Sr 0.955Al2-x B x Si2O8∶0.025Eu2+荧光粉的制备、晶体结构及发光性能
2019-06-06田一光
王 飞 田一光 张 乔
(1安徽三联学院实验中心,合肥 230601)
(2安徽大学化学化工学院,合肥 230601)
(3温州大学化学与材料工程学院,温州 325035)
0 Introduction
Since the advent of high-brightness Blu-ray emitting light-emitting diodes (blue-LEDs)[1],the efficacy of white light-emitting diode lamps(WLEDs,also called LEDs)based on InGaN blue chip and phosphor package has surpassed that of incandescent lamps[2].In the fields of flashlights,desk lamps,traffic lights,signs,decorations,display backlights,etc.,traditional incandescent and cathode fluorescent lamps have begun to be replaced by LEDs[3-5].Currently,the most common LED type is “fluorescence conversion”(pc-WLED),due to the low cost,simple structure,uniform performance and easy commercialization.pc-WLEDs will become an indispensable part of the next-generation lighting industry and display system.High-light color stability of phosphor materials has become a new challenge for researchers as the development of low-cost,high-efficiency and highcolor rendering[6-8].Silicates as a phosphor matrix,which can be excited by near-ultraviolet LED chips,have advantages of high energy efficiency, stable physicochemical properties,cheap and easy to obtain raw materials.Therefore,silicate-based phosphors have become a research hotspot[9-14].SrAl2Si2O8is a framework-like aluminosilicate structure in which silicon(aluminum)oxygen tetrahedrons are connected in a three-dimensional space in a common angular manner,and alkaline earth metal ions present in the voids of the skeleton[15].Recently,the main research focuses on the modulation of fluorescence spectra by adjusting the cation composition in the matrix.The emission peak of Eu2+is linearly red shifted,as Ti4+substituted for Si4+in CaAl2Si2O8[16].The researchers obtained a series of promising cyan,green,and yellow emission (Ba,Sr)3(Si,Al)6(O,N)15∶Eu2+phosphors by changing Sr2+to Ba2+,Al3+to Si4+and N3-to O2-[17].However,little research has been conducted on the changes in crystal structure and spectral changes caused by the changes in skeleton ions in the phosphor matrix.
In this work,a series of Sr0.955Al2-xBxSi2O8∶0.025Eu2+(x=0~0.9)samples were designed and synthesized.According to the X-ray powder diffraction data of the samples,the crystallographic parameters of the samples were calculated by Maud(2.14)software full-spectrum fitting method,and the fluorescence spectrum of the samples were fitted by Gaussian function with Origin 7.5 software.The correlation effect of Bバsubstituted for Alバon its crystal structure and spectral changes were discussed.
1 Experimental
1.1 Synthesis
We synthesized a series of phosphors based on the chemical composition of Sr0.955Al2-xBxSi2O8∶0.025Eu2+,in which x indicated the Bバconcentration.The raw materials were SrCO3(AR),H3BO3(GR),Al(OH)3(AR),H2SiO3(AR),and Eu2O3with a purity of 99.99%.The raw materials were thoroughly mixed with nSr∶nAl∶nB∶nSi∶nEu=0.955 ∶(2-x)∶x∶2 ∶0.025 in an agate mortar by grinding and then placed in a corundum crucible with a lid.Then the mixture was preheated at 1 000℃to the sufficient diffuse and infiltration,with a soaking time of 2.5 h in CO reducing atmosphere,meanwhile,carbon grains were used as a reducing agent and covered the samples during firing.Subsequently,the preheated mixtures were milled sufficiently again after cooling and sintered at 1 200℃for 4 h in CO reducing atmosphere.
