A practical approach to (2R,3R)-2,3- dimethoxy-1,1,4,4-tetraphenyl-1,4-diol
2019-04-04HUXiaoyunYINZhongyouTANGYanliuWULamei
HU Xiaoyun, YIN Zhongyou, TANG Yanliu, WU Lamei
(College of Chemistry and Materials, South-Central University for Nationalities, Wuhan 430074, China)
Abstract A practical approach to(2R,3R)-2,3-dimethoxy-1,1,4,4-tetraphenylbutane-1,4-diol, a C2 chiral diol, was developed based on highly regioselective 2,3-methylation of(2R,3R)-1,1,4,4-tetraphenylbutaneol with MeI/NaH. The results indicated that compared with the literature, this procedure avoided the treatment with acid, reduced the generation of poly-substituted THF derivative, and increased the yield. This report not only provided a practical approach to prepare(2R,3R)-2,3-dimethoxy-1,1,4,4-tetraphenylbutane-1,4-diol, but also presented a significant reference for the regioselective derivatization of(2R,3R)-1,1,4,4-tetra-substituted butanetetraols.
Keywords selective methylation;(2R,3R)-1,1,4,4-tetraphenylbutaneol;(2R,3R)-2,3-dimethoxy-1,1,4,4-tetraphenylbutane-1,4-diol;cycloetherization
A variety ofC2-symmetric chiral diols such as enantiopure 1,1′-bi-2-naphthols[1](BINOLs) andα,α,α′,α′-tetraaryl-2,2-dimethyl-1,3-dioxolan-4,5-dimethanols[2,3](TADDOLs), have been found to be excellent chiral inducers in different types of asymmetric transformation[4]. Thus the synthesis ofC2chiral diols is of interest to synthetic chemists.(2R,3R)-2,3-dimethoxy-1,1,4,4-tetraphenylbutane-1,4-diol1, which is aC2symmetrical chiral diol derived from dialkylL-tartrate, has attracted considerable attention due to its applications as a host compounds with gusts aniline[5], toluidine[6]and xylene[7]in supramolecular chemistry.
(2R,3R)-1was firstly synthesized and utilized as chiral host by Toda in 1993, in which(2R,3R)-1 was obtained in 18% yield via two step reactions from diethylL-tartrate[8].The low yield of(2R,3R)-1 in this synthesis impels us to develop a practical approach to(2R,3R)-1.(2R,3R)-1,1,4,4-tetraphenylbutaetetraol 2, which was firstly synthesized in 2010 by our group[9], is the parent compound of TADDOL, their hydroxyl group composition determines that they have rich reaction chemistry. During the past decade, our group has investigated regioselective derivatization of(2R,3R)-2and highly regioselective 1,4-cycloetherization[10], 1,3-cycloboration[11]and 2,3-spiroboration[12]2,3-sulfitation[13,14](Fig.1). Among these investigations,(2R,3R)-1,4-dimethoxy-1,1,4,4-tetraphenylbutane-2,3-diol3, which has been widely used as a chiral auxiliary in chiral boronic acids chemistry[15], could be readily prepared by highly regioselective 2,3-sulfitation[13]and 2,3-spiroboration[12]of(2R,3R)-2. In this work, we reported a practical procedure for preparing(2R,3R)-1via highly regioselective 2,3-methylation of(2R,3R)-2with NaH/MeI based on the differences in reactivity of secondary and tertiary hydroxyl groups and stereo-hindrance effect. It was found that the aftertreatment of regioselective 2,3-methylation reaction with different medium would afford different products, which may be related to the low yield of(2R,3R)-1in Toda′s synthesis.
