Tumor progression-dependent angiogenesis in gastric cancer and its potential application
2019-09-19HsiLungHsiehMingMingTsai
Hsi-Lung Hsieh,Ming-Ming Tsai
Abstract
Key words: Gastric cancer;Angiogenesis;Vascular endothelial cell;Angiogenic phenotype switch;Anti-angiogenesis;Tumor dormancy
INTRODUCTION
Gastric cancer (GC) has a high incidence throughout the world and a high mortality rate associated with malignant tumors[1-3].GC might not cause any clinical symptoms at the early stage,resulting in the fact that GC is rarely detected at the early stage[2,3].However,the five-year survival outcome for late-stage GC patients is only approximately 20%-30% after initial diagnosis[4],and gastrectomy is the major common treatment for GC.Thus,to improve the low survival outcome,it is necessary to develop novel therapeutic strategies for GC[5].
In recent decades,studies on the molecular mechanism of tumor development have focused on the genetic or epigenetic changes in tumor cells,such as the emergence of cancer stem cells (CSCs),epithelial-mesenchymal transition (EMT) and the expression of microRNAs (miRNAs)[6].However,several studies conducted in recent years found that the tumor microenvironment (TME) strongly influences tumor growth and progression and revealed that the tumor-host interactions determine tumor progression[7,8].The TME contains extracellular matrix and stromal cells,including ECs,tumor-associated fibroblasts and tumor-associated immune/inflammatory cells,which can regulate tumor progression through autocrine/paracrine cytokines or factors.Furthermore,cancer cells can support the angiogenesis of ECs,and ECs can also help cancer cell proliferation by releasing growth factors.Tumor-associated immune/inflammatory cells can control cancer cell proliferation and metastasis under different conditions,and cancer cells might induce immune cell dysfunction as well as proinflammatory cytokine release.Exosomal miRNAs can alter normal fibroblasts into TAFs for tumor survival,and TAFs can promote tumor proliferation and metastasis.Thus,the TME is also involved in multiple processes,including tumor angiogenesis,inflammation,immunosuppression and metastasis,as shown in Figure 1[9,10].
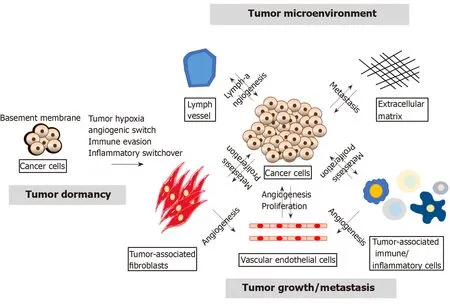
Figure1 The tumor microenvironment regulates tumor growth,relapse and metastasis.
In 1971,Dr.Folkman and Klagsbrun[11]provided a novel theory stating that all phases of rapid tumor growth are dependent on tumor angiogenesis.At present,it is known that tumor angiogenesis plays a key role in tumor progression,and the angiogenic switch is necessary for tumor growth,relapse and metastasis.Herein,we provide a review of tumor-associated angiogenesis,explore the molecular regulation of angiogenesis,and discuss various antiangiogenic drugs and their potential applications based on preclinical and phase III clinical trials for GC.
MOLECULAR REGULATION OF TUMOR ANGIOGENESIS IN GC
An increasing number of studies has revealed that tumor growth is strongly associated with tumor angiogenesis[12].Tumor growth,relapse and metastasis should turn on the “angiogenic switch” to induce tumor growth to a size greater than 1-2 mm.Numerous signals (e.g.,epigenetic changes,the TME,CSCs,EMT,and miRNAs)can disturb tumor dormancy,resulting in local tumor proliferation/recurrence or metastasis at a secondary site[13].The “angiogenic switch” is regulated by angiogenic activators and inhibitors[14,15],and the timing of the “angiogenic switch” can occur before,during or after tumor progression.As will be discussed in the following sections (Table 1),recent studies have shown that the available knowledge on the induction and molecular regulation of tumor angiogenesis has grown rapidly,and several growth factors,growth factor receptors,cytokines and signaling pathways have been identified in GC.