1.2 Characterization
The powder X-ray diffraction(XRD)patterns were collected with Bruker D8 advance diffractometer(Cu Kα1,λ=0.154 06 nm),in a Bragg-Brentano reflection geometry with a Sol-X detector over the scattering angle range of 10°≤2θ≤90°,a step of 0.02°,a potential of 40 kV,a current of 40 mA,and a scan speed of 0.12°·min-1.The Rietveld refinement against XRD data was fitted by the Maud(2.14)program.The measurements of photo luminescence were performed by FluoroMax-4 (HORIBA JobinYvon)using a Xe flash lamp with a scan speed of 1 nm·s-1.All of the characterizations were conducted at room temperature.
2 Results and discussion
2.1 XRD analysis
XRD patterns of the samples are shown in Fig.1.All the peaks could be indexed to SrAl2Si2O8phase according to PDF No.38-1454,which indicated that single-phase SrAl2Si2O8∶Eu2+phosphors were obtained,and the co-doped Eu2+and Bバions did not form a new phase besides the host materials.SrAl2Si2O8belongs to monoclinic system(space group C2/m(12)).The peaks of the(112),(220),(002)and(041)planes shifted to higher angles as the increase of Bバ(Fig.2),which might be ascribed to the smaller radius of Bバ(0.023 nm)than Alバ (0.051 nm)[18].When Al were occupied the B sites in the host,the smaller radius result in smaller crystal plane spacing d.According to the Bragg equation:

In the Formula (1),d is the inter planar spacing,θis the diffraction peak angle,n is the diffraction order,andλis the wavelength of the X-ray.The values of d(112),d(220)and d(002)decreased linearly as the increase of the substitution amount of Bバ(Fig.2 and 3).In addition,the non-crystalline content of samples increased as the increase of the substitution amount of Bバ(Fig.1).Topas3 software was used to calculate the XRD data of the samples,and the results show that the crystallinity of the samples were gradually decreased after the addition of Bバ,indicating the glass samples were generated with the increase of the boron(Fig.4).The crystallinity of the sample with x=0.9 was reduced by 37.4%compared to the sample with x=0.
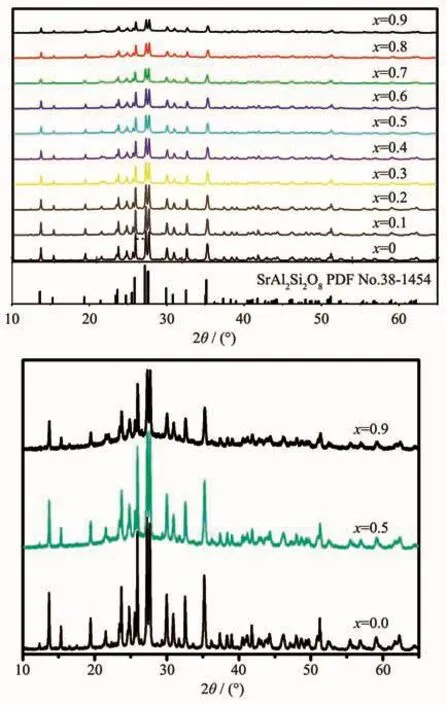
Fig.1 XRD patterns of Sr0.955Al2-x B x Si2O8∶0.025Eu2+(x=0~0.9)phosphors
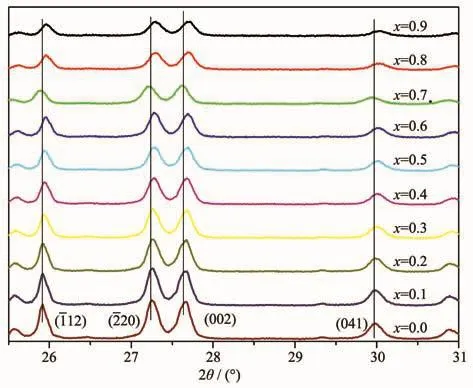
Fig.2 XRD patterns of Sr0.955 Al2-x B x Si2O8∶0.025 Eu2+(2θ=25.5°~31°)phosphors
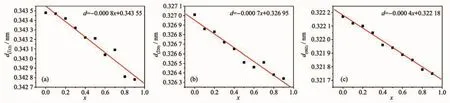
Fig.3 (a)d(111),(b)d(220)and(c)d(002)of Sr0.955Al2-x B x Si2O8∶0.025Eu2+(x=0~0.9)phosphors varied with x

Fig.4 Crystallinity of Sr0.955Al2-x B x Si2O8∶0.025Eu2+(x=0~0.9)phosphors varied with x
The grain size of each powder sample was calculated by the Scherrer formula from the half width of the diffraction peak at 2θ=27.248°of the sample:

In the Formula (2),L (nm)is the grain size in the direction perpendicular to the emitting surface(hkl);K is Scherrer constant,and the value is 0.89;W(rad)is FWHM of the sample diffraction peak.The value of W of the sample with x=0 is 0.005 2 rad,and the value of each parameter was brought into the Formula(2)which calculated that L is 46.004 4 nm;when x=1.0,W of the sample is 0.004 1 rad and the calculated L is 33.520 2 nm.The calculation results show that the grain size of the sample decreased as the increase of the substitution amount of Bバ.
