基于水分散纳米无定形水杨酸甲酯铽配合物的三价铬离子高灵敏荧光传感器
2019-08-08刘笑君孙丽婷刘艳珠周雪珍李永绣
刘笑君 孙丽婷 张 澍 周 晨 刘艳珠 周雪珍 李永绣
(南昌大学稀土与微纳功能材料研究中心,南昌 330031)
0 Introduction
The preparation and application of inorganic rare earth luminescent materials are classical research fields in chemistry,physics and material science.Therefore,a lot of papers on rare earth phosphors with high luminescence efficiency have been reported[1-2].However,in the past decades,luminescent lanthanide complexes (LLCs)have attracted more great attention due to their excellent photophysical properties and showed potential applications in many fields,such as drug delivery,bioimaging,optical imaging,luminescent sensors and OLEDs[3-7].Typically,an LLC contains organic ligand (an “antenna”to harvest energy)and central lanthanide ion(luminous center to emit characteristic light)[8-10].According to previous reports,LLCs of Tbバ and Euバ offer strong characteristic emission spectra and have advantage of long lifetime and large stokes shift[6,9,11].
To prepare LLCs with strongly luminescent properties,the organic ligands are usually selected according their coordination ability and excited triplet-state energy levels (ETSEL)because of the idea that only the ligands with strong coordination ability and suitable ETSEL can effectively sensitize the luminescent emitting of rare earth ions[12-17].However,most of the reported LLCs show poor water solubility and dispersion which limit their application as luminescent materials or sensors under aqueous conditions[18]. Therefore, the application fields of synthesized LLCs can be greatly extended when their stability,solubility or dispersion performance in aqueous systems are improved.
LLCs can be used as sensors for various metal ions and organic compounds[19-23].For this application,the enhancement and quenching of the luminescence of rare earth complexes by these alien species are more concerned about.Therefore,we suggest that coordination systems with insufficient luminescence intensity will provide more and better choices,because they are more susceptible to the external environment,ions and molecules,thus contributing to the construction of more sensitive and effective sensing systems and the development of new detection methods.
Methyl salicylate (MS)and its derivatives are a class of materials widely used in pharmaceutical molecules and optical functional materials[24-25].They are typical o-hydroxybenzoyl compounds with poor coordination ability towards metal ions and can form strong intramolecular hydrogen bond (IMHB)which produces ketone tautomer by excited-state intramolecular proton transfer (ESIPT)[26-27].Omagari et al.[28-30]have reported a series of salicylate terbium complexes with characteristic luminescence of Tbバattributed to the energy transfer process from ligands to Tbバions.The synthesized complex (molar ratio 16∶9 of MS to Tb)is a Tbバ nine-nuclear cluster (Tb9)compound.The distance between the Tb-Tb was investigated by doping gadolinium into the complex,and suggested that the increase in luminescence efficiency of terbium due to the enhanced energy transfer between Tb-Tb can cut down the energy back transfer from excited state to the ligand[29].However,their stability and solubility as well as the dispersion performance are not idea for their applications in systems containing water.
Chromium,one of the essential trace elements in the human body,plays an important role in maintaining human health[31-33].Naturally,it mainly includes Crバ and Crボ[34].As we know,Crバ ions are more important in maintaining life in animals and plants,and excess Crバions may destroy cellular components in the human body through bind to DNA[31,35].The standards of the European Community and World Health Organization regulate the maximum allowable level of Crバ in drinking water is 50 μg·L-1[36]. Several Crバ sensing methods have been developed,but most of them use organic molecules or multi-step modified nanomaterials as sensing platforms,and the preparation process is cumbersome[37-38].In addition,some sensing platforms have the disadvantage of poor selectivity and low luminous efficiency in aqueous solution[39-40].Therefore,we urgently need a simple,economical and good selective Crバdetection method[41].