TRANSCRIPTION FACTORS
Hypoxia and hypoxia-inducible factor
Preclinical trial:First,the basement membrane in growing tumor cells is injured locally,and tumor cells immediately experience destruction and hypoxia.Tumor hypoxia is a major force that triggers tumor angiogenesis and activates the expression of hypoxia-inducible factor-1 (HIF-1),which then induces the expression of various proangiogenic factors,including vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR),in cancer cells[16-19].Moreover,HIF-2 isoforms have similar functions as HIF-1,but HIF-2 mainly activates the expression of erythropoietin (EPO) in kidney and liver cells[20].Overall,HIF-1 is known as a potential target of anticancer therapy in many cancers[21].In addition,treatment with HIF-1-specific inhibitors has been studied in animal models,and it has been shown that this treatment results in slowed growth of tumors,decreasedangiogenesis and minor vessel maturation[22].Stoeltzinget al[23]obtained similar results using the dominant negative form of HIF-1 in GC.Chronic infection withHelicobacter pyloriinduces DNA damage by generating reactive oxygen species (ROS) in GC cells[24].Overaccumulation of ROS might stimulate HIF-1 accumulation and aid tumor angiogenesis in GC[25].
PROANGIOGENIC LIGANDS AND RECEPTORS
VEGF family
Preclinical trial:Growing cancer cells encourage the growth of new blood vessels by secreting VEGF and VEGFR into the surrounding TME,and secreted VEGF binds to VEGFR on the outer surface of ECs.ECs are activated by the VEGF signaling pathway,and this activation induces the growth,survival,vascular permeability and migration of ECs to encourage tumor angiogenesis[26].To date,various cytokines and a major proangiogenic factor of ECs have been found to be members of the VEGF-A family.The VEGF (homodimers) family of growth factors contains VEGF-A,B,C,D and E and placental growth factor (PIGF),and during angiogenesis[27,28],these growth factors bind to and activate the tyrosine kinase receptors (TKRs) VEGFR-1,VEGFR-2,and VEGFR-3,which are specifically expressed on the surface of ECs and have different affinities for the ligands.Consequently,the downstream TKR signaling proteins activate proliferation-mediating signaling pathways,such as the phosphatidylinositol 3 kinase (PI3K)/AKT,protein kinase C (PKC),and mitogenactivated protein kinase (MAPK;p38 and p42/44) pathways[29-31].In general,VEGF-A binds to VEGFR-1 and VEGFR-2,PlGF and VEGF-B bind to VEGFR-1,and VEGF-C and VEGF-D bind to VEGFR-2 and VEGFR-3[32-34].Carmelietet al[35]reported that among the VEGFs,thevegfagene can lead to embryonic lethality due to serious vascular defects after the loss of only a single allele in mice[34-36].Anin vitrotube formation assay using GC cells cocultured with human umbilical vein endothelial cells (HUVECs) demonstrated proangiogenesis function due to the upregulation of VEGF in GC cells[37].In a rat model,the blockage of VEGF by a specific siRNA led to reduced proliferation and cell cycle arrest[38].Moreover,the coreceptor of neuropilins in signaling pathways is activated by other growth factors or VEGFs,and neuropilins bind several growth factors and enhance their function;however,the molecular mechanisms affected by neuropilins remain unclear[39,40].The above data indicate that GC cells possess proangiogenic abilities by secreting angiogenic cytokines to both stimulate ECs and to support their own growth in an autocrine manner.Furthermore,the growth and invasion of GC cells are mainly controlled by the VEGF-mediated pathway.
Clinical application:These discoveries fromin vitroand animal models were confirmed in GC patients,and their diagnostic or prognostic abilities were tested in GC patients.Through ELISA,significantly higher preoperative plasma or serum VEGF levels were detected in GC patients compared with healthy control subjects.Importantly,a clinicopathological analysis revealed that higher VEGF expression in the plasma or serum of GC patients was significantly associated with advanced stage,distant metastasis and worse survival outcomes[21,41-47].
PIGF
Preclinical trial:PIGF is another member of the VEGF family and plays a proangiogenic role in the progression of some tumors[29,30,35,48].Akramiet al[49,50]reported that the knockdown of PlGF in AGS and MKN-45 cells inhibited the proliferation,self-renewal capacity,MMP activity,transcription activity and migration of these cells.