Cell dimensions of the compositions in the system were calculated by the full pattern fitting method(Fig.5).The crystal structure refinement of Sr0.955Al1.9B0.1Si2O8∶0.025Eu2+was done by taking SrAl2Si2O8as the starting model with space group I2/c[19].The observed,calculated,and difference patterns are shown in Fig.6.Additionally,the calculated residual factor values Rb,Rwp,Rexpand sig were 6.20%,8.16%,4.95%and 1.68,respectively,indicating good refinement quality.
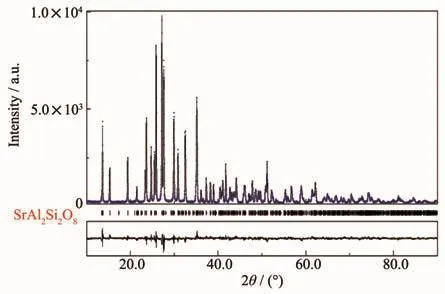
Fig.5 Observed(blue dashed line)and calculated(black line)XRD patterns of Sr0.955Al2B0.1Si2O8∶0.025Eu2+as well as the difference profile(bottom line)between them;Bragg reflection peak positions are shown as vertical bars;An ideal crystal structure is also shown in the inset
According to XRD patterns of the samples,both the lattice parameters(a,b,c,β)and unit cell volume(V)of Sr0.955Al2-xBxSi2O8∶0.025Eu2+(x=0~0.9)phosphors were calculated by the full pattern fitting method.It shows that the a,b,c and V of Sr0.955Al2-xBxSi2O8:0.025Eu2+(x=0~0.9)phosphors decreased linearly,and βincreased linearly with the increase of x(Fig.6),which may be attributed to the smaller radius of Bバthan Alバ It could be further explained that the Alバwere occupied by the Bバsite in the host which was benefit to reduce the volume from [AlO4]to[BO4],thus resulting in a decrease in unit cell parameters and volume.However,the change rate and variable quantity of lattice parameters are not consistent(Fig.6).With the substitution amount of Bバincreased from 0 to 0.9,the parameters a,b,c and V decreased by 0.000 9,0.002 1 and 0.002 5 nm,respectively.The variation range of c is significantly larger than a and b.βincreased linearly with the increase of x.
According to the standard crystal structure model data of SrAl2Si2O8[19],the structure diagram was made by using Diamond (3.1)software.In SrAl2Si2O8,the silicon(aluminum)tetrahedra constituted the skeleton of a,b plane direction along the c-axis layering.Sr2+ions arranged between the skeleton layers (Fig.7).It could be seen that the density of aluminum tetrahedron arranged on the c-axis direction was the highest,and that arranged on the b-axis was followed and a-axis was minimum.Therefore,the variation range of c was significantly larger than a and b as the increase of x.The(001)and(100)crystal plane constituted the crystal plane angle(β).The difference of change rate between the a-axis and c-axis caused the amplitude of the(001)plane changed to be larger than(100)plane,resulting in the increase ofβ.