In the present study,we synthesized a LLC directly in water using MS as ligand and Tb as emitting center.It was found that the synthesized solid product is a water dispersed nano-sized amorphous methyl salicylate terbium (A-MS-Tb)complex with particle size ranging from 50 to 100 nm and similar composition to the reported crystalline methyl salicylate terbium (C-MS-Tb)complex.This complex also shows strong green luminescence at 494,549,591,625 nm,and it can be used as strong green luminescence materials for display and sensors in systems containing water because its luminescent intensity in water suspension remains the same as that in ethanol.More interesting,the aqueous dispersion of nano-sized AMS-Tb shows more stability than that of C-MS-Tb,and the green emitting can been quenched with the addition of Cr3+,which is caused by the weakening of coordination between MS and Tb3+.Accordingly,a highly sensitive sensor for detecting Cr3+was established according to the relationship between the luminescence quenching amplitude and the concentration of chromium ion,and the selectivity and antiinterference ability were evaluated.
1 Experimental
1.1 Reagents and apparatus
All chemical reagents and solvents were obtained from commercial suppliers and used as received.Tb(NO3)3·6H2O was obtained from Siyu Chemical Co.,Ltd.MSwas purchased from Shanghai Jingxi Chemical Technology Co.,Ltd.NaOH,KNO3,NaNO3,Ca(NO3)2,Mn(NO3)2,Cd(NO3)2,HgI2,Cu(NO3)2,Al(NO3)3,Fe(NO3)3,Cr (NO3)3,K2CO3,K2CrO4,KBr,KCl,K2SO4,Ni(NO3)2,Fe(NO3)2, Zn (NO3)2, Mg(NO3)3,Co(NO3)3and 2-(4-(hydroxyethyl)-1-piperazinyl)-ethanesulfonic acid(HEPES)were purchased from China pharmaceutical chemical reagent Co.,Ltd.
Luminescence spectra were collected on an F-4600 spectrometer (Hitachi,Japan)with an excitation spectrum set at 380 nm.UV-Vis absorption spectra were examined on a UV-2550 spectrophotometer(Shimadzu,Kyoto,Japan)using a 1.0 cm quartz cell.Fourier transform infrared (FT-IR)spectra were recorded by an ALPHA FT-IR Spectrometer(Bruker,Germany)with KBr pellets.The luminescent lifetimes was determined on a C11367-11 Quantaurus-Tau(Hamamatsu,Japan).Transmission electron microscopy (TEM)image were measured by JEM-2100 transmission electron microscope (JEOL Ltd.Japan)with operational accelerating voltage being 200 kV.Powder X-ray diffraction (PXRD)patterns were measured by XD-3diffractometer(Beijing Purkinje General Instrument Co.,Ltd.)with Cu Kα radiation (λ=0.154 06 nm)operating at 40 kV and 15 mA with a Kβfoil filter in a range of 5°~90°.Thermogravimetric analysis (TG)was carried on a NETZSCH TG 209 F1 thermogravimetric analyzer at a heating rate of 10℃·min-1under a vacuum atmosphere.Contents of C,H,N were determined by a VarioELcube elemental analyzer.Particle size distribution was obtained by laser nanoparticle size analyzer NPA152.All luminescence images were taken under 365 nm UV lamp.All determination experiments were conducted at room temperature.
1.2 Preparation of salicylate sodium
MS and sodium hydroxide were dissolved in an appropriate amount of absolute ethanol with a molar ratio of 1∶1.After the dissolution was completed,the sodium hydroxide was slowly poured into MS while stirring.After 30 min,the mixture was filtrated,and the precipitate was finally placed in an oven at 60℃,taken out and allowed to cool naturally into a fluffy shape,placed in a desiccator containing silica gel for use.
1.3 Preparation of nano-sized amorphous methyl salicylate terbium (A-MS-Tb)comp lex
In general,2 mL Tb(NO3)3aqueous solution (10 mmol·L-1)and 2 mL MS-Na aqueous solution (10 mmol·L-1)were fully mixed for reacting.One hour later,the white precipitate was separated by centrifuging and washed several times[26].The obtained precipitate was dried in air and re-dispersed in 4 mL HEPES (pH=6.7,0.1 mmol·L-1)to prepare an A-MS-Tb suspension for further measurement and testing.
