基于金属有机骨架前驱体制备纳米磷化镍催化剂
2019-08-08朱良奎付煜荣符小文李海霞
徐 丹 朱良奎 周 丹 付煜荣 符小文 陈 榕 李海霞
(1海南医学院药学院,海口 571199)
(2吉林大学无机合成与制备化学国家重点实验室,吉林 130012)
0 Introduction
In the past few decades,the production of environmentally friendly energy has become a critical concern due to increasing environmental pollution and energy demand[1-3].Hydrogen is considered to be one of the most ideal and cleanest energy sources[4].It is considered to be the main energy carrier due to its high energy storage,ideal combustion efficiency and non-toxicity.Water electrolysis is an alternative process and a desirable way to produce molecular hydrogen,but both half-reactions of water splitting,namely,the oxygen evolution reaction (OER)and the hydrogen evolution reaction (HER),still remain technical challenges[5].Although,platinum (Pt)-based materials are most active and stable catalysts for HER,but their prohibitive cost,scarce reserves,and poor durability significantly prohibit widespread application in energy conversion[6-7].It is therefore highly imperative and challenging to develop nonprecious metal electrocatalysts for HER to achieve efficient overall water splitting.
At present,metal phosphide (TMP)catalysts composed of transition elements such as Fe,Co,Ni and Mo are the alternative to precious metal catalysts and achieve high efficiency of HER catalytic activity[8-12].In particular,nickel phosphides (Ni3P,Ni2P,Ni5P2,Ni12P5and Ni5P4)of different phases exhibit promising HER catalytic performance in strongly acidic electrolytes.Schaak′s research group synthesized nanostructured Ni2P with a high accessible surface area and a high density of exposed (001)facets.The active Ni2P nanoparticles investigated for electrocatalytic activity and stability for the HER in acidic solutions,which had the highest HER activity among the non-noble metals at that time[13].Dismukes′s group reported microcrystalline Ni3P as a non-precious metal electrocatalyst for hydrogen evolution reaction (HER),whose catalytic activity was second only to the activity of Pt[14].
Metal-organic frameworks (MOFs),with high porosity,large surface areas and inherent presence of heteroatoms have been proved to be ideal sacrificial templates for fabricating electrocatalyst by changing the thermal conditions in the different atmosphere[15-17].Liu′s group prepared carbon-cobalt hybrid materials through the thermolysis of the Co-ZIF precursor at 750℃which has been explored as a good catalyst for oxygen reduction reaction (ORR)[18].Dong′s group prepared porous CoP concave polyhedrons (CoPCPHs)via low-temperature multi-step calcination using Co-MOF (ZIF-67)polyhedrons as the precursor[19].The eletrocatalyst nanomaterials prepared by this novel MOF-templated route possessed many advantages such as large internal surface area,high crystallinity,tailorable porous structure and more catalytic centers.More and more researchers focuse on fabricating desired catalysts derived from MOFs precursors.
In this work,Ni-based metal-organic framework(Ni-MOF)as a precursor is used to carbonize and synthesize a nickel-carbon composite (Ni@C)under a nitrogen atmosphere at 500℃.Next,the nickel phosphide nanocatalysts are obtained by phosphorization of Ni@C composite using phosphorus vapor without complicated chemical reactions and post-treatment steps involved.The MOF-derived crystalline Ni1P1-500 nanostructure guarantees the highly exposed active sites,and the charge properties of Ni and P contributes to a consecutive electrical conductivity,which is crucial for electron transfer.As a result,the Ni1P1-500 nanocatalyst exhibited excellent HER activity in acid solution with overpotentials of 178 mV at 10 mA·cm-2.More importantly,this facile two-step synthetic method reported here has the characteristics of simple synthesis and large-scale production,can be extended to other MOFs to synthesize metal phosphides.
1 Experimental
1.1 Synthesis
1.1.1 Synthesis of 1,4,5,8-naphthalenetetracarboxylic acid (NTCA)
1,4,5,8-Naphthalenetetracarboxylic dianhydride(NTCDA,1 mmol)was dissolved in NaOH (2 mol·L-1,50 mL)solution and stirred at 60~70 ℃.After hydrolyzing for 1 h,the solution was cooled to room temperature,and 1 mol·L-1HCl solution wasadded dropwise to adjust the pH value to 5~6.Then the precipitate was filtered and dried at room temperature[20].
