小檗碱对氧糖剥夺/复氧诱导的大鼠体外血脑屏障损伤的保护作用
2020-04-20王美华王甲韩丹
王美华 王甲 韩丹
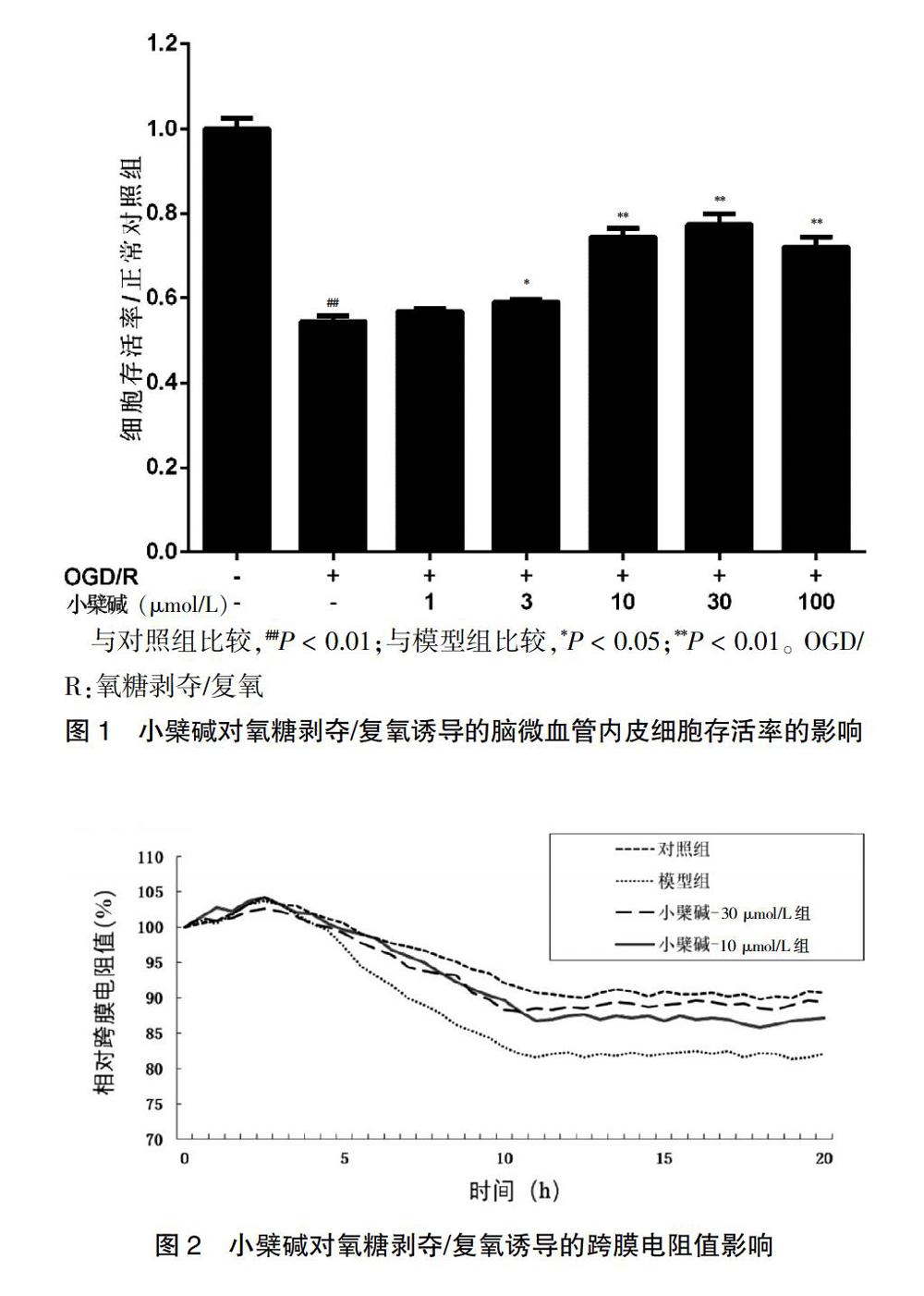
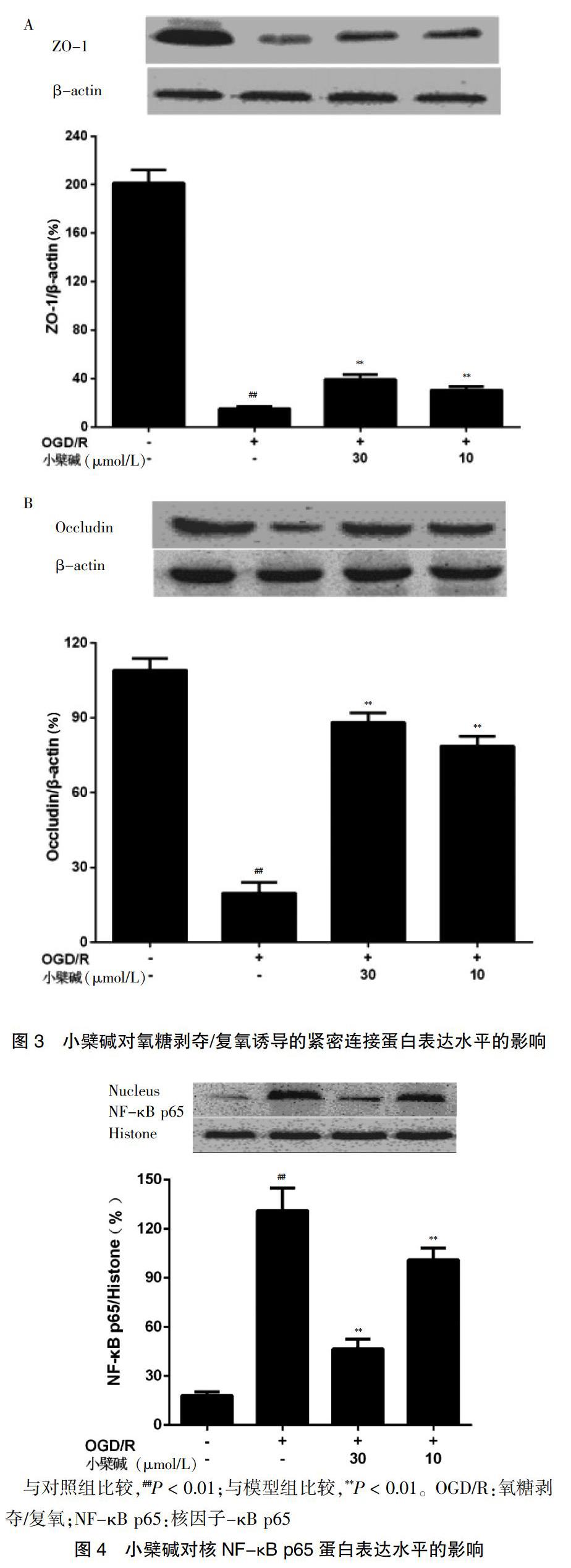
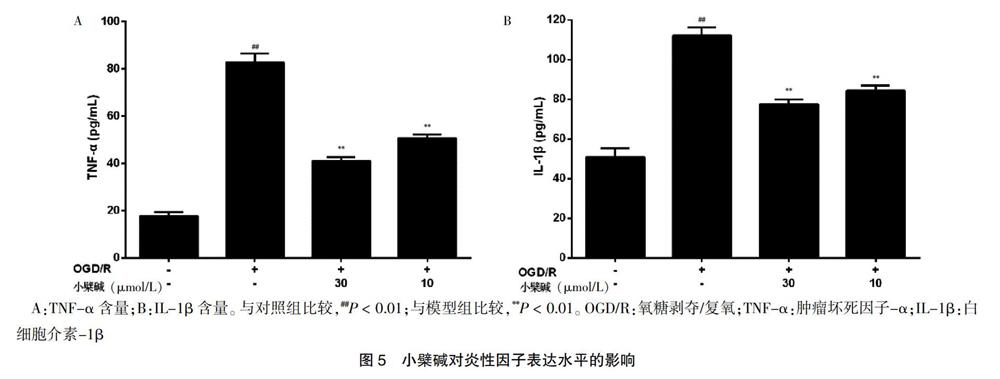
[摘要] 目的 探究小檗堿对氧糖剥夺/复氧(OGD/R)诱导大鼠体外血脑屏障损伤的保护作用及其机制。 方法 将原代培养的大鼠脑微血管内皮细胞(rBMECs)分为对照组、模型组、小檗碱给药组。对照组加入新鲜培养基;模型组和小檗碱给药组给予不同浓度(0、1、3、10、30、100 μmol/L)的小檗碱预孵24 h后更换低糖培养基并将其放在不含氧气的三气培养箱中(95%N2/5%CO2)培养2 h(氧糖剥夺,OGD),之后换成新鲜培养基,置于37℃、5%CO2培养箱内继续培养2 h(复氧,R)。利用MTT法检测细胞存活率,跨膜电阻法测定细胞紧密连接程度,Western blot法检测紧密连接蛋白ZO-1、occludin以及核因子-κB p65(NF-κB p65)的蛋白表达量,ELISA法检测炎性因子肿瘤坏死因子-α(TNF-α)、白细胞介素-1β(IL-1β)含量。 结果 与对照组比较,模型组rBMECs细胞存活率、ZO-1表达量、occludin表达量显著降低,跨膜电阻值呈下降趋势,NF-κB p65表达量及IL-1β、TNF-α含量显著升高,差异均有高度统计学意义(均P < 0.01);与模型组比较,小檗碱给药组rBMECs细胞存活率不同程度提高,小檗碱10、30 μmol/L浓度组ZO-1表达量、occludin表达量显著升高,跨膜电阻值显著提高,差异有统计学意义(P < 0.05或P < 0.01),同时NF-κB p65表达量及IL-1β、TNF-α含量显著降低,差异均有高度统计学意义(均P < 0.01)。结论 小檗碱通过抑制NF-κB入核、降低炎性因子释放量、改善OGD/R诱导的rBMECs的损伤,发挥对OGD/R损伤的血脑屏障保护作用。
[关键词] 小檗碱;血脑屏障;核因子-κB p65;炎症
[中图分类号] R285.5 [文献标识码] A [文章编号] 1673-7210(2020)03(c)-0016-05
