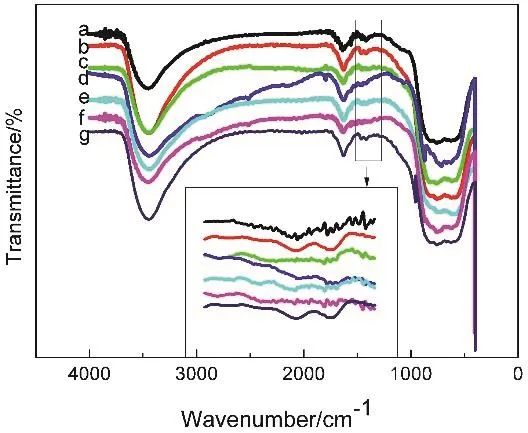
Fig. 4 FTIR spectra of Au/Al2O3, Pt/Al2O3, and Pt@Aux nanoparticles.

Fig. 5 The overall XRD pattern for different molar ratios of Pt@Au/Al2O3.
The H2-TPR pro files of a series of the Al2O3supported monoand bimetallic catalysts with various molar ratio were showed in Fig. 6. The sample of Pt/Al2O3single mental catalyst showed a broad reduction peak around 459 °C, related to reduction of PtOxspecies42. For the Au/Al2O3, the H2consumption peak at 400 to 750 °C was observed, indicating the AuxOyreduction18. In addition, all the bimetallic Pt@Aux/Al2O3catalysts only showed one reduction peak. And the bimetallic Pt@Aux/Al2O3of various molar ratios showed the lower reduction temperature which was from 300 to 450 °C compared to single mental catalyst. We can conclude that the reduction peak was shifted to a lower temperature, indicating a strong interaction between Pt and Au metal, due to the core-shell structure.

Fig. 6 H2-TPR profiles of Pt/Al2O3 (a), Pt1@Au1/Al2O3 (b),Pt1@Au2/Al2O3 (c), Pt1@Au3/Al2O3 (d), Pt1@Au4/Al2O3 (e),Au/Al2O3 (f).
The surface chemical state and composition of the different molar ratios were obtained by XPS showed in Fig. 7, which was showed the spectra of the four samples, revealing the presence of Pt, Au and Al elements in the Pt@Aux/Al2O3samples. Owing to the Al 2p peak overlapped with the Pt 4f peak and the Au 4d peak overlapped with the Pt 4d in the range of 68-80 eV and 300-360 eV, it was necessary to separate the Al 2p peak, Pt 4f,Au 4d and Pt 4d from the spectra. The spectra of Au 4f was showed in Fig. 7C. The peak of Pt1@Au1/Al2O3centered at 84.59 eV belonged to Au0and the other located at 85.09 eV was corresponding to Auδ+43,44, indicating that part of Au shell was oxidized, which was consistent with the result of H2-TPR. The spectra of Al 2p&Pt 4f was showed in Fig. 7A. The doublet of Pt1@Au1/Al2O3at 71.79 and 74.59 eV was assigned to metallic Pt. And the doublet at 71.19 and 74.39 eV was assigned to oxidized Pt45,46. Fig. 7B showed the spectra of Au 4d &Pt 4d.As for Au 4d of the sample of Pt1@Au1/Al2O3, the components at BE = 335.79 eV and 354.39 eV were due to the surface Au0species, while the ones at BE = 335.99 eV and 353.79 eV were due to the surface Auδ+. The coexistence of Pt0and Pt2+was evidenced by a shoulder observed on the peak at 315.49 eV,321.79 eV and 317.59 eV, 330.49 eV of Pt 4d spectra. Compared to the Au 4f, Pt 4f, Au 4d and Pt 4d peaks of Pt@Aux/Al2O3sample, the Pt1@Au1/Al2O3had the lowest energy, revealing the strong interaction between Pt and Au, due to its core-shell structure, which revealed the result of catalytic oxidation of toluene. And the Au0/Auδ+and Pt0/Pt2+proportion of Pt1@Au1/Al2O3were the largest, which was concluded that the Au0and Pt0contents occupied the majority of the catalyst,indicating that Au0and Pt0were the main active species on the catalyst surface.

Fig. 7 Pt 4f & Al 2p, Au 4f and Au 4d & Pt 4d XPS spectra of (A-a, B-a)Pt/Al2O3, (A-b, B-b, C-b)Pt1@Au1/Al2O3, (A-c, B-c, C-c)Pt1@Au2/Al2O3,(A-d, B-d, C-d)Pt1@Au3/Al2O3, (A-e, B-e, C-e)Pt1@Au4/Al2O3, (B-g, C-a)Au/Al2O3, (A-f, B-f, C-f)after stability test Pt1@Au1/Al2O3.