1.4 Cr 3+sensing
Cr3+detection was carried out under the following experimental conditions.10μL of different concentrations of Cr3+aqueous solution were added to the above prepared nano-sized A-MS-Tb suspension(1 mL,2.5 mg·mL-1,pH=6.7),then incubated for 3 minutes (time experiment excepted)at room temperature.Subsequently,the fluorescence spectrum was collected.
9.3 后熟:早熟品种后熟期20~30天,中早熟品种后熟期30~40天。温度控制在15~18℃,空气相对湿度控制在40%~50%。
2 Results and discussion
2.1 Synthesis and characteristic of nano-sized A-MS-Tb
The transmission electron microscope (TEM)image of A-MS-Tb is shown in Fig.1(a),indicating the morphology of A-MS-Tb is irregular with particle size ranging from 50 to 100 nm.The size distribution obtained by DLS measurement (Fig.1(b))showed that the diameter of A-MS-Tb was 171 nm,which is larger than that observed from TEM,indicating the existing of aggregation.The PXRD of the synthesized A-MSTb is shown in Fig.1(c),and the broad weak peak ranging from 10°to 35°indicates that the assynthesized A-MS-Tb is amorphous.Fig.1(d)is the TG curve of A-MS-Tb.The first weight loss occurred between 30 and 230℃with a weight loss of 9.36%,corresponding to the loss of the moisture or water.The next weight loss of 44.29% is the combustion decomposition of MS.According calculation,the total weight loss was around 53.65%,and the Tb content was about 39.39%.The results of elemental analysis were C 41.23%,H 3.31%,N 0.37%.Given the composition of the isolated A-MS-Tb is Tb9C128H128O48,and the theoretically calculated elemental contents are C 39.76%,H 3.34%,Tb 37.04%,indicating that the isolated solid A-MS-Tb had similar composition as that of the reported C-MS-Tb[30].

Fig.1 Transmission electron microscope image (a),histograms of intensity contribution versus diameter (b),PXRD pattern (c)and TG curve (d)of A-MS-Tb
The luminescence spectra of A-MS-Tb and CMS-Tb were comparatively measured.As shown in Fig.2(a),they exhibited considerable luminescence intensity with a little stronger for A-MS-Tb.The fluorescence spectra of C-MS-Tb and A-MS-Tb dispersed in water over time are shown in Fig.2 (b)with the photos of their suspension under radiation of 365 nm.It is clear that the fluorescence intensity of C-MS-Tb declines very fast in the first 30 minutes because the crystals are poorly dispersed in water,while that of A-MS-Tb remainssubstantially unchanged due to its well dispersion in water.Furthermore,as shown in Fig.2(c),no obvious difference was observed for the luminescence properties of A-MS-Tb in water or EtOH,indicating that the synthesized A-MS-Tb shows high stability towards water,which is different from most of the reported complexes of Tb because the water molecule is a strong quencher for the luminescence of Tb due to the strong vibration of O-H bonds. Therefore, A-MS-Tb can be used as luminescent materials in aqueous medium,which is very important to expand its application scope.
The UV-Vis spectra of A-MS-Tb and MS-Na were measured and comparatively shown in Fig.3(a).It was noticed that the MS-Na aqueous solution had a characteristic absorption peak at 306 nm (ε=2.5×103L·mol-1·cm-1)in the UV region owing to the transition of n electrons in O atom of MS to theπ*orbit.After coordinated by Tb ion,a new absorption peak occurred at around 340 nm accompanied with the peak of 306 nm shifting to 312 nm with a little intensity decreasing.Among them,the 340 nm peak is related to the coordination of phenolic hydroxyl oxygen with terbium ion,and the shift and intensity decrease of the 306 nm peak indicate that the ester carbonyl oxygen is also involved in the coordination with Tb.