[Abstract] Objective To investigate the protective effect of berberine on oxygen glucose deprivation/reoxygenation (OGD/R) induced blood-brain barrier injury in vitro in rats and its mechanism. Methods Primary cultured rat brain microvascular endothelial cells (rBMECs) were divided into the control group, the model group and the berberine administration group. Fresh medium was added to the control group. The model group and the berberine administration group were preincubated with berberine at different concentrations (0, 1, 3, 10, 30, 100 μmol/L) for 24 h, then the low glucose medium was replaced and the culture medium was placed in a three-gas incubator without oxygen (95%N2/5%CO2) for 2 h (oxygen glucose deprivation, OGD). then replaced with fresh medium, at 37℃ and 5%CO2 incubator continue to develop 2 h (Reoxygenation, R). Cell survival was determined by MTT assay, cell tightness was determined by transendothelial electrical resistance, protein expression levels of tight junction protein ZO-1, occludin and nuclear factor-κB p65 (NF-κB p65) were detected by Western blot assay, and levels of inflammatory factors tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) were detected by ELISA assay. Results Compared with the the control group, rBMECs cell survival rate, ZO-1 expression level and occludin expression level in the model group were significantly reduced, transendothelial electrical resistance showed a decreasing trend, and NF-κB p65 expression level, IL-1β, and TNF-α levels were significantly increased in the model group, the differences were highly statistically significant (all P < 0.01). Compared with the model group, the survival rate of rBMECs cells in the berberine administration group increased to different degrees, the ZO-1 expression, occludin expression, the transmembrane electrical resistance increased significantly in the berberine 10 and 30 μmol/L concentration groups, with statistically significant differences (P < 0.05 or P < 0.01). Meanwhile, the NF-κB p65 expression and the levels of IL-1β and TNF-α were significantly decreased in the berberine 10 and 30 μmol/L concentration groups, the differences were highly statistically significant (all P < 0.01). Conclusion Berberine plays a protective role of blood brain barrier on OGD/R injury by inhibiting NF-κB entry into the nucleus, reducing the release of inflammatory factors, and improving the damage of OGD/R-induced rBMECs.