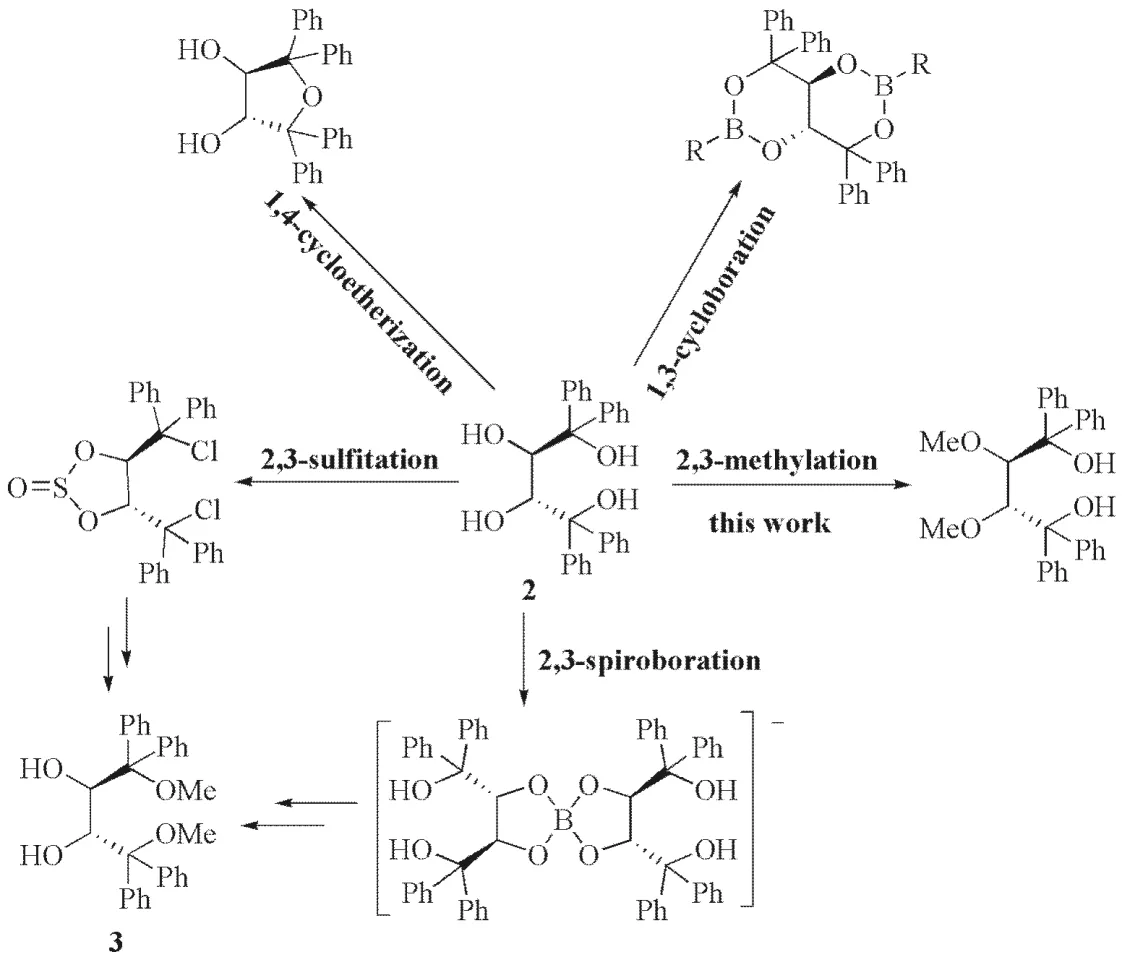
Fig.1 Synthesis of(2R,3R)-1 and regioselective derivation of(2R,3R)-2 图1 (2R,3R)-1的合成及(2R,3R)-2的区域选择性衍生化
1 Experimental
1.1 Reagents and apparatus
(2R,3R)-diethyl tartrate was prepared from(2R,3R)-tartaric acid and ethanol. Sodium hydride and methyl iodide were purchased and used directly.
1H and13C NMR spectra were performed on a Varian Mercury VS 300. MS was recorded on a VG ZAB-HF-3F spectrometer. Optical rotations were measured on a PE-341 Mc polarimeter. Melting points were determined on a VEB Wagetechnik Rapio PHMK05 instrument and were uncorrected.
1.2 Preparation of(2R,3R)-2
Under Ar, a freshly dried two-necked round bottomed 250 mL flask equipped with a magnetic bar, 100 mL pressure-equalizing dropping funnel and reflux condenser with oil seal were charged with Mg turnings(2.64 g, 0.11 mol) and dry THF(20 mL). The pressure-equalizing dropping funnel was charged with a solution of phenyl bromide(10.5 mL, 0.1 mol) in dry THF(40 mL). A quarter of the solution was added to the two-necked round bottomed and stirred vigorously. After the initiation of the Grignard reaction, the solution was added dropwise so that THF was gently refluxing. After complete addition, the mixture was continued to stir vigorously for 2 h and refluxed for an additional 1 h, then cooled to 0 ℃, followed by carefully dropwise addition of a solution of(2R,3R)-diethyl tartrate(2.6 g, 12.5 mmol) in 20 mL dry THF under vigorous stirring. After addition, the mixture was allowed to stir for additional 1 h, and then warmed up to reflux for 1.5 h. After cooled to r.t., saturated aqueous NH4Cl was added with stirring. The organic layer was separated and the aqueous layer was extracted with Et2O(10 mL×3), the combined extracts were dried over Na2SO4, followed by removal of most of the solvent, then steam distillation was carried out. The residue was recrystallized with 80% ethanol to yield 2.6 g of(2R,3R)-2as colorless crystals, 48% yield, m.p. 149-150 ℃9. [α]D20=+154.2(c0.5, CHCl3).1H NMR(300 MHz, CDCl3):δ7.37-7.13(m, 20H), 4.61(s, 2H, disappeared after adding D2O), 4.45(d,J=5.1 Hz, 2H), 3.74(d,J=4.5 Hz, 2H, disappeared after adding D2O).13C NMR(75 MHz, CDCl3): 143.8, 142.7, 134.6, 131.5, 129.3, 128.3, 128.2; 127.9, 127.5, 126.7, 125.5, 81.1, 69.7.