Clinical application:Higher PIGF and VEGF levels were detected by ELISA in GC tissues compared with paired noncancerous mucosa tissues.A clinicopathological analysis showed that higher expression of only PIGF in GC patients was significantly associated with tumor stage,distant metastasis and worse survival outcomes[51].
Fibroblast growth factors,epidermal growth factor,hepatocyte growth factor,and insulin-like growth factor
Preclinical trial:The fibroblast growth factor (FGF) family is a large cytokine family,and some of these cytokines,e.g.,FGF-1/-2,bind to different fibroblast growth factor receptors,e.g.,FGFR 1-4,to activate the PI3K/AKT/mTOR (mammalian target of rapamycin) pathway.Furthermore,these cytokines can regulate tumor angiogenesis,proliferation,migration and antiapoptosis/survival activities bothin vitroandin vivo[31,52-54].epidermal growth factor (EGF),hepatocyte growth factor (HGF) and insulin-like growth factor (IGF) reportedly stimulate proangiogenic,proliferation and survival activities similarly to those induced by VEGF[55].
Platelet-derived growth factor
Preclinical trial:Pericytes and smooth muscle cells secrete platelet-derived growth factor (PDGF)-BB,which then binds to PDGFR-β and thereby modulates tumor angiogenesis in ECs[56,57].
GP130,interleukin-6,and interleukin-6R
Preclinical trial:In a mouse model,the blockage of GP130 inhibits tumor development in the epithelium of the glandular stomachviathe STAT 1/3-mediated angiogenesis pathway.These results suggest that the TME and cancer cells secrete interleukin-6 (IL-6)viaautocrine or paracrine binding to GP130 or IL-6R[58].
Human epidermal growth factor receptor 2/Neu (HER-2/neu) and EGFR
Preclinical trial:In tumor cells,EGF binds to EGFR and HER-2/neu to activate the PI3K/AKT and RAS-MAPK-mediated pathways,which are involved in the overexpression of VEGF-A.The secretion of VEGF-A from cancer cells can be mediated through the activation of various signaling pathways.Furthermore,these factors act as central regulators of tumor growth and tumor angiogenesis in GC[59-62].
Angiopoietin-1,2,3,and 4 (Ang-1,-2,-3,and -4)
Preclinical trial:Ang-1,-2,-3,and -4 biologically serve as growth factors for ECs and can strongly regulate competitive interaction with TIE-2 (TKR),which is expressed on the surface of ECs[63,64].The binding of Ang-1 to TIR-2 activates TIE-2 phosphorylation via the Ang-1/Tie2-cascade pathway and is involved in the proliferation,migration,inflammation and survival of ECs.Ang-2 is then released from activated ECs and serves as a significant antagonist[65,66].Additionally,TIE-1 (an orphan receptor) can form a complex with TIE-2 to form heterodimers and compete with Ang-1/TIE-2 interactions and thereby promote inflammation in ECs[66-69].Inhibition of Ang-1 or Ang-2 shows similar inhibition of cell proliferation in GC cell lines[70-73].
Clinical application:Blanket al[74]found that high expression levels of Ang in serum and tissue from GC patients are associated with poor survival.In addition,the Ang/VEGF ratio in GC and esophageal cancer patients serves as an independent proangiogenic biomarker for the clinical response to chemotherapy[75].Another group of researchers found that Ang-2 can serve as an independent predictor of OS and liver metastasis in GC patients[76].Moreover,Aktaşet al[77]found that VEGF,PIGF,and Ang-1 are strongly correlated with OS;thus,these angiogenesis prognostic indices(APIs) could predict survival outcomes in GC patients.
IL-8
Preclinical trial:Tumor-infiltrating macrophages secrete IL-8 and upregulate VEGF to activate EC angiogenesis in GC,as demonstrated in anin vitroassay[37].
IL-17
Preclinical trial:IL-17 stimulates the STAT3-mediated angiogenesis pathway to upregulate VEGF in GC[78].