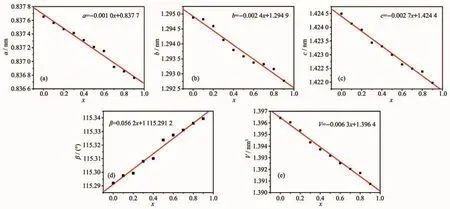
Fig.6 Lattice parameters and unit cell volumes of Sr0.955Al2-x B x Si2O8∶0.025Eu2+(x=0~0.9)phosphors varied with x
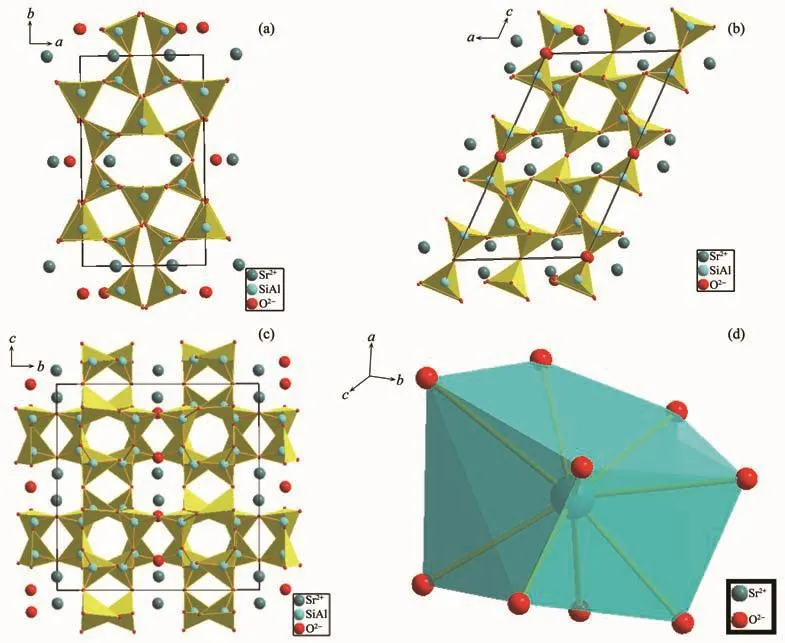
Fig.7 Illustrations of crystal structures of SrAl2Si2O8
2.2 Fluorescence spectra analysis
The excitation spectrum of Sr0.955Al2-xBxSi2O8∶0.025Eu2+located around 225~400 nm.The maximum emission wavelength located at 350 nm and FWHM was 90 nm.The four excitation peaks(270,314,340 and 364 nm)when x=0 were fitted by Gauss.With the substitution amount of Bバincreased from 0 to 0.9,the positions of the fitted peaks at 270 and 314 nm remained unchanged,however,the peak intensity decreased at 270 nm and increased at 314 nm.The excitation peaks at 340 and 364 nm showed clear blue-shift.The peak intensity increased at 340 nm and significantly reduced at 364 nm,making the apparent excitation peak width at half maximum increased to 102 nm(Fig.8).