[Key words] Berberine; Blood-brain barrier; Nuclear factor-κB p65; Inflammation
脑卒中是全球主要致死疾病之一,其中80%~85%的患者是缺血性脑卒中[1-2]。迄今为止,建立早期再灌注是唯一被认可的有效治疗缺血性脑卒中的方法。然而缺血再灌注会导致血脑屏障(blood-brain barrier,BBB)损伤[3],引发继发性的脑损伤,包括脑水肿、神经功能障碍,甚至脑梗死[4-5]。因此,改善BBB损伤有望成为防治缺血性脑卒中的治疗方法。
小檗碱是一种中药黄连衍生的异喹啉生物碱,具有广泛的药理作用[6-8],然而,小檗碱对脑缺血再灌注损伤后BBB的保护作用及其机制尚不清楚。本研究拟探究小檗碱对氧糖剥夺/复氧(OGD/R)损伤的大鼠脑微血管内皮细胞的保护作用及其可能的机制,为临床防治缺血性脑卒中提供新思路。
1 材料与方法
1.1 材料
occludin、ZO-1、核因子-κB(NF-κB)抗体IgG购自Santa Cruz公司(批号:K-2712、B-2513、J-1612);肿瘤坏死因子-α(TNF-α)、白细胞介素-1β(IL-1β)ELISA试剂盒购自南京建成生物工程研究所(批号:E-20219、E-20212)。
1.2 方法
1.2.1 大鼠脑微血管内皮细胞的原代培养方法 原代培养大鼠脑微血管内皮细胞(rat brain microvessel endothelial cells,rBMECs),方法参照文献[9]。接种后静置培养,待细胞长成致密单层,细胞呈长梭型。
1.2.2 MTT法检测小檗碱对OGD/R诱导的rBMECs损伤细胞存活率影响 将rBMECs分为对照组、模型组、小檗碱给药组(1、3、10、30、100 μmol/L)。对照组加入新鲜DMEM-F12培养基;模型组和小檗碱给药组给予不同浓度(0、1、3、10、30、100 μmol/L)的小檗碱预孵24 h后换为DMEM低糖培养基,并把培养板放在不含有氧气的三气培养箱中(95%N2/5%CO2)培养2 h(氧糖剥夺,OGD),之后换成DMEM-F12培养基,置37℃、5%CO2培养箱内继续培养2 h(复氧,R)。除去培养板内的培养基,加入无血清培养基和MTT溶液(5 mg/mL),孵育4 h。弃掉含MTT的培养基之后,加入DMSO液,在酶标仪570 nm处测定各孔的OD值,以实验组OD值/对照组OD值反映细胞的存活情况。
1.2.3 跨膜电阻法检测小檗碱对OGD/R诱导的损伤后rBMECs紧密连接程度的影响 在Transwell小室内接种细胞测定跨膜电阻值(TEER),TEER趋于稳定说明rBMECs在Transwell孔底形成单层屏障。在TEER不再增长之后按照上述预孵给药及造模条件处理细胞,以测试点TEER/造模起始点TEER表示细胞紧密连接程度。
1.2.4 Western blot法检测小檗碱对核NF-κB p65、ZO-1、occludin表达水平的影响 经上述预孵给药及造模处理后,收集细胞并裂解,12000 g、4℃离心15 min,收集上清液。BCA法测定蛋白浓度后,制备12%分离胶、5%浓缩胶进行SDS-PAGE电泳,转膜。BSA封闭后,抗体孵育。接下来ECL显色,Image J软件进行定量分析。
1.2.5 ELISA法测定IL-1β、TNF-α的释放含量 经上述预孵给药和造模处理后,吸取培养基,3000 g离心10 min,取上清液,按照说明书操作,测定IL-1β、TNF-α含量。
1.3 统计学方法
采用SPSS 20.0对所得数据进行统计学分析,計量资料采用均数±标准差(x±s)表示,多组间比较采用方差分析,组间两两比较用Bonferroni检验。以P < 0.05为差异有统计学意义。
2 结果
2.1 小檗碱对大鼠脑微血管内皮细胞存活率影响
与对照组比较,模型组细胞存活率显著降低,差异有高度统计学意义(P < 0.01)。与模型组比较,小檗碱给药组细胞存活率不同程度提高,其中小檗碱3、10、30、100 μmol/L浓度组细胞存活率显著提高,差异有统计学意义(P < 0.05或P < 0.01)。见图1。