Tryptase
Preclinical trial:Tumor-infiltrating mast cells (TIMs) secrete tryptase by binding to proteinase-activated receptor-2 (PAR-2) and then produce VEGF to stimulate tumor angiogenesis and EC proliferation,as demonstrated throughin vitroandin vivoassays[79,80].
Clinical application:TIMs can release tryptaseviaPAR-2 activation and are involved in tumor angiogenesis.Ammendolaet al[81]suggested that an increased mast cell density positive for tryptase (MCDPT) and a higher general vascularized area are related to poor survival outcome and can thus serve as potential targets in both primary tumor and lymph node metastases in GC patients.
RESULTS FROM PRECLINICAL AND CLINICAL STUDIES OF ANTIANGIOGENIC THERAPIES FOR GC
According to the results of studies on the molecular mechanism of tumor angiogenesis,we can develop a novel antiangiogenic strategy that could reduce tumor angiogenesis and limit tumor growth instead of eradicate the tumors and thereby delay the progression of precancer/primary lesion to metastases/aggressive cancers.The purpose of antiangiogenesis therapy is not to directly target cytotoxic tumor cells but rather block the supply of oxygen,growth factors and nutrition from blood vessels[109].Thus,this section will focus on several tumor angiogenic factors that could serve as potential targets for antiangiogenic drugs that are currently being investigated in preclinical (the section only highlights the most common antiangiogenic drugs;Table 1) and clinical studies on GC patients.Due to the metabolic changes and stemness of malignant cells lacking oxygen supply in various tumors,tumors appear to escape antiangiogenic therapy within a short time owing to the manipulation of alternative pathways[110],vasculogenic imitation[111]and recruitment of bone marrow-derived cells[112,113].Various clinical trials have not shown a statistically significant extension of survival outcomes.Thus,most of the antiangiogenesis strategy can be ineffective.In phase III clinical trials,only ramucirumab (anti-VEGFR) and apatinib (VEGFR-TKI) have reported to improve ORR and prolong OS and PFS outcomes when used as a 2nd-line regimen combined with chemotherapy treatment in advanced GC (Table 2).
INHIBITORS OF PROANGIOGENIC LIGANDS AND RECEPTORS
Bevacizumab (avastin,genentech,rhumad)
Preclinical trial:As demonstrated in a preclinical model,this drug,which is a recombinant monoclonal antibody against VEGF-A,serves as a powerful and effective antiangiogenesis agent in several cancers[83-85].Anin vitrostudy revealed that treatment with bevacizumab reduced cell growth and pro-apoptosis in GC cell lines[86].Yamashita-Kashimaet al[87]performed anin vivostudy and found that bevacizumab could be effective against GC and select biomarkers in the MKN-45 human gastric xenograft model.A study with mouse models revealed that treatment with bevacizumab significantly reduced the tumor size[88,89].In the future,we will explore the effects of the antibody-mediated blockage of VEGF-mediated tumor angiogenesis in GC to obtain a more in-depth understanding.
Clinical trial:Ohtsuet al[114]explored the effect of bevacizumab,which is a VEGF blocker.The AVAGAST clinical trial indicated that the 1st line treatment of advanced GC patients (multiethnic population) with bevacizumab in combination with chemotherapy (Cisplatin;Cis/Capecitabine;Cap) resulted in significantly improved ORR (P =0.0315) and extended PFS (P =0.0037) outcomes compared with those achieved with chemotherapy alone (Table 2).However,the AVATAR clinical trial showed that the 1st line treatment of advanced GC patients (China) with bevacizumab in combination with chemotherapy (Cis/Cap) did not significantly prolong the survival outcomes compared with those achieved with chemotherapy alone[115].In contrast,Maet al[116]assessed the effects of bevacizumab in combination with chemotherapy (Docetaxel;Doc/Oxaliplatin;Oxa/5-FU) compared with those of the 1st line treatment of chemotherapy alone in advanced GC patients (China) and observed significantly improved ORR (P =0.0436) and extended PFS (P =0.013)outcomes compared with those achieved with chemotherapy alone.The other group,the ST03 clinical trial,showed that the perioperative treatment of advanced GC patients (United Kingdom) with bevacizumab in combination with chemotherapy(Cis/Cap/Epirubicin;Epi) had no positive results compared with those achieved with chemotherapy alone[117].However,the differences in the outcomes achieved after bevacizumab treatment among the different populations remain unknown.