Fig.2 (a)Luminescence spectra of C-MS-Tb and A-MS-Tb;(b)Luminescence intensity dispersed in water with time of C-MS-Tb and A-MS-Tb;(c)Luminescence spectra of A-MS-Tb in H2O or EtOH
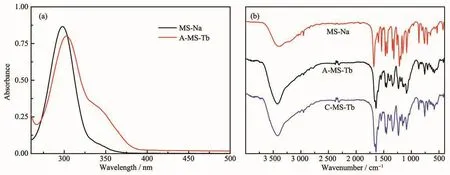
Fig.3 (a)UV-Vis absorption spectra of MS-Na,A-MS-Tb aqueous solution;(b)FTIR spectra of solid MS-Na,A-MS-Tb and C-MS-Tb
Fig.3(b)shows the FT-IR spectra of MS-Na,AMS-Tb and C-MS-Tb.The broad strong band at approximately 3 574 cm-1corresponds to O-H stretching vibrations,whereas the band at 1 641 cm-1is assigned to the H-O-H bending mode.The bands observed in the low-frequency region of the spectrum are interpreted as lattice vibration modes and may be attributed to M-O (850~600 cm-1)[42-43].Compared with the 1 680 cm-1peak on the carboxyl group of MS-Na,the strong absorption peak around 1 666 cm-1for AMS-Tb is attributed to the symmetrical stretching vibration of C=O,and the obvious red shift is due to the coordination with Tb which weaken the C=O bond.The asymmetric stretching vibration of C-O-C on the ester group appearing at about 1 160 and 1 100 cm-1were alsoobserved for the complex,meaning that no direct coordination is found for ester oxygen.The absorption peak of phenolic hydroxyl C-O shifted from 1299 to 1 327 cm-1,which indicates that phenolic hydroxyl oxygen of MS has a strong coordination with Tb3+,accompanied with the ionization of hydrogen ion.It is noticed that the adsorption of the nitrate anions at around 1 380 cm-1was not observed,which allows us to conclude that no nitrate anion exists in A-MS-Tb complex.The other main IR absorption bands correspond to the stretching vibration of C-C and the C-H bending vibration of aromatic rings.
2.2 Lum inescence properties of A-MS-Tb
As shown in Fig.4(a),MS-Na emits blue light(wide band from 400 to 500 nm)under excitation of 380 nm.However,the emission spectrum of A-MS-Tb were centered at 494,549,591,625 nm,which are attributed to the characteristic luminescence of Tb3+,belonging to the energy level transition of5D4→7FJ(J=6,5,4,3)[44].The varnish of blue phosphorescence attributing to MS-Na demonstrates the occurrence of energy transformation from MS to Tb3+.Therefore,AMS-Tb exhibited strong green luminescence.
As showed in Fig.4(b),the decay profiles of the excited states5D4(Tb3+)in A-MS-Tb was determined.The corresponding luminescence lifetimes were obtained by fitting the decay profiles with a oneexponential form.The results show that the lifetime of A-MS-Tb is approximately 0.579 ms,reaching millisecond level,indicating that Tb3+is effectively sensitized by MS and the energy transfer process from MS to Tb3+occurs.
It was reported that the difference between the excited triplet-state energy level (ETSEL)of ligand and the excited state energy level (ESEL)of lanthanide ions is the key factor on the transformation efficiency.According to Latva′s rules,an optimal ligand-to-metal energy transfer process for a lanthanide ion needs ΔE=(E3ππ*-E5D)of 2 500~4 500

Fig.4 Emission spectra of MS-Na,A-MS-Tb aqueous solution (10 mmol·L-1)under excitation of 380 nm (a)and the decay curves of A-MS-Tb and it in the presence of Cr3+ (b)
2.3 Lum inescence quench by Cr 3+
The characteristic green luminescence of A-MSTb gives us the possibility to construct a luminescent probe or sensor.As shown in Fig.5(a),A-MS-Tb had superior response to Cr3+,the luminescence spectra showed obviously change before and after Cr3+was added.

