脑梗死占比对大面积脑梗死患者脑疝形成的预判价值
2020-07-04杜小燕刘庆军赵立波龚洪敏吴林魏静谭庆赵瑞
杜小燕 刘庆军 赵立波 龚洪敏 吴林 魏静 谭庆 赵瑞


[摘要] 目的 探讨脑梗死占比对大面积脑梗死患者脑疝形成的预判价值。 方法 回顾性分析2017年1月~2019年1月于重庆医科大学附属永川医院住院治疗的71例大面积脑梗死患者的临床资料。根据脑疝形成与否分为脑疝组(17例)和非脑疝组(54例)。对临床资料与影像学资料进行单因素和二元逻辑回归分析,采用ROC曲线评价诊断效果,寻找最佳预测值。 结果 两组临床资料比较,差异无统计学意义(P > 0.05)。脑疝组的脑梗死体积、脑梗死占比、缓冲体积均大于非脑疝组,差异有统计学意义(P < 0.05);两组的脑组织体积、颅腔体积比较,差异无统计学意义(P > 0.05)。脑梗死占比为大面积脑梗死患者脑疝形成的独立危险因素(OR = 7.749,95%CI: 1.539~38.284,P < 0.05)。脑梗死占比、脑梗死体积曲线下面积分别为0.818、0.808,当脑梗死占比为21%时为最佳拐点,敏感度为77%,特异度为76%。 結论 脑梗死占比为大面积脑梗死患者脑疝形成的独立危险因素,对判断大面积脑梗死患者脑疝形成有一定预测价值。
[关键词] 大面积脑梗死;脑梗死占比;脑疝;预判价值
[中图分类号] R743 [文献标识码] A [文章编号] 1673-7210(2020)05(b)-0023-04
Predictive value of the ratio of cerebral infarction on cerebral hernia formation in patients with large area cerebral infarction
DU Xiaoyan1,2 LIU Qingjun1,2 ZHAO Libo1,2,3 GONG Hongmin1,2 WU Lin1,2 WEI Jing1,2 TAN Qing1,2 ZHAO Rui1,2
1.Department of Neurology, Yongchuan Hospital Affiliated to Chongqing Medical University, Chongqing 402160, China; 2.Chongqing Key Laboratory of Cerebrovascular Disease Research, Chongqing 402160, China; 3.Department of Neurology, the Third People′s Hospital of Chongqing City, Chongqing 400010, China
[Abstract] Objective To assess predictive value of the ratio of cerebral infarction on cerebral hernia formation in patients with large area cerebral infarction. Methods The clinical data of 71 patients with massive cerebral infarction hospitalized in Yongchuan Hospital Affiliated to Chongqing Medical University from January 2017 to January 2019 were retrospectively analyzed. They were divided into brain hernia group (17 cases) and non brain hernia group (54 cases) according to the formation of brain hernia. Single factor and binary logistic regression analysis were carried out for clinical data and imaging data. ROC curve was used to evaluate the diagnosis effect and find the best predictive value. Results Clinical data between two groups was not statistically significant (P > 0.05). The volume of cerebral infarction, the proportion of cerebral infarction and the buffer volume of cerebral hernia group were larger than those of non cerebral hernia group, the differences were statistically significant (P < 0.05). There were no significant differences in volume of brain tissue and cranial cavity between two groups (P > 0.05). The proportion of cerebral infarction was an independent risk factor for the formation of cerebral hernia in patients with massive cerebral infarction (OR = 7.749, 95%CI: 1.539-38.284, P < 0.05). The proportion of cerebral infarction and the area under the volume curve of cerebral infarction was 0.818 and 0.808 respectively. When the proportion of cerebral infarction was 21%, it was the best turning point, the sensitivity was 77%, and the specificity was 76%. Conclusion The cerebral infarction proportion is an independent risk factor for the formation of cerebral hernia in patients with massive cerebral infarction, which provides a certain predictive value for judging the formation of cerebral hernia.
