Evidence for using dextromethorphan-quinidine for the treatment of agitation in dementia
2020-07-11
Rajesh R Tampi,Department of Psychiatry and Behavioral Sciences,Cleveland Clinic Akron General,Akron,OH 44106,United States
Rajesh R Tampi,Cleveland Clinic Lerner College of Medicine of Case Western Reserve University,Cleveland,OH 44195,United States
Pallavi Joshi,Department of Psychiatry and Behavioral Sciences,Northwell Health-Staten Island University Hospital,Staten Island,NY 10306,United States
Padmapriya Marpuri,Department of Psychiatry,Einstein Medical Center,Philadelphia,PA 19141,United States
Abstract
Key words:Dextromethorphan;Quinidine;Agitation;Dementia;Behavioral and psychological symptoms of dementia
INTRODUCTION
Behavioral and psychological symptoms of dementia (BPSD) are a group of psychological reactions,psychiatric symptoms and behaviors including agitation and aggression that are unsafe,disruptive,and impair the care of the individual in a given environment[1].BPSD is seen in one-third of individuals with dementia who live in the community and in up to 80% of individuals with dementia who live in skilled nursing facilities[2].The prevalence of agitation in dementia can be up to 46%,and is associated with decreased quality of life and increased risk of institutionalization for these individuals,in addition to greater caregiver burden and higher social and economic burden of caring for these individuals[3,4].
Non-pharmacological interventions are recommended as first-line for management of agitation in dementia.Pharmacotherapy including antipsychotics,antidepressants,anticonvulsants and cognitive enhancers are used when non-pharmacological interventions are ineffective[5].Unfortunately,these medications are burdened with a significant side effect profile including QTc interval prolongation,weight gain,anticholinergic effects and cardiovascular adverse effects that carry substantial risks for the geriatric population[5].Therefore,there is a great need for identifying efficacious and safe pharmacological treatments that are suitable for managing agitation in dementia.
Dextromethorphan is a low-affinity,uncompetitive N-methyl-D-aspartate receptor antagonist,σ1 receptor agonist,serotonin and norepinephrine reuptake inhibitor,and neuronal nicotinic α3β4 receptor antagonist[6].Dextromethorphan has low and variable bioavailability when administered alone because of its rapid first-pass metabolism and subsequent elimination.The addition of quinidine,a potent inhibitor of the cytochrome P450 (CYP) liver enzyme CYP2D6,inhibits dextromethorphan metabolism and yields greater bioavailability[6].The combination of dextromethorphan and quinidine sulfate is approved for the treatment of pseudobulbar affect in the United States and European Union.Evidence suggesting a potential effect of dextromethorphan-quinidine for agitation in dementia comes from controlled clinical trial data in non-demented patients with pseudobulbar affect[7].
The aim of this editorial is to review the literature on published randomized control trials (RCTs) that evaluated the efficacy and tolerability of dextromethorphanquinidine for the management of agitation in dementia.
EVIDENCE FOR USIGN DEXTROMETHORPHAN-QUINIDINE FOR AGITATION IN DEMENTIA
A review of literature found only 1 RCT that evaluated the use of dextromethorphanquinidine for the management of agitation in dementia (Table 1)[8].The study was assessed as being of good quality based on JADAD criteria (Table 2).The details of the study are described in Table 3.
DISCUSSION
Available data from RCTs on the use of dextromethorphan-quinidine for themanagement of BPSD is extremely limited.The only trial that we found in our literature review evaluated the efficacy of dextromethorphan-quinidine in reducing severity of agitation among individuals with Alzheimer's disease when compared to placebo.This is the first dementia-related trial to use a sequential parallel comparison design[8].In studies using this design,the first stage randomizes more patients to placebo than to active treatment.In the second stage,placebo non-responders from stage 1 are rerandomized and are included in the primary analysis.Pooled analysis of both stages maximizes the power to detect treatment differences and reduces the required sample size[8].