1.1.2 Synthesis of Ni-MOF
Ni(NO3)2·6H2O (0.116 g,0.4 mmol)was dissolved in a mixture solvent of 1.4 mL of dimethylformamide(DMF),1.4 mL of ethanol,1.4 mL of water.Then NTCA (0.015 g,0.05 mmol)was added into the above solvent under sonicating.After uniformly dispersing,the resulting mixture was transferred into a Teflon autoclave and placed in an oven at 100℃for 3 d.After cooling,the product was suction filtered under reduced pressure,washed successively with distilled water,DMF and ethanol,and then dried at room temperature.The as-prepared powders were called as Ni-MOF.
1.1.3 Synthesis of nickel phosphide nanocatalyst
A certain amount of Ni-MOF powder was placed in a quartz crucible,and then the crucible was placed in the middle part of the tube furnace[21].The powder was carbonized under the protection of N2.The heating temperature was raised to 500℃at a heating rate of 5℃·min-1and maintained for 3 h.The black powder named as Ni@C.Ni@C and red phosphorus were ground and mixed in a mortar according to the mass ratio in Table 1,and carbonized in a tube furnace.At this time,the heating rate was changed to 10℃·min-1,and the holding time was changed to 1 h to obtain different compositions of nickel phosphide sample.The resultant samples were called Ni x P y-T (x∶y represents the mass ratio of Ni@C to red phosphorus;T represents pyrolysis temperature).
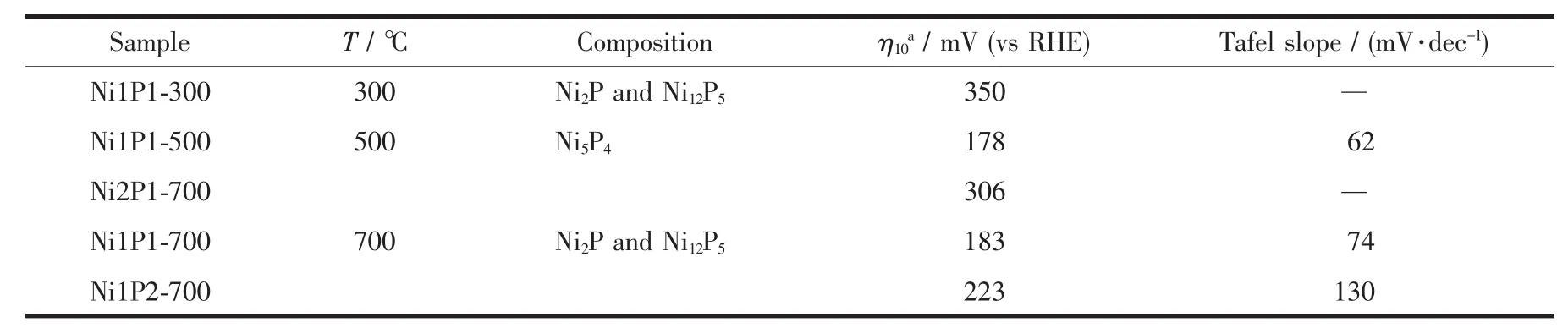
Table 1 Experimental conditions and electrocatalytic performance of nickel phosphide sam ples
1.2 Characterization methods
Powder X-ray diffraction (PXRD)was carried out using an XRD-6000 powder X-ray diffractometer manufactured by Shimadzu Corporation of Japan.The PXRDwas performed with an accelerating voltage of 40 kV and a tube current of 30 mA,and Cu Kαradiation(λ=0.154 18 nm)in the 2θrange of 4°~80°with a step size of 0.02°and a time of 4 second per step.The transmission electron microscope (TEM)images were performed on a JEM-2100 with a field emission gun operating at 200 kV,manufactured by JEOL Ltd.The TEM image was recorded by an A Gatan 794 CCD camera.An X-ray photoelectron spectroscopy (XPS)wasmeasured by an ESCALAB 250 (Thermo VGUSA)multi-function surface analyzer.The gas used in the carbonization and phosphating process was high purity nitrogen.