[Key words] Massive cerebral infarction; Proportion of cerebral infarction; Formation of cerebral hernia; Predictive value
脑卒中为最常见的成人致残性脑血管病[1-2]。大面积脑梗死(MCI)为急危重症。脑组织恶性水肿可致脑疝形成,危及生命,探究脑疝形成因素意义重大。有研究报道,恶性水肿主要与梗死体积和/或脑萎缩程度有关,脑萎缩程度越重,缓冲空间越大,越不易导致脑疝[3-5]。有研究认为,梗死体积相同而脑萎缩程度不同,脑疝形成风险不同。脑组织体积与脑萎缩呈年龄相关,可代表脑萎缩程度[6]。近期有研究将脑梗死体积/脑组织体积定义为脑梗死占比,该值可消除脑萎缩的影响[7]。采用脑梗死占比探究MCI患者脑疝形成的影响因素可能更合理,但目前鲜有类似报道。本研究拟探讨脑梗死占比对MCI患者脑疝形成的预判价值。
1 资料与方法
1.1 一般资料
回顾性分析2017年1月~2019年1月于重庆医科大学附属永川医院神经内科住院的MCI患者71例的临床资料。MCI诊断标准参考2017年《大脑半球大面积梗死监护与治疗中国专家共识》[8]。脑疝形成标准:MCI伴瞳孔不等大、意识障碍加深。排除标准:出血性梗死、幕下梗死。观察时间:入院后1周。根据是否形成脑疝将患者分为脑疝组(17例)和非脑疝组(54例)。回顾性收集MCI患者临床及影像学资料。前者包括性别、年龄、高血压病、房颤、冠心病、糖尿病、吸烟、饮酒、美国国立卫生研究院卒中量表(NIHSS)评分、入院体温、最高体温、D2聚体、溶栓人数。后者为入院时和1周内复查的头颅CT图像。
1.2 方法
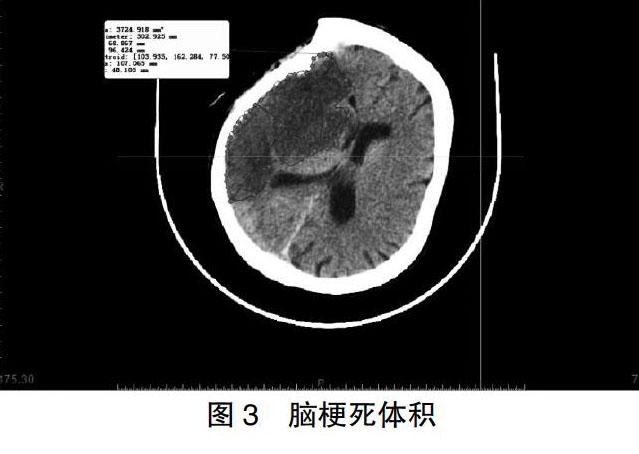
采用Mimics软件计算颅腔体积和脑组织体积(图1~2)。梗死体积=1/2×病灶层面梗死灶长径×短径×高[9](图3)。
1.3 统计学方法
采用SPSS 19.0软件进行分析。计数资料比较采用χ2检验。正态分布计量资料以均数±标准差(x±s)表示,采用t检验。采用二元逻辑回归分析脑疝形成危险因素,ROC曲线评价诊断效果,约登指数确定最佳预测值。以P < 0.05为差异有统计学意义。
2 结果
2.1 两组患者临床资料比较
两组临床资料比较,差异无统计学意义(P > 0.05)。见表1。
2.2 两组患者影像学资料比较
脑疝组的脑梗死体积、脑梗死占比、缓冲体积均大于非脑疝组,差异有统计学意义(P < 0.05);两组的脑组织体积、颅腔体积比较,差异无统计学意义(P > 0.05)。见表2。
2.3 MCI患者脑疝形成二元逻辑回归分析
以是否脑疝形成为因变量行二元逻辑回归分析,并将脑疝形成赋值为1,将单因素比较差异有统计学意义的指标(脑梗死体積、脑梗死占比、缓冲体积)作为自变量进行二元逻辑回归分析,结果显示,脑梗死占比为MCI患者脑疝形成的独立危险因素(OR = 7.749,95%CI:1.539~38.284,P < 0.05)。见表3。
2.4 脑梗死占比、脑梗死体积预测MCI脑疝形成ROC曲线
脑梗死占比、脑梗死体积曲线下面积分别为0.818、0.808,当脑梗死占比为21%时为最佳拐点,敏感度为77%,特异度为76%。见图4。
3 讨论
MCI为颈内动脉远端或大脑中动脉主干闭塞引起脑组织缺血坏死,病死率高[10-12],存活患者遗留不同程度神经功能缺损症状[8]。大脑中动脉急性闭塞所致脑卒中患者中1%~10%可被归类为“恶性缺血性脑卒中”。恶性缺血性脑卒中指缺血脑组织水肿引起颅内压显著升高,超过自身的代偿,可导致脑疝,危及生命[13]。脑疝形成加重颅内血管受压及脑组织机械损伤,增大死亡风险。目前MCI患者脑疝形成危险因素仍不明确。