Table2 Overview of phase-lll clinical trials in gastric cancer including vascular endothelial growth factor,vascular endothelial growth factor receptor and vascular endothelial growth factor receptor tyrosine kinase inhibitor blockers

A P value less than 0.05 indicates statistical significance according to the Mann-Whitney U test.VEGF:Vascular endothelial growth factor;VEGFR:Vascular endothelial growth factor receptor;TKI:Tyrosine kinase inhibitor;ORR:Median overall response rate;DCR:Median disease control rate;PFS:Median progression-free survival;OS:Median overall survival;Cis:Cisplatin;Cap:Capecitabine;Doc:Docetaxel;Oxa:Oxaliplatin;5-FU:5-Fluoropyrimidin;Epi:Epirubicine;Pla:Polylactic acid;Pac:Paclitaxel;HR:Hazard ratio;CI:Confidence interval.
Interferon,rapamycin,and neovastat
Preclinical trial:The interferon family contains multifunctional cytokines that exhibit antiviral and antitumor properties,induce regulatory cell apoptosis and immune responses and inhibit proangiogenic factors.Abdel-Rahmanet al[90]evaluated bevacizumab in combination with other anticancer agents,such as mTOR inhibitors and interferon (IFN),as a more effective treatment for gastrointestinal tract and pancreatic tissues.Preclinical and clinical trials showed that other mTOR inhibitors,such as rapamycin,also display antiangiogenic activity in GC[91].Moreover,Neovastat is a multifunctional drug that blocks VEGF,MMPs and proapoptotic activity in ECs.One MMP inhibitor (Marimastat) has been shown to induce positive outcomes in phase III clinical trials with advanced GC patients.The other MMP inhibitors are continuing to be investigated in clinical trials[92].
Clinical trial:A clinical phase II trial showed that the treatment of advanced GC patients with interferon-alpha 2B (IFN) and folinic acid (FA) in combination with 5-fluorouracil (5-FU) chemotherapy also resulted in significantly prolonged PFS outcomes compared with those achieved with chemotherapy alone[122].Al-Batranet al[123]demonstrated that mTOR-mediated inhibitors (e.g.,rapamycin) blocked the growth of GC cells and delayed tumor progression in cell lines and mouse models.Additionally,the mTOR inhibitor rapamycin has also yielded better survival outcomes in phase I/II studies of metastatic GC patients than do treatment without rapamycin.
Ramucirumab
Preclinical trial:Ramucirumab is a VEGFR-2-targeted monoclonal antibody that inhibit VEGFR-2 signaling.Anin vitrostudy showed that treatment with ramucirumab also inhibited cell growth and promoted apoptosis in GC cell lines and animal models[95,96].Thus,both bevacizumab and ramucirumab inhibit VEGFmediated pathways in GC.Additionally,anin vivostudy showed that the effects of combination therapy involving anti-VEGFR and anti-EGFR agents resulted in a significantly decreased tumor size in a GC mouse model[97].
Clinical trial:Fuchset al[118]attempted to explore the effect of ramucirumab,which blocks VEGFR signaling.The REGARD clinical trial indicated that the treatment of advanced GC patients (multiethnic) with ramucirumab in combination with chemotherapy (Pla/5-Fu) resulted in significantly extended PFS (P< 0.0001) and OS(P =0.047) outcomes compared with those achieved with placebo.Moreover,the RAINBOW clinical trial showed that the treatment of advanced GC patients(multiethnic) with ramucirumab in combination with chemotherapy (Paclitaxel;Pac)also resulted in significantly improved ORR (P< 0.0001) and DCR (P< 0.0001),extended PFS (P< 0.0001) and OS (P =0.0169) outcomes compared with those achieved with chemotherapy alone[119].In contrast,the RAUNFALL clinical trial showed that the treatment of advanced GC patients (multiethnic) with bevacizumab in combination with chemotherapy (Cis/5-Fu) had no positive results compared with those achieved with chemotherapy alone[120].Ramucirumab was approved by the United States Food and Drug Administration (FDA) in 2014 as a 2nd-line treatment of advanced GC due to the REGARD and RAINBOW clinical trials and has beneficial effects on PFS and OS for advanced GC.