1.3 Preparation of(2R,3R)-1
A dried round bottom flask were charged with(2R,3R)-2(0.459 g,1.07 mmol),sodium hydride(0.053 g,2.2 mmol) and dried THF(12 mL). The mixture was stirred at room temperature for 2 h,and then added methyl iodide(0.304 g,2.14 mmol) and stirred overnight at room temperature. To the stirred reaction mixture was added distilled water(10 mL) and diethyl ether(15 mL) to form a two phase solution. The upper organic phase was separated,dried over anhydrous sodium sulfate,then the solution was concentrated to dryness and the solid residue was recrystallized ethanol to afford a colorless crystal of(2R,3R)-1(0.387 g),89% yield,m.p. 123-125 ℃(Lit.8m.p. 125-126 ℃),[α]D25= -151(c0.8,CHCl3).1H NMR(300 MHz,CDCl3): 7.63-7.55(m,8H,Ph-H),7.43(t,J=7.5 Hz,4H),7.32-7.12(m,8H),4.91(s,2H,disappeared after adding D2O),4.43(s,2H),2.53(s,6H).13C NMR(75 MHz,CDCl3): 145.9,145.0,128.7,128.2,127.5,127.0,126.3,126.2,126.1,85.62,85.57,80.19,61.35,61.26. ES-MS: 454([M455-1]+).
1.4 Preparation of(3R,4R)-4
According to similar procedure to the preparation of(2R,3R)-1, the mixture resulting from the reaction of(2R,3R)-2, sodium hydride and methyl iodide in THF was worked up with 2 mol/L HCl, stirred to form a deep brown solution. To it was added with excess Na2S2O3with stirring to furnish a yellowish solid-liquid mixture, filtered, the solid was extracted with Et2O, and the combined organic phases were dried over anhydrous sodium sulfate, concentrated, and then recrystallized from ethanol to afford a colorless crystal of(3R,4R)-3,4-dimethoxy-2,2,5,5-tetraphenyltetrahydrofuran4, 83% yield,m.p. 170-172 ℃(Lit.16m.p. 170-172 ℃), [α]D25= -207(c0.6, CHCl3).1H NMR(300 MHz, CDCl3): 7.54(d,J=7.8 Hz, 4H), 7.39-7.30(m, 8H), 7.18-7.10(m, 8H), 4.51(d,J=6.9 Hz, 1H), 4.45(s, 2H), 3.65(s, 6H).13C NMR(CDCl3, 75 MHz): 145.7,143.0,127.8,127.5,127.7,126.7,126.3,126.1,88.0,83.9,59.1. HRMS: Cacld. for C30H28O3: 436.2038,found: 436.2042.
2 Results and discussion
During our ongoing research on the chemistry ofchiral 1,1,4,4-tetrasubstituted butanetetraols, selective 2,3-methylation of(2R,3R)-2was investigated. To a dried THF solution of(2R,3R)-2was added with sodium hydride(2.1 equiv), and stirred at room temperature for 0.5 h, followed by adding methyl iodide and reacted overnight. The reaction mixture was worked up with distilled water and diethyl ether, the organic phase was separated and evaporated, and the residue was recrystallized in ethanol, a colorless crystal was afforded. Its ES mass spectra showed the ionic peak of 454 corresponding to C30H30O4, namely, it might be a dimethylation product of(2R,3R)-2. The1H and13C NMR spectra revealed that this molecule had a symmetric structure, and was in full conformity with those of(2R,3R)-1. Its melting point and specific rotation were also identical with the reported ones[8]. It could be concluded that the product was(2R,3R)-1.
Interestingly, if the reaction mixture resulting above was worked up with diluted hydrochloric acid, a deep brown solution was obtained.Investigation found that the brown solution was caused by the oxidation of I-in the acidic medium. After treatment with Na2S2O3, a novel product rather than(2R,3R)-1was obtained. The1H and13C NMR spectra revealed that the novel product also had a symmetric structure, and two tertiary hydroxyl groups disappeared. Its ES mass spectrashowed the ionic peak of 437 corresponding to C30H28O3. Compared to the ES mass spectra of(2R,3R)-1, one molecule of H2O was lost. Based on these spectra data, we deduced the novel product was(3R,4R)-4(Fig.2), and its melting point and specific rotation were also identical with the reported ones[16].