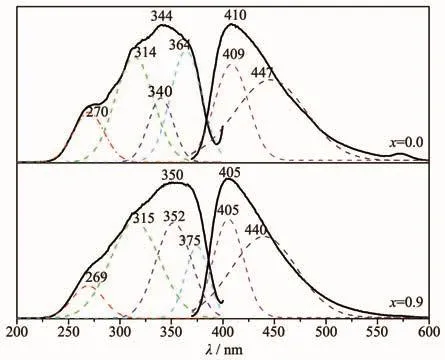
Fig.8 Luminescence spectra of Sr0.955Al2-x B x Si2O8∶0.025Eu2+
Luminous characteristics of Eu2+was studied in the sulfides,halides and aluminate matrix by Van Uitert[20].Studies have shown that the 5d excited state band edge of Eu2+has an effect on its coordination number,and the empirical formula is as follow:
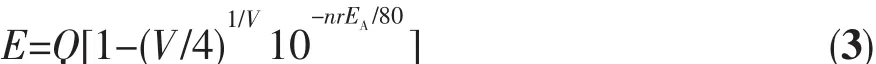
Equation(3)was taken the logarithm of both sides of Equation(4):

where E represents the position of the d-band edge in energy for the rare-earth ion(cm-1),here,the emission peak of Eu2+is 410 nm,so E is 24 390 cm-1;Q represents the position of the lower d-band edge in energy for the free ion,Q is 34 000 cm-1for Eu2+;V is the valence of the ‘active’ cation,here Eu2+as the activator ions,so V is 2;EA(eV)is the electron affinity of the atoms that form anions.Van Uitert′s research shows that the value of EAis related to the type of coordination ion,and when the species of coordination ions are different,Eais the smaller in the coordination ions.The EAof simple compounds of oxygen is 1.17 eV,and the EAof Y3Al5O12and GdAlO3aluminate system is 1.60 eV.EAincreased as the increase of the amount of charge in the central atom in the oxyanion group.For example SiO44-,PO43-and WO42-have higher EAvalues.Because SrAl2Si2O8belongs to aluminum silicate system,in which the Sr2+taken by the activator has two ligands,silicate and aluminate,EAvalue is 2.65 eV.In the crystal,n is the number of coordinated anions and r is the radius of the host cation replaced by the‘active’ cation.The above-mentioned parameter values were brought into the Equation(4):nr=1.227 0,the nine-coordinated Sr2+ion radius is 0.131 nm and the calculated coordination number is 9.36,which can be inferred that Sr2+is nine-coordinated in SrAl2Si2O8.
The emission spectrum,which was also a broadband between 370 and 600 nm,could be fitted by two peaks at 409 and 447 nm,and its apparent peak located at 409 nm.The apparent peak was blue-shifted from 410 to 405 nm as well as the two fitted apparent peaks were blue-shifted linearly with the increase of the substitution amount of Bバ),and the slopes of the lines are-3.944 0 and-6.814 3,respectively(Fig.9).The emission peak area of luminescence center Eu2+1in 409 nm (AEm1)and the emission peak area of luminescence center Eu2+2in 447 nm(AEm2)decreased linearly with the increase of the amount of the substitution amount of Bバ(Fig.10).The rate of change of the displacement and intensity of the fitted peak at 447 nm was larger than at 409 nm,indicating that Bバentered into the lattice and has a greater impact on the luminescence centers at 447 nm.When the substitution amount of Bバwas 1.0,two fitting peak positions were blue-shifted to 405 and 440 nm,and FWHM reduced from 74 to 65 nm.