Fig.5 (a)Emission spectra of A-MS-Tb in the absence and presence of 3 μmol·L-1 Cr3+;(b)Absorbance spectra of A-MS-Tb responding to Cr3+with different concentrations
To explain the luminescence change of A-MS-Tb caused by Cr3+,UV-Vis spectra of A-MS-Tb in the presenceof different Cr3+concentrationsweremeasured.As shown in Fig.5(b),the absorbance at 340 nm greatly decreased,while a weak increase was observed at 306 nm.We infer that this absorbance change is caused by the competitive coordination of Cr3+,which weaken the coordination of MSwith Tb3+and perturb the energy transfer process from Tb3+.Besides,the absorbance showed a regular change with the concentration of Cr3+increasing.
The formation of the ground state complex in static quenching tends to cause a change in the absorption spectrum of the fluorescent substance.From Fig.5(b),we find that the absorption spectrum changed significantly after the addition of Cr3+,which imply that the quenching type is of static quenching.Besides,the presence of quenching agent during static quenching does not change the excited state lifetime of the fluorescent molecule.We determined the5D4excited state lifetime of Tb3+before and after the addition of Cr3+by A-MS-Tb (Fig.4 (b)).The5D4excited state lifetime of Tb3+was 0.579 ms.When Cr3+was added,the lifetime was 0.578 ms.The lifetime has not changed significantly.Based on the above two points,we judge that the quenching of A-MS-Tb caused by Cr3+is static quenching type.
2.4 Optim ization of sensor condition
In order to obtain the best sensitivity,the solution pH value and the response time for determination were optimized.As shown in Fig.6(a),with the pH value decreasing from 8.5 to 5.0,especially from 7.5 to 6.5,the luminescent intensity of A-MS-Tb decreased because of the weak acidity of MS which results in the dissociation of A-MS-Tb in acidic condition.Meanwhile,the addition of Cr3+resulted in an evident decrease of luminescence intensity.However,the quench degree of green luminescence by the addition of Cr3+showed an optimum value at pH 6.7(Fig.6(b)),indicating that the largest quenching does not appear at the conditions when the A-MS-Tb in its best stability or instability states,but at the transition state from stable to unstable.Therefore,the optimal pH value is selected at 6.7.

Fig.6 Effect of pH value on PL intensity (a)and intensity variation with and without Cr3+addition (b);Dependence of intensity with the time after adding different amounts of Cr3+ (c)
The results in Fig.6 (c)indicated that the response of A-MS-Tb to Cr3+is also affected by the reaction time.It is clear that after the addition of Cr3+,the luminescent intensity of A-MS-Tb in aqueous suspension solution rapidly decreased at first 2 minutes and reached a platform after 3 minutes.So,the optimal response time was set as over 3 minutes.
2.5 Effect of potentially interfering ions
Good selectivity and anti-interference ability are very important parameter for evaluating a detecting method.To evaluate the selectivity of this detection system,some metal ions (Cr3+,K+,Na+,Ca2+,Mn2+,Cd2+,Cu2+,Hg2+,Al3+,Fe3+,Zn2+,Mg2+,Co3+,Ni2+,Fe2+)and anions (Cl-,Br-,SO42-,CO32-,Cr2O72-)were selected to test their effect on the luminescence of A-MS-Tb and evaluate their interfering for the detection of Cr3+.As shown in Fig.7(a),we found that the relative emission intensity showed obviously decrease after Cr3+was added,while most of other metal ions and anions showed no significant changes,and only Cu2+,Mn2+,Al3+,Fe3+,SO42-had little influence.When Cr3+and potentially interfering ions coexist,A-MS-Tb still maintains a good response to Cr3+.The presence of following amounts of different substances compared with the concentration of Cr3+resulted in less than±5%error:10-folds Cu2+,Fe3+,Hg2+;50-folds Fe2+,Cr2O76-,100-folds Al3+,Ca2+,Cd2+,Ni2+,Co3+,Mn2+,SO42-,CO32-,500-folds Na+,K+,Zn2+,Mg2+,Cl-,Br-.The above results indicated that the proposed luminescence assay possessed excellent selectivity for the detection of Cr3+.We determined the UV absorption spectrum of A-MS-Tb after adding other metal ions,and found that the addition of other ions did not cause a significant change in the absorption spectrum.Only the addition of Cr3+weakened the absorption at 340 nm,which probably because chromium ions weaken the coordination between MS and Tb3+.This also explains why the luminescence of Tb3+was quenched.