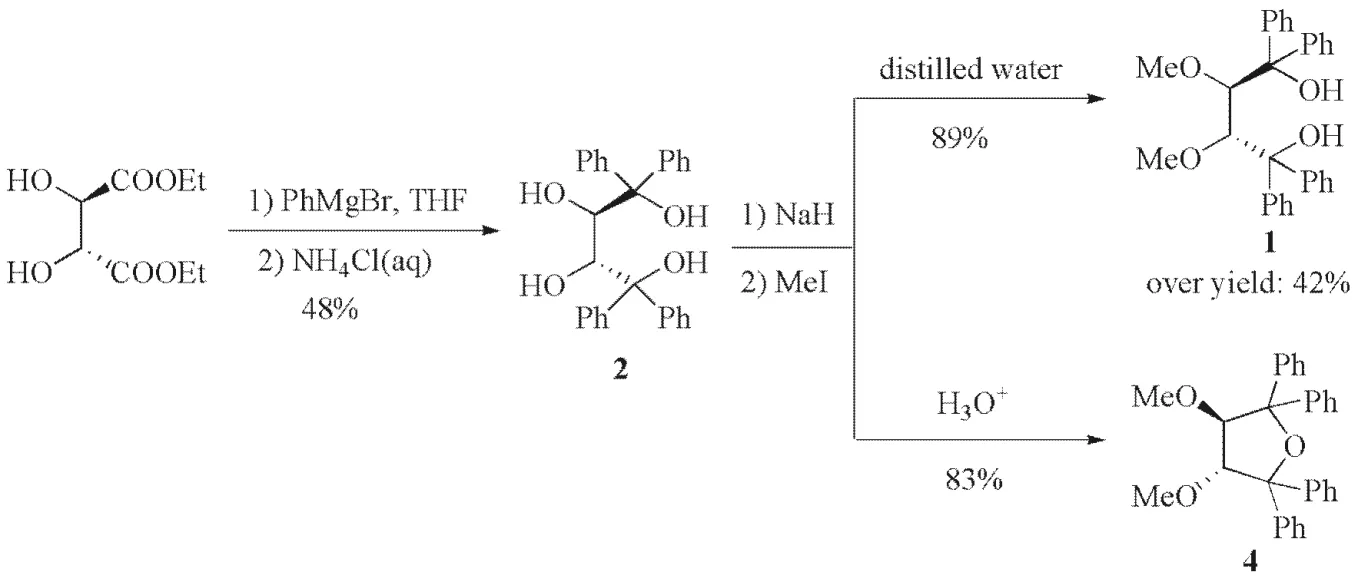
Fig.2 Regioselective 2,3-methylation of(2R,3R)-2图2 (2R,3R)-2的区域选择性2,3-甲基化
As shown in
Fig.3, a possible mechanism for the formation of(3R,4R)-4in acidic medium was proposed. One tertiary hydroxyl would be firstly protonated in acidic medium, and then another tertiary hydroxyl attacked this tertiary carbon and one molecular of H2O was lost and afforded(3R,4R)-4.
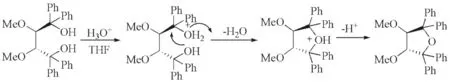
Fig.3 Possible mechanism for generating(3R,4R)-4图3 生成(3R,4R)-4的可能机理
Considering the forming of poly-substituted THF derivative(3R,4R)-4by treatment with acidic medium, we deduced the low yield of(3R,4R)-4in Toda′s synthesis might be closely related with the aftertreatment with acidic medium(Fig.4). Acidic medium is usually required in the aftertreatment of the Grignard reaction, and side reactions brought by acidic medium undoubtedly lead to the low yield. In this work, highly regioselective 2,3-methylation of(2R,3R)-2was achieved based on steric hinderance of phenyl substituents and the different reactivity of the secondary and tertiary hydroxyls of(2R,3R)-2. This synthetic protocol avoids the aftertreatment with acidic medium and efficiently improves the yield of aimed product.
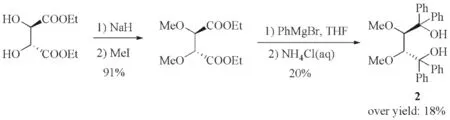
Fig.4 Synthesis of(2R,3R)-1 by Toda′s procedure 图4 Toda制备(2R,3R)-1的合成方案
3 Conclusion
In summary, based on the differences in reactivity of secondary and tertiary hydroxyl groups and stereo-hindrance of tetrasubstituents, a practical approach toC2chiral diol(2R,3R)-1was developped via the highly regioselective 2,3-methylation of(2R,3R)-2with NaH/MeI. A poly-substituted THF derivative(3R,4R)-4tended to be formed if the reaction was treated with acidic medium. These results not only provide us a practical approach to(2R,3R)-1, but also give us important information for functional groups transformation of chiral 1,1,4,4-tetrasubstituted butanetetraols.