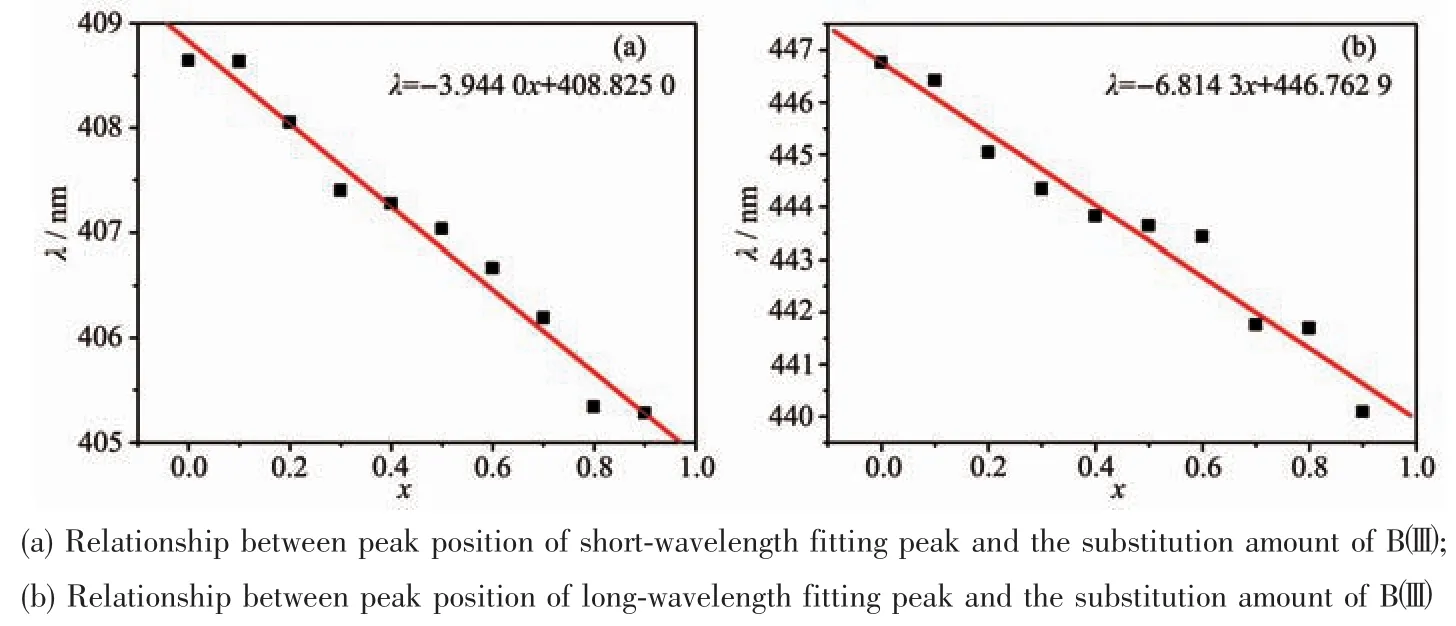
Fig.9 Relationship between spectra position of Gaussian fitted and the substitution amount of Bバ

Fig.10 Area ratio of emission spectra from two luminescent centers vared with the substitution amount of Bバ
2.3 Luminescence mechanism of Eu2+
The luminescence mechanism of Eu2+in the matrix crystal SrAl2Si2O8can be well illustrated by the bit-shaped coordinate model diagram.The luminescence of Eu2+is mainly due to the transition of 4f65d→4f7electrons.Since Eu2+entered the SrAl2Si2O8,two different energy levels of 4f65d were formed,resulting in the existence of two different luminescent centers of Eu2+land Eu2+2in the phosphor SrAl2Si2O8∶Eu2+.Therefore,when the electrons transfered from the excited state back to the ground state level,the energy released was different,and the wavelength at which the fluorescence was generated was different.Eu2+lwas in a higher excited state energy position and produced a short wavelength emission of 406 nm;Eu2+2was in a position with low excited state energy and produced a long wavelength emission of 447 nm(Fig.11).Esis the energy difference between the lowest energy levels of Eu12+and Eu22+.