1.3 Electrochem ical test
5 mg nickel phosphide sample was dispersed in a mixture of 1 mL of distilled water and ethanol (3∶1,V/V),and then 10 μL of 5%(w/w)Nafion was added.The mixture was ultrasonicated for 30 min to form a uniform ink dispersion.5μL of the mixture was drop-casted onto the glassy carbon electrode with the diameter of 3 mm for the electrochemical measurements.The test procedure was performed on an electrochemical workstation using a typical three-electrode mode:the Pt wire was the counter electrode,the Ag/AgCl electrode(saturated KCl)was the reference electrode,the glassy carbon loaded with the nickel phosphide catalyst was the working electrode,and the electrolyte was a 0.5 mol·L-1H2SO4solution.Before the electrochemical test,in order to eliminate the influence of dissolved O2on the catalytic reaction,Ar was first introduced until a stable CV cycle curve was obtained at a scanning rate of 50 mV·s-1,and then high-purity H2was introduced until a stable LSV curve was obtained at 5 mV·s-1.The LSV curve was obtained by a potential interval of 0.01 to-0.7 V (corrected under RHE)with a scan speed of 2 mV·s-1.The low scan rate overcomes the effect of material capacity on the catalytic performance.All potentials measured were converted to the reversible hydrogen electrode (RHE)scale according to the Nernst equation:in a 0.5 mol·L-1H2SO4solution,E (vs RHE)=E(Ag/AgCl)+(0.197+0.059pH).
2 Results and discussion
2.1 General properties of the Ni-MOF
Nickel nitrate (Ni(NO3)2)and NTCA were selfassembled to form a Ni-MOF at a solvothermal temperature of 100℃.The PXRD pattern of the crystalline powder was shown in Fig.1.The position of the diffraction peak and the relative intensity of diffraction were consistent with the Ni-MOF pattern reported in the literature[22],indicating that the Ni-MOF crystal was successfully synthesized.
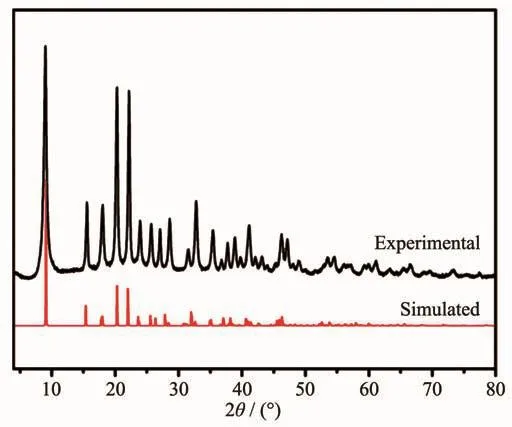
Fig.1 PXRD patterns of Ni-MOF
2.2 General properties of the Ni@C
The synthesized Ni-MOF precursor underwent pyrolysis at 500℃in N2atmosphere for 3 h to afford Ni@C composites[23].One low broad peak centered at 2θ=25°assigned to amorphous carbon[24],the other three prominent peaks near at 2θ=44.3°,51.6°and 76.2°assigned to the (111),(200)and (220)crystalline planes of cubic-phased Ni (PDF No.04-0850),respectively(Fig.2A).TEM showed that the Ni nanoparticles were uniformly dispersed in the carbon matrix with almost no agglomeration (Fig.2B).