2.2小檗碱对大鼠脑微血管内皮细胞紧密连接程度的影响
与对照组比较,造模5 h后,模型组TEER有所降低;与模型组比较,小檗碱10、30 μmol/L浓度组TEER不同程度的提高。在造模10 h后,小檗碱10、30 μmol/L浓度组提高TEER的趋势呈现稳定状态。见图2。
2.3 小檗碱对紧密连接蛋白ZO-1、occludin蛋白表达水平的影响
与对照组比较,模型组ZO-1蛋白表达水平显著降低,差异有高度统计学意义(P < 0.01)。与模型组比较,小檗碱10、30 μmol/L浓度组ZO-1蛋白水平显著提高,差异有高度统计学意义(P < 0.01)。见图3A。与对照组比较,模型组occludin蛋白表达水平显著降低,差异有高度统计学意义(P < 0.01)。与模型组比较,小檗碱10、30 μmol/L浓度组occludin蛋白水平显著提高,差异有高度统计学意义(P < 0.01)。见图3B。
2.4 小檗碱对 NF-κB p65入核水平的影响
与对照组比较,模型组的NF-κB p65表达水平显著升高,差异有高度统计学意义(P < 0.01)。与模型组比较,小檗碱10、30 μmol/L浓度组NF-κB p65入核水平不同程度降低,差异有高度统计学意义(P < 0.01)。见图4。
2.5 小檗碱对脑微血管内皮细胞释放炎性因子TNF-α、IL-1β水平的影响
与对照组比较,模型组细胞上清液中TNF-α含量显著升高,差异有高度统计学意义(P < 0.01)。与模型组比较,小檗碱10、30 μmol/L浓度组细胞上清液中TNF-α含量显著降低,差异有高度统计学意义(P < 0.01)。见图5A。与对照组比较,模型组细胞上清液中IL-1β含量显著提高,差异有高度统计学意义(P < 0.01);与模型组比较,小檗碱10、30 μmol/L浓度组细胞上清液中IL-1β水平显著降低,差异有高度统计学意义(P < 0.01)。见图5B。
3 讨论
随着我国老龄化人口增加以及人们生活水平的提高,缺血性脑卒中患者的人数持续增长。BBB对脑组织内环境稳态的维持起着重要作用[10-11],脑微血管内皮细胞在此过程中尤为重要[12-13]。本研究采用OGD/R体外诱导rBMECs损伤模型模拟脑缺血/再灌注导致的BBB损伤[14],探究小檗碱对缺血性脑卒中/溶栓后BBB的保护作用。
本研究结果显示小檗碱给药组能提高OGD/R损伤的rBMECs细胞存活率,选取其中效果较好的小檗碱10 μmol/L以及30 μmol/L浓度组进行后续实验,实验数据进一步提示,与模型组比较,小檗碱10、30 μmol/L浓度组可提高TEER。脑微血管内皮细胞和其他内皮细胞的区别在于它能够特异性表达紧密连接蛋白[15]。本实验结果显示小檗碱显著性上调紧密连接蛋白ZO-1、occludin表达量,以上结果揭示小檗碱对OGD/R损伤的体外BBB具有保护作用。
缺血/再灌注损伤会导致炎性反应,NF-κB信號通路介导的炎性反应在脑损伤过程中起着至关重要的作用[16-17]。在静息细胞的胞质中,NF-κB p65位于胞浆中;当细胞受到外界刺激时可导致NF-κB p65转位至细胞核。NF-κB p65入核后促使炎性因子TNF-α、IL-1β等的转录,这一过程在脑缺血中起着破坏性的作用[18-20]。本研究结果提示小檗碱给药组显著性抑制NF-κB p65入核,并降低炎性因子TNF-α、IL-1β释放量。
综上所述,本研究采用OGD/R诱导的rBMECs损伤模型探究小檗碱对体外BBB的保护作用。实验结果显示,小檗碱通过抑制NF-κB入核、降低炎性因子释放量、改善OGD/R诱导的rBMECs的损伤,发挥对OGD/R损伤的BBB保护作用。因缺乏体内实验的验证,本研究结果具有一定局限性,但是本研究为小檗碱的进一步开发以及抗缺血性脑卒中药物的研发提供依据。
[参考文献]
[1] Vidale S,Consoli A,Arnaboldi M,et al. Postischemic Inflammation in Acute Stroke [J]. J Clin Neurol,2017,13(1):1-9.