DIRECT ACTION ON ECs
Regorafenib,apatinib,and foretinib
Preclinical trial:Regorafenib,apatinib and foretinib belong to the family of multitargeting TKIs.Blockage of the effects of VEGF by silencing RNA in GC cell lines led to reduced tumor volume after implantation of these GC cells into nude mice[98].The same effect was observed in mice treated with apatinib after tumor grafting[99].
Clinical trial:First,Liet al[121]explored the effect of apatinib,which VEGFR TKI blockade.A 116 clinical trial (3rdline) indicated that the treatment of advanced GC patients (China) with apatinib resulted in significantly improved ORR (P< 0.001),extended PFS (P< 0.001) and OS (P =0.0149) outcomes compared with those achieved with placebo.In a phase II study,the tumor-angiogenesis inhibitor regorafenib,which targets VEGFR,TIE and multiple kinases,was evaluated in advanced GC patients,and the results showed that treatment with this inhibitor resulted in significantly prolonged PFS outcomes compared with those achieved with placebo[124].Thus,regorafenib will be investigated in a phase III study.However,another antiangiogenic drug,foretinib,which inhibits VEGFR2 and TIE-2,did not yield any benefits in the survival outcomes of GC patients[125].In addition,Shanet al[126]reviewed information from clinical trials evaluating antiangiogenic agents (with a focus on multitargeting TKIs) in advanced GC and found that only apatinib yielded a positive effect on PFS.
Orantinib (SU5416,SU6668),Pazopanib,Sorafenib (Nexavar),Sunitinib (Sutent),Telatinib (Erbitux,Cetuximab)
Preclinical trial:Orantinib (SU5416 SU6668)[94],pazopanib[100],sorafenib(Nexavar)[101,102],sunitinib (Sutent)[103,104]and telatinib (Erbitux,Cetuximab)[105]block tyrosine kinases and belong to the family of multitargeting TKIs.Suppressing the effects of VEGF by silencing RNA in GC cell lines led to decreased tumor angiogenesis and growth after these cells were implanted into nude mice.
Clinical trial:Chenet al[71]summarized the results from clinical trial phase II studies of antiangiogenic drugs,including VEGF ligands,VEGFRs and multitarget TKIs,in advanced GC.The treatment of advanced GC patients with orantinib[127],pazopanib[128,129],sorafenib[130-133],sunitinib[134-136],telatinib[137-141]and vandetanib resulted in significantly extended OS and PSF.
Aflibercept
Preclinical trial:Aflibercept traps VEGF and PlGFin vivoand is currently being investigated in a clinical trial (NCT01747551) as a supplement to standard chemotherapy for GC patients[22].In addition to VEGF-specific inhibition,the effect of HIF-1 blockage has been investigated in animal models in several studies.The treatment of subcutaneous xenografts with an inhibitory HIF-1 compound results in smaller and less vascularized tumors after implantation into nude mice.
Trastuzumab
Seidmanet al[62]reported that the antibody trastuzumab blocks the Her2/neu receptor through the RAS-MAPK proliferation signaling pathway.A log-rank test showed improved survival outcomes in breast cancer patients.The comparison of two different Her2 and VEGF inhibitors revealed that the effect of tumor growth inhibition on Her2-overexpressing GC xenografts through the combination of Her2 and VEGF inhibitors was better than that achieved with either inhibitor alone[59].
Nonsteroid anti-inflammatory drugs
In an animal model,nonsteroid anti-inflammatory drug (NSAID)-mediated cyclooxygenase (COX) inhibition resulted in reduced tumor angiogenesis,and decreased HIF-1 expression was detected in GC cells after treatment with NSAIDs[25].