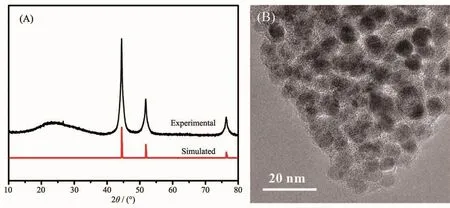
Fig.2 PXRD patterns (A)and TEM image (B)of Ni@C
2.3 PXRD and TEM analysis of nickel phosphide
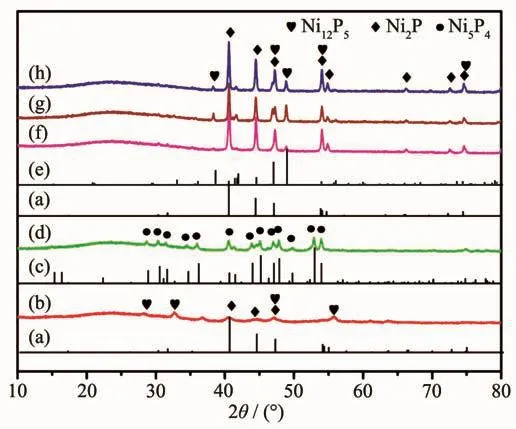
Fig.3 PXRD patterns of Ni2P phase(PDF No.03-065-3544)(a),Ni1P1-300 (b),Ni5P4 (PDF No.03-065-2075)(c),Ni1P1-500 (d),Ni12P5 phase (PDF No.03-065-1623)(e),Ni1P2-700 (f),Ni2P1-700 (g)and Ni1P1-700 (h)
Ni@C was further phosphatized to prepare nickel phosphide nanocatalysts.Five nickel phosphide nanocatalysts were synthesized according to the different mass ratios and phosphating temperatures (Table 1).In our experiments,the phosphating temperature was found to be a key factor that affected the phase structure of the nickel phosphide nanocatalysts.The crystalline phase structure and the purity of the assynthesized nickel phosphide materials at different phosphating temperatures were characterized by PXRD (Fig.3).When phosphidation treatment of Ni@C was conducted at a calcination temperature of 300℃for 1 h,the Ni2P (PDF No.03-065-3544)and Ni12P5(PDF No.03-065-1623)nanoparticles could be prod-uced (Fig.3(a,b,e)).Interestingly,the Ni5P4nanoparticles could be obtained via a heat treatment at 500℃for 1 h.As shown in Fig.3(c,d),all diffraction peaks matched well with the hexagonal structure of Ni5P4(PDF No.03-065-2075)and no extraneous peaks existed.Upon further increasing the phosphating temperature to 700℃,the product was a mixture of hexagonal Ni2P and hexagonal Ni12P5phases ((Fig.3(a,e~h)).Among five nickel phosphide materials,the crystallinity increased with the increase of temperature.The morphology and structure of the nickel phosphide were further characterized by TEM (Fig.4).The Ni2P and Ni12P5particles in the Ni1P1-300 material were uniform in size and encapsulated in the carbon matrix (Fig.4(A,B)).The HRTEM image (Fig.5A)showed the lattice fringes with a distance of 0.221 nm,corresponding to the (111)lattice plane of Ni2P.As the phosphating temperature increased,the Ni5P4particles grew to ~20 nm (Fig.4(C,D)).The distance of the adjacent lattice fringes was calculated to be about 0.332 nm,corresponding well to the (110)lattice plane of Ni5P4(Fig.5B).When the temperature rises to 700℃,the biphasic Ni2P and Ni12P5nanoparticles were obtained with an average particle size of 100 nm and escaped from the carbon matrix (Fig.4(E,F)).
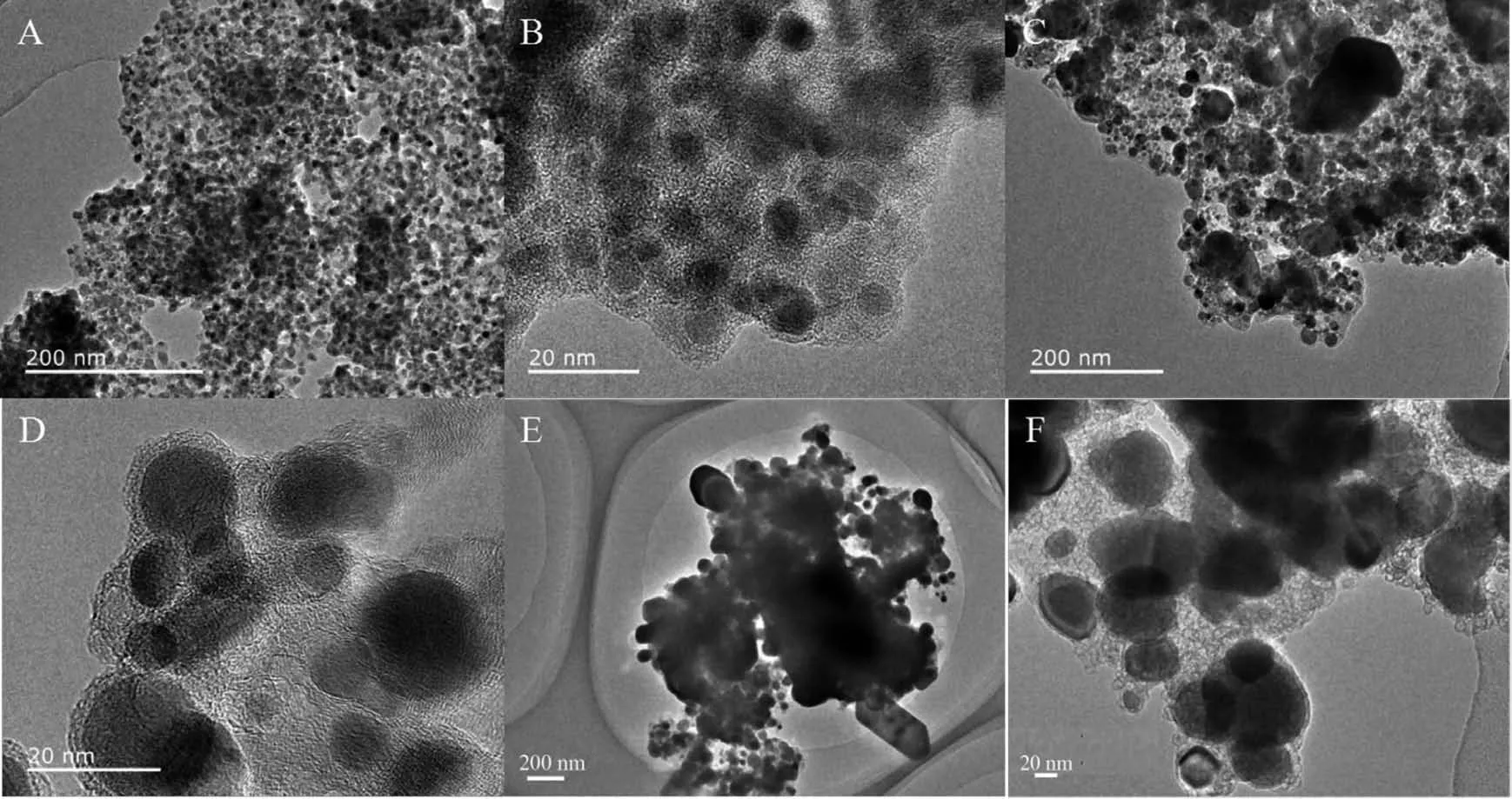
Fig.4 TEM images of Ni1P1-300 (A,B),Ni1P1-500 (C,D)and Ni1P1-700 (E,F)