[2] Li Y,Zhong W,Jiang Z,et al. New progress in the approaches for blood-brain barrier protection in acute ischemic stroke [J]. Brain Res Bull,2019,144:46-57.
[3] Ji B,Zhou F,Han L,et al. Sodium Tanshinone IIA Sulfonate Enhances Effectiveness Rt-PA Treatment in Acute Ischemic Stroke Patients Associated with Ameliorating Blood-Brain Barrier Damage [J]. Transl Stroke Res,2017, 8(4):334-340.
[4] Wu W,Zhong W,Lang B,et al. Thrombopoietin could protect cerebral tissue against ischemia-reperfusion injury by suppressing NF-kappaB and MMP-9 expression in rats [J]. Int J Med Sci,2018,15(12):1341-1348.
[5] Jiang X,Andjelkovic AV,Zhu L,et al. Blood-brain barrier dysfunction and recovery after ischemic stroke [J]. Prog Neurobiol,2018,163-164:144-171.
[6] Kumar A,Ekavali,Chopra K,et al. Current knowledge and pharmacological profile of berberine:An update [J]. Eur J Pharmacol,2015,761:288-297.
[7] Ahmed T,Gilani AU,Abdollahi M,et al. Berberine and neurodegeneration:A review of literature [J]. Pharmacol Rep,2015,67(5):970-979.
[8] Pang B,Zhao LH,Zhou Q,et al. Application of berberine on treating type 2 diabetes mellitus [J]. Int J Endocrinol,2015,2015:905749.
[9] Xue Y,He JT,Zhang KK,et al. Methamphetamine reduces expressions of tight junction proteins,rearranges F-actin cytoskeleton and increases the blood brain barrier permeability via the RhoA/ROCK-dependent pathway [J]. Biochem Biophys Res Commun,2019,509(2):395-401.
[10] Lv J,Hu W,Yang Z,et al. Focusing on claudin-5:A promising candidate in the regulation of BBB to treat ischemic stroke [J]. Prog Neurobiol,2018,161:79-96.
[11] Haley MJ,Lawrence CB. The blood-brain barrier after stroke:Structural studies and the role of transcytotic vesicles [J]. J Cereb Blood Flow Metab,2017,37(2):456-470.
[12] Abbott NJ,Patabendige AA,Dolman DE,et al. Structure and function of the blood-brain barrier [J]. Neurobiol Dis,2010,37(1):13-25.
[13] Sandoval KE,Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke [J]. Neurobiol Dis,2008, 32(2):200-219.
[14] Zhou YF,Li YN,Jin HJ,et al. Sema4D/PlexinB1 inhibition ameliorates blood-brain barrier damage and improves outcome after stroke in rats [J]. FASEB J,2018, 32(4):2181-2196.
[15] Abdullahi W,Tripathi D,Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke:targeting tight junctions and transporters for vascular protection [J]. Am J Physiol Cell Physiol,2018,315(3):C343-C356.
[16] Jin R,Liu L,Zhang S,et al. Role of inflammation and its mediators in acute ischemic stroke [J]. J Cardiovasc Transl Res,2013,6(5):834-851.
[17] Li X,Su L,Zhang X,et al. Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury[J]. Neurol Res,2017, 39(4):367-373.
[18] Simmons LJ,Surles-Zeigler MC,Li Y,et al. Regulation of inflammatory responses by neuregulin-1 in brain ischemia and microglial cells in vitro involves the NF-kappa B pathway [J]. J Neuroinflammation,2016,13(1):237.
[19] Gao S,Mo J,Chen L,et al. Astrocyte GGTI-mediated Rac1 prenylation upregulates NF-kappaB expression and promotes neuronal apoptosis following hypoxia/ischemia [J]. Neuropharmacology,2016,103:44-56.
[20] Chen AQ,Fang Z,Chen XL,et al. Microglia-derived TNF-alpha mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke [J]. Cell Death Dis,2019,10(7):487.
(收稿日期:2019-09-25 本文編辑:顾家毓)