Fig.7 (a)Emission intensity of A-MS-Tb in the presence of different potentially interfering substances (3 μmol·L-1,orange column)and the mixture of Cr3+and potentially interfering substances (pink column);(b)Absorption spectra of various substances(Cr3+,K+,Na+,Ca2+,Mn2+,Cd2+,Cu2+,Hg2+,Al3+,Fe3+,Zn2+,Mg2+,Co3+,Ni2+,Fe2+,Cl-,Br-,SO42-,CO32-,Cr2O72-)mixed with A-MS-Tb
2.6 Quantitative analysis of Cr 3+
Under optimized assay conditions at room temperature,quantitatively analysis based on the emission intensity at 549 nm was carried out.As shown in Fig.8,the fluorescence intensity of A-MS-Tb decreased gradually with the increase of Cr3+concentration,and shows a good linear relationship in a range of 0.4~2.8 μmol·L-1.The correlation equation is y=-881.9lg x+489.6 (R2=0.998),where y is the value of intensity and x is the concentration of Cr3+.The detection limit (LOD)calculated by 3S0/K is 35 nmol·L-1,where S0is the standard deviation of blank measurements (n=10)and K is the slope of calibration line.
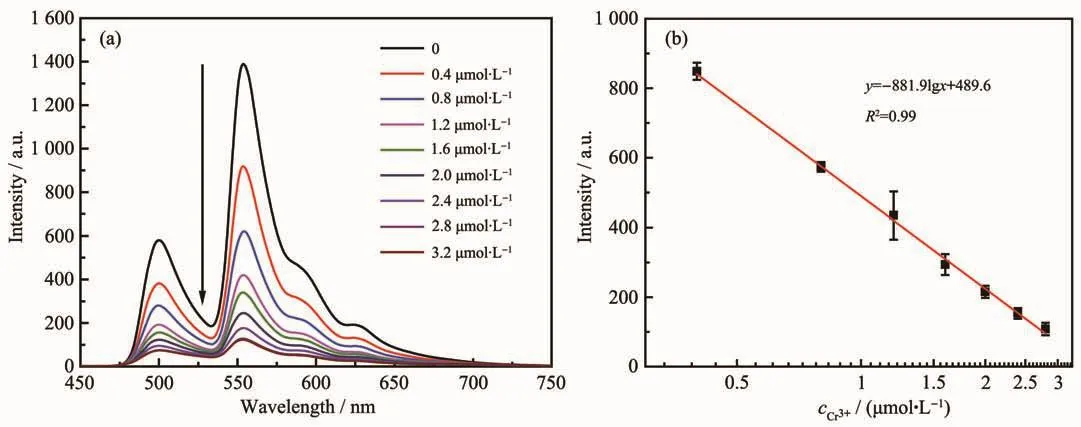
Fig.8 (a)Emission spectra of A-MS-Tb in the presence of varying concentrations of Cr3+from 0 to 3.2 μmol·L-1;(b)Linear relationship between luminescence intensity and the logarithm values of Cr3+concentration
3 Conclusions
In summary,we have developed a new sensing platform for Cr3+by its quenching property for the luminescence of A-MS-Tb,which exhibited good luminescence performance in water and high sensitivity and selectivity towards Cr3+.When Cr3+was added to the water suspension solution of A-MS-Tb,the coordination between MS and Tb3+was weakened.And the luminescence of Tb3+was quenched and exhibited a good linear relationship between the fluorescence intensity and the logarithm value of Cr3+concentration.The quenching mechanism is owing to the complex dissociation promoted by Cr3+,which blocks the energy transformation from MS to Tb3+.In all,simple fabrication and good analytical performance make the probe promising for the detection of complex samples.
Conflict of interest:There are no conflicts of interest to declare.
Acknow ledgments:This work is financially supported by the National Natural Science Foundation of China (Grants No.21161014,51864033).