OTHER ASSOCIATED CHEMOTHERAPIES
In clinical phase trials,cancer patients are typically administered combination therapy consisting of antiangiogenic agents with chemotherapeutic agents.However,antiangiogenic therapy sometimes elicits several adverse effects,such as hypertension[142,143]or proteinuria[144],but the factors responsible for these adverse effects remain unknown.In general,the results from several studies on some antiangiogenic therapies,such as the inhibition of VEGF,Ang-1 and PlGF,indicate that antiangiogenic therapy not only inhibits EC migration and proliferation but also enhances chemotherapy ability.Hwanget al[145]indicated that the inhibition of VEGFR enhances paclitaxel sensitivity in GC cells.Another group of researchers showed that the upregulation of HIF-1 promotes chemotherapy and the antiapoptosis ability in GC cells by inducing miR-27a- or p53- and NF-kB-mediated pathways[146-148].Additionally,compared with normal blood vessels,tumor vessels exhibit heterogeneity,versatility,high permeability and vascular properties that benefit chemotherapy[149].Thus,antiangiogenic therapy could exert an adjuvant effect in chemotherapy.
CONCLUSION
Tumor angiogenesis involves a complex multistep process.In general,the available knowledge indicates that proangiogenic and pro-oncogenic (such as proliferation,anti-apoptosis,migration and invasion) pathways are linked to each other.Thus,tumor angiogenesis occurs at different stages of tumor progression,including tumor growth,metastasis and recurrence.This connection can be clearly observed by the administration of combination therapy against angiogenic and proliferative pathways,such as the VEGF-,EGFR- and STAT3-mediated pathways[16-19,31,52-54,58].These transcription factors regulate cell growth,migration and angiogenesis in multiple ways.
First,we investigated the expression of angiogenic factors in GC through preclinical trials [cell line (in vitro)/animal model (in vivo)] and thus determined whether these factors could serve as predictive factors/biomarkers for proliferation,invasion or metastasis and/or have diagnostic or prognostic value[7,8].An increasing number of studies has revealed that antiangiogenic agents attack tumor ECs as their target instead of tumor cells themselves,which is the final goal of tumor dormancy therapy.Moreover,the therapeutic target of antiangiogenic agents is tumor ECs,which are more genetically stable,show increased homogeneity and have a lower alteration level;antiangiogenic drugs can interact with ECs directly,resulting in higher potency,decreased drug resistance and fewer side effects[150].We explored the combination of antiangiogenic drugs and cytotoxic anticancer (chemical) drugs to develop a highly effective strategy for the management of advanced GC[13-15].Thus,antiangiogenic drugs might be valuable for the long-term management of tumor dormancy because they do not induce the development of antiangiogenic drug resistance,and these drugs present fewer side effects.A few recent clinical trials have revealed that antiangiogenic therapy could potentially extend the survival outcomes of advanced GC patients[109].
DISCUSSION
In assessing the effectiveness of antiangiogenesis therapy,a clinical phase III trial showed that only ramucirumab (an anti-VEGFR antibody) and apatinib (VEGFR TKI blocker) achieved positive results (Table 2).Although both ramucirumab and bevacizumab are anti-VEGF drugs,bevacizumab (AVAGAST,AVATAR,ST03,Maet al[116]) had no positive results on OS,while ramucirumab (REGARD,RAINBOW) was more effective targeted drug and exerted more positive results for OS in advanced GC.We suggested that this is because bevacizumab only binds to VEGF-A,whereas ramucirumab binds to VEGFR-2,which blocks more VEGFs.Therefore,ramucirumab could exert more effective antiangiogenic function due to the inhibition of more VEGF molecules.One possible reason is the differences in the targets of the antiangiogenic action.However,the differences in the ability of these two anti-VEGF drugs remain partially unknown.Furthermore,the different populations of GC patients might be another factor that affects the benefits of these drugs.In the AVAGAST and RAINBOW studies,the non-Asian subgroup (66.5%;RAINBOW) achieved a greater benefit in OS from antiangiogenic therapy than did the Asian subgroup (51%;AVAGAST).However,the effect of ramucirumab still lacks 1st-line chemotherapy evidence.The extent of the usefulness of ramucirumab still requires exploration in further trials in different ethnicities and upon delivery as a 1st-,2nd- or 3th-line chemotherapy.Additionally,in evaluating the safety of antiangiogenic therapy,most adverse events related to antiangiogenesis are tolerable and controllable,including hypertension,neutropenia and wound healing (Table 2).Conversely,the Cougar-02 trial,a Doc+best supportive care (BSC) study,has a similar result for OS as the REGARD trial and was more cost effective[151].Finally,of the VEGFR TKIs,only apatinib in the phase III clinical trial showed extended PFS and OS in advanced GC patients.We recommend that chemotherapy in combination with ramucirumab (anti-VEGFR) and apatinib (VEGFR TKI) significantly improves the outcome in ORR,expended PFS,and OS in the management of advanced GC.