Fig.5 HRTEM images of Ni1P1-300 (A)and Ni1P1-500 (B)
2.4 Electrocatalytic activity
The electrocatalytic activities of nickel phosphide nanocatalysts in different phases towards HER was evaluated.The polarization curves of catalysts in 0.5 mol·L-1H2SO4with a slow scan rate of 2 mV·s-1using a three electrode setup were shown in Fig.6A.As a comparison,commercial 20%(w/w)Pt/C catalyst was also performed under the identical measurements.The glassy carbon electrode of the nickel phosphide catalysts measured under the reversible hydrogen electrode had a large current density.Generally,the overpotential(η)required for the current density of 10 mA·cm-2(η10)was a matric relevant to solar fuel synthesis,commonly used as a standard for evaluating the HER activity of the catalyst[25].The Ni1P1-500 required an η10of 178 mV,while η10of Ni1P1-700,Ni1P2-700,Ni2P1-700,and Ni1P1-300 were 193,223,306,and 350 mV,respectively.These datas showed that nickel phosphide with different phases had good electrocatalytic properties,and that the Ni1P1-500 catalyst consisting of Ni5P4exhibited the highest catalytic activity.This superior HER activity can be attributed to the positive charge of Ni and the ensemble effect of P in nickel phosphide catalysts[26].The small positive charge of Ni was beneficial to improve the catalytic activity of HER.The XPSshowed that δ(Ni12P5)<δ(Ni2P)<δ(Ni5P4)(δ is the value of charges)[27],which indicated that the catalytic activity of Ni1P1-500 (Ni5P4)was better than other four catalysts.In addition,for the phosphide materials,the increase of the radio of P to Ni on the surface can also improve the catalytic activity[28].This effect could be one explanation why a higher phosphorous content was found to be beneficial for the activity and stability of transition metal phosphides[29-30].The molar ratios of P to Ni were 1∶2.4,1∶2 and 1∶1.25 in Ni12P5,Ni2P and Ni5P4,respectively,which was in accordance with our electrocatalytic results.In previous study,Ni and P sites in the nickel phosphides represented the hydride acceptor and proton acceptor center,respectively,to facilitate catalysis of the HER[13].Moreover,the P could also facilitate the formation of Ni-hydride via electrochemical desorption and offer more active sites[27].Therefore,Ni1P1-500 were expected to be better HER catalysts because of more positive charge of Ni and more P-rich nature.