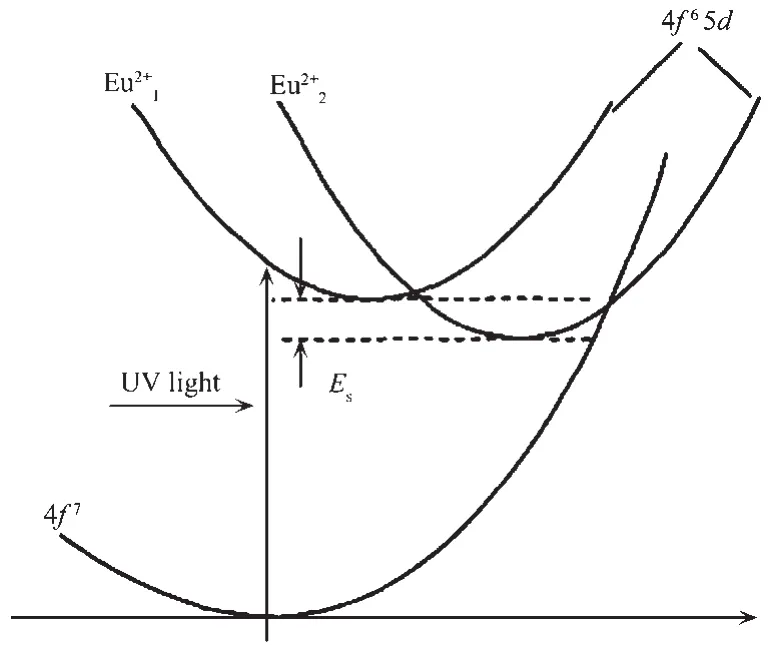
Fig.11 Configuration coordination model for the Eu2+l and Eu2+2 sites in the SrAl2Si2O8∶Eu2+
The 4f65d configuration of Eu2+electrons was formed by the strong coupling of 4f and 5d electrons under the action of electrostatic force.The 5d electrons of Eu2+did not have shielding effect and were in a bare state,so they were sensitive to the changes in the surrounding crystal field environment[21]. With the increase of the substitution amount of Bバ,the number of titanium oxide tetrahedrons[BO4]formed in the matrix crystal gradually increased,because the volume of titanium oxide tetrahedron[BO4]is smaller than that of the silicon tetrahedron[AlO4].The gap between the tetrahedrons increased,and the distance between the luminescence center Eu2+in the gap and the ligand tetrahedron became larger.According to the fifth power of the crystal field intensity(Dq)around Eu2+and the distance(R)from Eu2+to O,the Dqof Eu2+wasweakened and the 5d energy level splitting became smaller[22],making the lowest emission level of Eu2+shifted downward.The center of gravity of the emission level shifted upward,resulting in a blue shift of the two fitted peaks of the 4f65d→4f7emission band of the luminescence center Eu2+.
3 Conclusions
The complete solid solutions were formed in the whole range ofx=0~0.9,as Bバ substituted for Alバentered to SrAl2Si2O8.The lattice parameters (a,b,c)and unit cell volume(V)of Sr0.955Al2-xBxSi2O8∶0.025Eu2+(x=0~0.9)phosphors decreased linearly,the crystal plane spacing(112),(220),(002))and lattice parameter(β)increased linearly with the increase of the substitution amount of Bバ.
The broad-band excitation spectrum of the samples could be fitted to four peaks which were in the range of 225~400 nm,and the apparent peak was around 350 nm.The emission spectrum was a broad-band in the range of 370~600 nm,which could be fitted by two peaks at 409 and 447 nm,and its apparent peak was at 409 nm,FWHW was 74 nm,and Sr2+was ninecoordinated in SrAl2Si2O8.As the substitution amount of Bバincreased,the center of gravity of the emission level shifted upward,resulting in a blue shift of the two fitted peaks of the 4f65d→4f7emission band of the luminescence center Eu2+,and FWHW was reduced from 74 to 65 nm.
Acknowledgements:This study was supported by the National Natural Science Foundation of China (Grant No.20771086),the Key projects of the Department of Education of Anhui Province(Grant No.KJ2016A893),the Support Project for Outstanding Young Talents in Colleges and Universities of Anhui Province(Grant No.gxyq2018126),the Scientific Research Project of Anhui Sanlian University (Grant No.KJZD2018004,2015Z001,2013Z001),the Quality Engineering Project of Anhui Sanlian University(Grant No.15zlgc008,16zlgc060,17zlgc030),and the National College Students Innovation and Entrepreneurship Training Program (Grant No.201710959055,201710959074,2018109050).