Here,this review only included phase III clinical trials published in English.Previous studies have found that the combination of antiangiogenic agents with chemotherapy may be beneficial for advanced GC in OS,but potential publication bias should be considered when construing these results.To reduce possible publication bias,we tried to search in multiple databases.Nevertheless,some restrictions were present in this systemic review and statistical analysis (e.g.,metaanalysis)[152,153]such as the small size of included studies,multiple drugs implemented and the high heterogeneity between different studies.Therefore,a larger cohort size,more standardized research and high statistical quality should be implemented in future studies to identify patients who would most likely benefit from antiangiogenic treatment.Thus,this review will provide basic (tumor angiogenesis) and clinical(antiangiogenic drugs) research for the survey of the management of GC treatments.
FURTHER CHALLENGES OF ANTIANGIOGENIC THERAPY
Although several phase III clinical trials have reported positive results,new vessels in tumors have pleomorphic features,including heterogeneity,flexibility,penetrability,various vascular biomarkers,and turbulent blood flow with no lymphatic vessels,and these unusual features make the delivery of therapeutic drugs difficult.Hence,there remain several obstacles regarding the translation of antiangiogenic strategies from animal models to clinical trials[92,108,154].
The current problems regarding preclinical to clinical trials and the future directions for antiangiogenic therapy are discussed below.
In preclinical trials,we usually perform experiments in animals with xenografts of various tumor cells,but these models cannot represent spontaneous and orthotopic human cancers,particularly highly metastatic tumors[155].Therefore,antiangiogenic drugs are not effective for every organ in the body.Antiangiogenic drugs often yield different results or side effects in preclinical and clinical trials.
In advanced GC,the tumor develops several ways of escaping treatment and rapidly activating angiogenic pathways.Eboset al[156]reported that enhanced metastasis was treated with sunitinib in a mouse model.Another group found a similar result[157].This may partly fail to translate to a survival benefit of antiangiogenic drugs in localized or nonmetastatic GC.Therefore,it is crucial to develop novel biomarkers that are able to predict the prognosis of antiangiogenic treatments for advanced GC.In clinical trials,to assess antiangiogenic therapies,newer imaging systems and/or substitute biomarkers should be established for monitoring tumor vessel functions.Antiangiogenic drugs induce tumor dormancy,which is different from the results of chemotherapy[155].
The aims of managing GC are to reduce drug toxicity and adverse events and prolong survival.Therefore,the optimal biological dose and therapeutic schedule of antiangiogenic drugs should be established.Moreover,antiangiogenic drugs can be combined with chemotherapy and/or radiotherapy[149].
According to previous studies,the clinical effect is quite different in individuals due to heterogeneity of the tumor.It is unclear which patients benefit most from angiogenesis inhibitors.The race/ethnicity of patients seems to influence the efficacy of antiangiogenic treatments on OS.The patients should be selected,and angiogenic factors should be detected before the administration of antiangiogenic drugs.Individual angiogenic profiling according to an individual’s genetic background remain a problem that need to be addressed.
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Advancements and challenges in treating advanced gastric cancer in the West
- Premalignant lesions and gastric cancer:Current understanding
- Current status of adjuvant chemotherapy for gastric cancer
- MicroRNA-331 inhibits development of gastric cancer through targeting musashi1
- Correlation between invasive microbiota in margin-surrounding mucosa and anastomotic healing in patients with colorectal cancer
- Colorectal cancer fecal screening test completion after age 74,sources and outcomes in French program