有研究提出,MCI患者脑疝形成与脑梗死体积和脑萎缩程度相关[3]。因脑梗死缺血性水肿的占位效应不仅取决于病变大小,还取决于可代偿的颅内容积储备[14],主要由脑萎缩程度决定[4]。对于MCI而言,脑组织体积越小,即脑萎缩程度越重,其代偿空间越大,即缓冲体积越多,越不易形成脑疝[4,15]。也有研究报道,脑萎缩与年龄相关[16],随年龄增长,侧脑室和皮质沟扩大,脑室或颅内脑脊液体积增加,脑组织体积减少。有学者认为,脑组织体积一定程度上可代表脑萎缩程度[6]。有理由认为,梗死体积越大,水肿越重,脑疝风险越高;不同个体不同年龄脑萎缩程度不一,同样梗死体积、缓冲空间不一,脑疝风险不同。脑梗死占比可消除脑萎缩因素,用脑梗死占比探讨MCI脑疝形成危险因素可能更为合理。
Goto等[7]提出,采用弥散加权成像(DWI)测得脑梗死体积、脑组织体积,用脑梗死占比来预测MCI患者恶性水肿形成的可能,其最佳预测值为7.8%,敏感度、特异度分别为86%、87%。目前认为,DWI是测量脑梗死核心体积最精确的技术[17]。然而,磁共振较CT检查耗时长,风险大,尤其是对血氧饱和度低和/或有气管插管危重患者,DWI检查可行性受到限制。而CT耗时短,风险小,简单易行。
在本回顾性研究中,采用Mimics软件定量测量CT图像脑梗死体积、脑组织体积,得出脑梗死占比,将临床资料、影像学资料进行单因素和二元逻辑回归分析,结果提示,仅脑梗死占比为MCI患者脑疝形成的独立危险因素,与脑疝形成密切相关。根据ROC曲线分析结果,当脑梗死占比为21%时是脑疝形成最有力的预测指标,敏感度、特异度分别为77%、76%。即当MCI患者脑梗死占比接近21%时,意味着脑疝形成概率较大,需要更加积极降颅压治疗,甚至外科手术减压。有研究提出,对于MCI可能导致脑疝形成的患者,尽早采取手术干预可以有效降低颅内压,并降低一定的死亡率和改善预后[18-20]。故对于MCI患者,需密切随访CT,若有脑疝形成趋势,可在脑梗死占比上升未达到21%之前尽早采用各种方法积极降颅压治疗。
本研究得出脑梗死占比阈值为21%,而Goto等[7]研究得出阈值为7.8%,相差较大,其原因可能是:①本研究采用CT,Goto等[7]采用DWI;CT平扫所显示低密度区域为核心梗死区和水肿区之和,而DWI显示高信号区域为核心梗死区。②本研究CT图像采集时间在1周内,而Goto等[7]报道的DWI图像采集时间在48 h内,本研究图像采集时间较长,水肿更严重,故脑梗死占比更大。本研究阈值敏感度、特异度尚不足够高,本研究组推测在脑疝形成前更早期采集CT图像,得到脑梗死占比来预测脑疝形成,可能提高敏感度和特异度,才能更好地指导临床决策。
综上,脑梗死占比可在一定程度上预测MCI患者脑疝形成,当CT检查提示脑梗死占比接近21%时,脑疝形成可能性较大,需拟定进一步的治疗方案。但本研究为单中心回顾性研究,尚需随机、多中心、大样本量、前瞻性研究进一步验证。
[参考文献]
[1] Naghavi M,Abajobir AA,Abbafati C,et al. Global,regional,and national age-sex specific mortality for 264 causes of death,1980-2016:a systematic analysis for the Global Burden of Disease Study 2016 [J]. Lancet,2017,390(10100):1151-1210.