To further investigate the catalytic reaction kinetics of HER,the Tafel slope for different nickel phosphide nanocatalysts were examined by deriving from polarization curves.Under different overpotentials,the Tafel curve fitted well with the Tafel equation (η=b lg|j|+a,ηrepresents the overpotential,b represents the Tafel slope,and j represents the current density).The Tafel slope of Pt/C was 25 mV·dec-1,which was consistent with that reported in the literature[13].In contrast,the Tafel slope of Ni1P1-500,Ni1P1-700,and Ni1P2-700 electrodes (Fig.6B)were 62,74,130 mV·dec-1.The Tafel slope was a useful indicator of reaction kinetics.The smaller Tafel slope of the HER catalyst, the faster kinetics for electrochemical reactions[31].Thus,the reaction kinetic of the HER on Ni1P1-500 was much faster than those on other samples.According to the literature reports,nickel phosphides exhibit good catalytic stability[32-33].
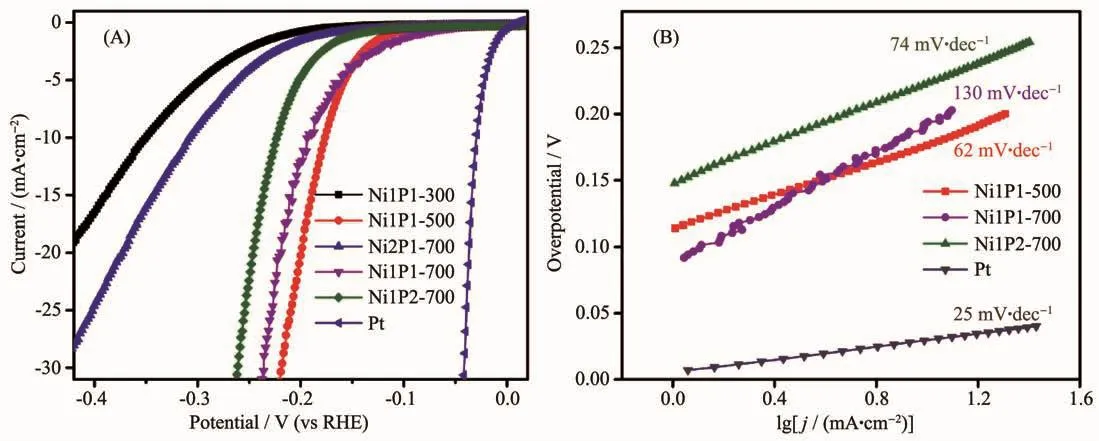
Fig.6 Polarization data (A)and corresponding Tafel plots (B)for nickel phosphide,Pt electrodes in 0.5 mol·L-1 H2SO4
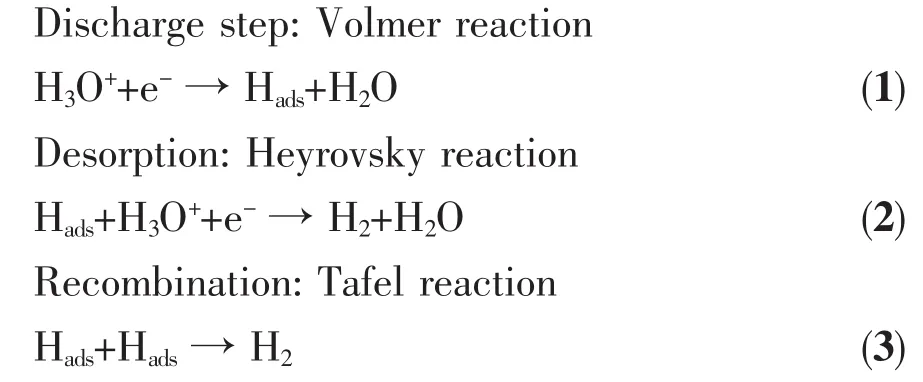
An overview of the hydrogen evolution reaction in acidic medium was given below with three possible rate limiting steps[34]:If Equation (1)is the rate determining step,the expected slope would be 120 mV·dec-1.On the other hand,if the kinetics of the HER is limited by the Equation (2),one would expect a Tafel slope of 40 mV·dec-1.Finally,if the Equation (3)is the rate limiting step,a slope of 30 mV·dec-1is expected[35].In our studies,the observed Tafel slopes of Ni1P1-500 were 62 mV·dec-1,which indicated that the HER reaction was limited by Desorption-Heyrovsky reaction.Hadsrepresents an H atom absorbed at the active site of the catalyst.