Fig. 8 Catalytic oxidation of toluene with different molar ratios of Pt@Aux/Al2O3, Pt/Al2O3 and Au/Al2O3.
3.2 Catalytic activity of Pt@Aux/Al2O3, Pt/Al2O3 and Au/Al2O3 catalysts for toluene oxidation
Catalytic oxidation of toluene with different molar ratios of Pt@Aux/Al2O3were showed in Fig. 8. In this study, all experimental runs were operated until reaching the steady state condition before taking data. For all the samples CO2and water were the only products detected under the applied reaction conditions. The reaction temperatures for 98% toluene conversion were 518, 533, 523, 528, 538 and 568 K for the Pt1@Au1/Al2O3, Pt1@Au2/Al2O3, Pt1@Au3/Al2O3,Pt1@Au4/Al2O3, Pt/Al2O3and Au/Al2O3catalysts, respectively.Among the as-prepared catalysts, Pt1@Au1/Al2O3gave the lowest T98 of 518 K and the highest TOF of 0.084 s-1at 170 °C,which was lower than those of other catalysts. And it also illustrated the coexistence of Au and Pt in the bimetallic Pt1@Au1/Al2O3catalyst had higher activity for toluene oxidation compared with monometallic Pt36,47. The authors proposed that the high activity of the catalysts was related to the particle size and synergistic effect of bimetal.

Fig. 9 Stability test of toluene oxidation (a)and selectivity to CO2 (b)with time-on-stream over Pt1@Au1/Al2O3, Pt/ Al2O3 and Au/Al2O3 catalyst at T98 and Pt1@Au1/Al2O3 at T90.
3.3 Catalyst stability test of Pt/Al2O3, Au/Al2O3 and Pt1@Au1/Al2O3
In order to investigate the influence of single metal and bimetallic catalysts on the long-term activity of the Pt1Au1/Al2O3, Pt/Al2O3and Au/Al2O3samples, catalytic experiments were performed up to 50 h. Catalyst stability test was carried out at temperature which resulted in toluene conversion of 98%, providing a more sensitive indication of changes of the catalyst performance with time on stream. As showed in Fig. 9, the core-shell Pt1@Au1/Al2O3, Au/Al2O3and Pt/Al2O3catalysts at constant temperature of T98 and Pt1@Au1/Al2O3catalyst at constant temperature of T90,exhibited a steady-state conversion of about 98%-100% and 89%-91%, indicating high stability of these catalysts under used reaction conditions. The structure characterization of catalyst that after stability test was shown in Fig. 4g, Fig. 5f and Fig. 7A-f, B-f, C-f. The FTIR spectra of the after stability test catalyst was similar to the fresh catalyst, which indicated that the structure was preserved. The result of XRD was similar as the FTIR. The Pt 4f binding energy of after stability test Pt1@Au1/Al2O3catalyst was 0.36 eV higher than fresh Pt1@Au1/Al2O3catalyst, which all confirmed that after long stability test, the structure of Pt1@Au1/Al2O3catalyst was preserved.
As can be seen from the above, the activity of Pt1@Au1/Al2O3did well in dry gas. However, water, as a main product of toluene catalytic oxidation, always had a harmful influence on the catalytic activity. As shown in Fig. 10, the toluene conversion decreased by 13% with the addition of 5% (volume fraction)water vapor. When the water vapor was cut off, the toluene conversion rate gradually increased, which showed a strong competitive adsorption on the surface of the catalyst in the three components of water vapor, toluene and oxygen.

Fig. 10 Effect of water vapor on toluene conversion at reaction temperatures over Pt1@Au1/Al2O3 catalyst under the conditions of toluene concentration 1000 × 10-6, water vapor concentration 5%(volume fraction), and SV = 18000 mL·g-1·h-1.
4 Conclusions
In summary, for the first time, we developed a general, facile and eco-friendly method for core-shell Pt@Au nanomaterials.By using the Pt nanocrystals (Pt NPs)as metal cores, a monolayer-like Au skin surface could be formed on the surface of Pt NPs under the Pt surface catalysis. In the successive hydrogen reduction process, propylene carbonate (PC)was used as a “green” solvent, dispersant and stabilizer, which could effectively control the particle size and crystal structure of the core-shell nanoparticles. According to Mapping and the Fourier transform of HRTEM, the nanoparticles prepared by this method did have the characteristics of core-shell structure. The catalytic efficiency of toluene combustion over Pt@Aux/Al2O3, Pt/Al2O3and Au/Al2O3had been extensively investigated. The catalytic activity for toluene was given in the order of Pt1@Au1/Al2O3>Pt1@Au3/Al2O3> Pt1@Au4/Al2O3> Pt1@Au2/Al2O3>Pt/Al2O3> Au/Al2O3. Under the adopted conditions, the Pt1@Au1/Al2O3catalyst exhibited high activity and stability for toluene oxidation. According to the result of XPS and H2-TPR,Au0and Pt0were the main active species on the catalyst surface and the binding energy of Pt1@Au1/Al2O3was the lowest, which revealed the high catalytic activity for toluene oxidation. These evidences suggested that the active sites might be formed on the core-shell Pt@Au catalyst by particle size and metal synergy.