[2] Mozaffarian D,Benjamin EJ,Go AS,et al. Executive summary:heart disease and stroke statistics—2016 update:a report from the American Heart Association [J]. Circulation,2016,127(1):143-152.
[3] Thomalla G,Hartmann F,Juettler E,et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset:a prospective multicenter observational study [J]. Ann Neurol,2010,68(4):435-445.
[4] Minnerup J,Wersching H,Ringelstein EB,et al. Prediction of malignant middle cerebral artery infarction using computed tomography-based intracranial volume reserve measurements [J]. Stroke,2011,42(12):3403-3409.
[5] Oppenheim C,Samson Y,Manai R,et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging [J]. Stroke,2000,31(9):2175-2181.
[6] 車旭东,何朝晖.蛛网膜下腔出血后脑萎缩与认知功能障碍相关性研究进展[J].重庆医科大学学报,2016,41(8):869-872.
[7] Goto Y,Kumura E,Watabe T,et al. Prediction of malignant middle cerebral artery infarction in elderly patients [J]. J Stroke Cerebrovasc Dis,2016,25(6):1389-1395.
[8] 中华医学会神经病学分会神经重症协作组,中国医师协会神经内科医师分会神经重症专委会.大脑半球大面积梗死监护与治疗中国专家共识[J].中华医学杂志,2017, 97(9):645-652.
[9] Sims JR,Gharai LR,Schaefer PW,et al. ABC/2 for rapid clinical estimate of infarct,perfusion,and mismatch volumes [J]. Neurology,2009,72(24):2104-2110.
[10] Huttner HB,Schwab S. Malignant middle cerebral artery infarction:clinical characteristics,treatment strategies,and future perspectives [J]. Lancet Neurol,2009,8(10):949-958.
[11] Heiss WD. Malignant MCA infarction:pathophysiology and imaging for early diagnosis and management decisions [J]. Cerebrovasc Dis,2016,41(1/2):1-7.
[12] Su Y,Fan L,Zhang Y,et al. Improved neurological outcome with mild hypothermia in surviving patients with massive cerebral hemispheric infarction [J]. Stroke,2016, 47(2):457.
[13] Stokum JA,Gerzanich V,Simard JM. Molecular pathophysiology of cerebral edema [J]. J Cerebr Blood F Met,2016,36(3):513-538.
[14] Kauw F,Bennink E,de Jong HWAM,et al. Intracranial cerebrospinal fluid volume as a predictor of malignant middle cerebral artery infarction [J]. Stroke,2019.[Epub ahead of print].
[15] Beck C,Kruetzelmann A,Forkert ND,et al. A simple brain atrophy measure improves the prediction of malignant middle cerebral artery infarction by acute DWI lesion volume [J]. J Neurol,2014,261(6):1097-1103.
[16] Bektas H,Wu TC,Kasam M,et al. Increased blood-brain barrier permeability on perfusion CT might predict malignant middle cerebral artery infarction [J]. Stroke,2010,41(11):2539-2544.
[17] Copen WA,Yoo AJ,Rost NS,et al. In patients with suspected acute stroke,CT perfusion-based cerebral blood flow maps cannot substitute for DWI in measuring the ischemic core [J]. PLoS One,2017,12(11):e0188891.
[18] Bongiorni GT,Hockmuller MCJ,Klein C,et al. Decompressive craniotomy for the treatment of malignant infarction of the middle cerebral artery:mortality and outcome [J]. Arq Neuropsiquiatr,2017,75(7):424-428.
[19] Shah A,Almenawer S,Hawryluk G. Timing of decompressive craniectomy for ischemic stroke and traumatic brain injury:a review [J]. Front Neurol,2019,10:11.
[20] Dasenbrock HH,Robertson FC,Vaitkevicius H,et al. Timing of decompressive hemicraniectomy for stroke:a nationwide inpatient sample analysis [J]. Stroke,2017,48(3):704-711.
(收稿日期:2019-10-09 本文編辑:李亚聪)