2.5 XPS analysis
Based on previous density functional theory(DFT)calculation,the highly active HER of the Ni1P1-500 was attributed to the presence of the proton acceptor and hydride acceptor centers on the surface of Ni5P4[36].In order to reveal the cause of HER reaction,we further analyzed the XPS spetra of Ni1P1-500.The binding energy of 853.0,856.6 and 861.4 eV for the Ni2p3/2energy level were observed (Fig.7A),corresponding to Niδ+,oxidized Ni species and the satellite of Ni2p3/2peak,respectively[37].It is noteworthy that the Ni2p3/2binding energy (853.0 eV)in this work was slightly higher than that in nickel metal(852.5~852.9 eV)[38],which suggested that the Ni in Ni1P1-500 had a very small positive charge.For the P2p region,one peaks at 133.9 eV was attributed to oxidized P species originated from the superficial oxidation of phosphide(Fig.7B)[39],and the other peaks at 129.6 eV was attributed to P in Ni1P1-500,which suggested that the related P species had a very small negative charge because this binding energy was less than that of elemental P (130.0 eV)[40].The results clarified that the HER catalytic activity of Ni1P1-500 may be related to the intrinsic charge properties of Ni and P[41].Moreover,the higherδ+value of phosphate and higher levels of phosphorus may be utilised for the HER in acidic medium[28].
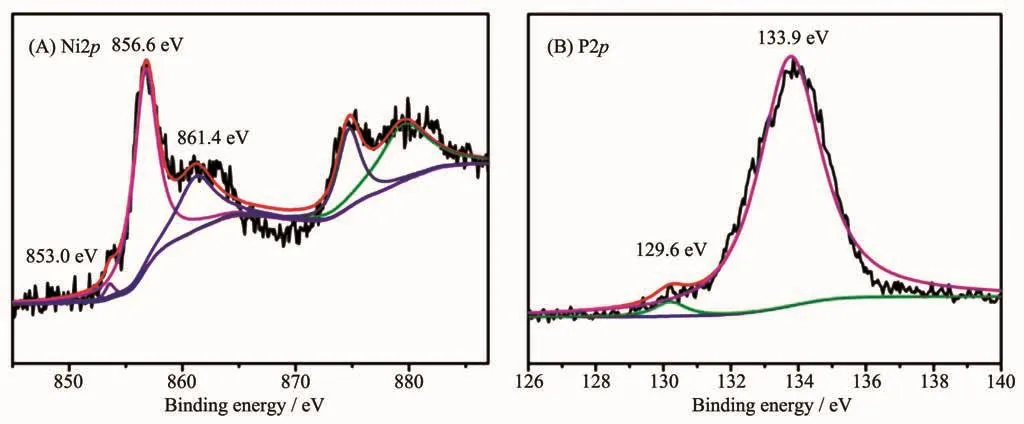
Fig.7 XPSspectra of the Ni1P1-500 nanoparticles
3 Conclusions
In conclusion, we have developed nickel phosphide nanocatalysts for HER,via a MOF pyrolysis and subsequent phosphating process.The resultant nickel phosphide catalysts exhibited superior HER catalytic performance in acid solution.The sample obtained by phosphating at 500 ℃ (Ni1P1-500)was composed of Ni5P4,which exhibited excellent electrocatalytic performance in HER.The Tafel slope was 62 mV·dec-1and the overpotential was 178 mV at a current density of 10 mA·cm-2.The excellent electrocatalytic performance might be ascribed to the presence of the proton acceptor (P site)and hydride-acceptor(Ni site)centers on the surface of nickel phosphide.More importantly,this strategy has the characteristics of simple synthesis,large-scale production and practical application,and it can also be extended to synthesize metal phosphides from other MOFs.