Table1 Summary of included studies
In this trial,treatment with dextromethorphan-quinidine demonstrated statistically significant decrease in agitation and aggression when compared to placebo.The reduction in agitation was considered clinically significant as measured by clinician rated scales.While the Alzheimer disease-related agitation characteristics of patients in this study were generally consistent with the International Psychogeriatric Association definition of agitation[8],patient emotional distress in patients was not directly measured.
Dextromethorphan-quinidine was generally well tolerated in this elderly population and was not associated with cognitive impairment.Most adverse events,including dizziness and diarrhea,were consistent with those observed in dextromethorphan-quinidine trials for pseudobulbar affect[8].Falls were more common among patients receiving dextromethorphan-quinidine when compared to placebo.This may be explained by greater duration of exposure to dextromethorphan-quinidine and the lack of randomization to groups based on fall risk.
The strengths of the study include the use of the sequential parallel comparison design;inclusion of stable concomitant medications,including psychotropic medications,high retention rate,blinding of study sites to all aspects of the study,use of prespecified sensitivity analyses to corroborate the primary efficacy end point,and consistent results among multiple secondary outcomes and primary end point.Limitations of this trial include a short duration (10 wk),and a dose-escalation schedule that limited evaluation of dose-response relationships.Exclusion of concomitant drugs related to quinidine,tricyclic antidepressants,monoamine oxidase inhibitors,or phenothiazines,as well as cardiac parameters limit the generalizability of study findings.Finally,the patient population was predominantly outpatient,with only 5.5% of study participants domiciled in nursing homes.The treatment response may not be generalizable to patients in nursing homes and should be further explored.
In the study reviewed,the combination of dextromethorphan-quinidine was clinically efficacious for agitation and was generally well tolerated.However,evidence-based trials are limited,as is the generalizability of the results to wider clinical and nursing home populations.This highlights the need for further research on both the efficacy and safety of dextromethorphan-quinidine and other pharmacological interventions for agitation in dementia.
CONCLUSION
This review indicates that there is a scarcity of evidence for the use of dextromethorphan-quinidine for the management of agitation in dementia.There is only one available trial,which demonstrated a decrease in agitation and aggression compared with placebo.However,the trial had a limited number of participants and low representation of patients in nursing home,which further restricts the generalizability of the results.The need to further investigate the effectiveness of different pharmacotherapeutic modalities for the management of agitation in dementia is,therefore,essential.
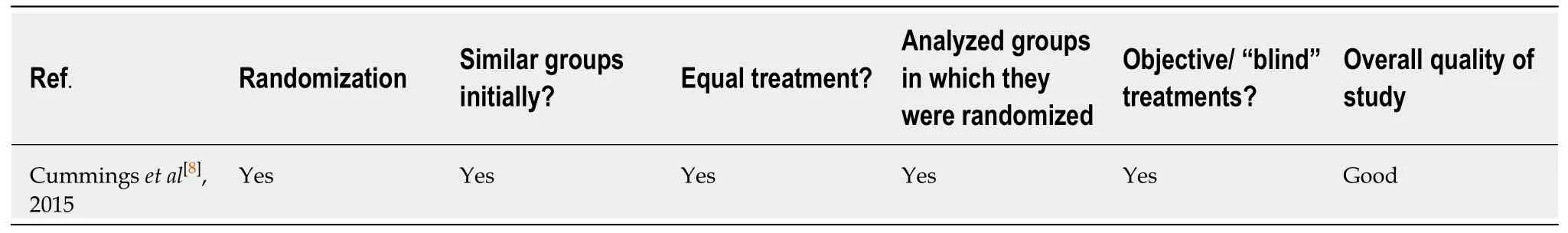
Table2 Quality of included studies
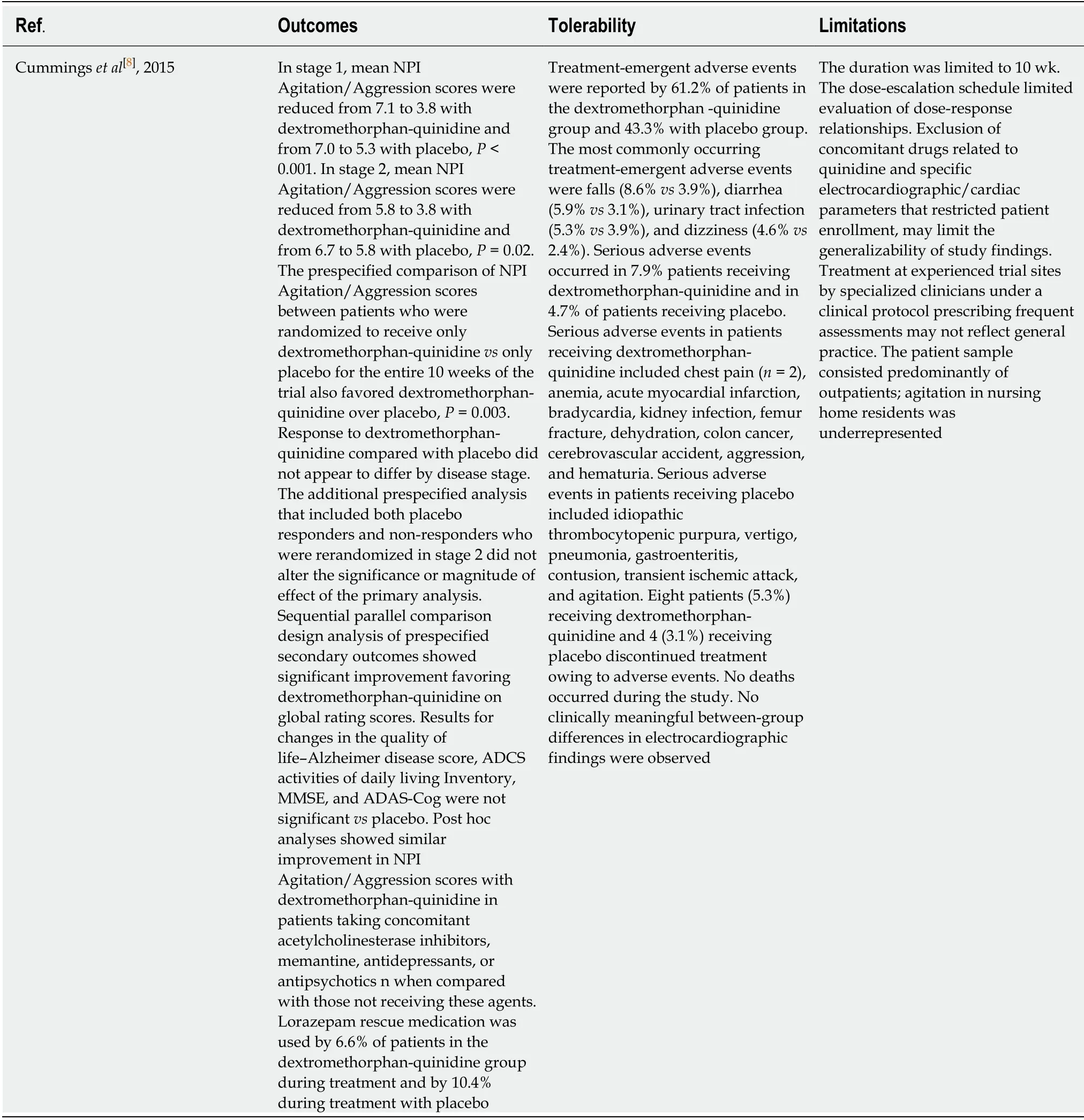
Table3 Results summary from included studies
杂志排行
World Journal of Psychiatry的其它文章
- Moderators and mediators of antipsychotic response in delusional disorder:Further steps are needed
- Comparison of novel tools with traditional cognitive tests in detecting delirium in elderly medical patients
- Risk factors for depression in patients with chronic obstructive pulmonary disease
- Diagnostic accuracy and clinical utility of non-English versions of Edinburgh Post-Natal Depression Scale for screening post-natal depression in lndia:A meta-analysis
