A composite absorption liquid for simultaneous desulfurization and denitrification in flue gas
2019-02-09
School of Chemical Engineering,Changchun University of Technology,Changchun 130000,China
Keywords:Flue gas Absorption Chemical reaction Reaction mechanism NaClO/KMnO4
ABSTRACT A composite-liquid absorbent(CLA),NaClO/KMnO4,for simultaneous desulfurization and denitrification(SDD)was studied in a homemade bubbling reactor.The experimental results showed that the CLA configured by sodium hypochlorite(NaClO)and potassium permanganate(KMnO4)had a very good synergistic effect on SDD.The effects of NaClO concentration(CNa),KMnO4concentration(CK),gas space velocity(Vg),initial pH value,and temperature of the absorption liquid(Ts)on efficiencies of the SDD were investigated.Under the optimal reaction conditions,the best removal efficiencies were 100%for sulfur dioxide(SO2)and above 94%for nitric oxide(NO).The ion chromatography and titration were used to analyze the changes of both the ion species and concentrations in the liquid before and after the reaction.According to the experiment results and related literature,the reaction mechanism of the SDD based on the CLA was proposed.
1.Introduction
With the rapid development of economy and society,the demand for energy,especially for the coal energy,is increasing sharply.In 2014,73.2%of the world's energy consumption was produced by coal[1].The main source of SO2and NO in atmosphere is the combustion of coal,which is the important precursor of acid rain pollution.Furthermore,NO can react with oxygen(O2)to form nitrogen dioxide(NO2)which is an important source for photo-chemical smog formation[2].At present,wet flue gas desulfurization(WFGD)and selective catalytic reduction(SCR)are widely used in large enterprises,for example,coal-fired power stations for desulfurization and denitrification[3].However,the further development of these technologies will be limited due to their complex system,large occupying land,high equipment investment and operating costs,etc.[4,5].Alternatively,selective non-catalytic reduction(SNCR),circulating fluidized bed(CFB)system,electric beam(EB)and non-thermal plasma(NTP)technologies are also common treatment methods for flue gas.However,these technologies have many disadvantages such as low removal efficiency and high operating and power costs[3-12].Therefore,it is necessary to develop a low-cost,high-efficiency process for desulfurization and denitrification.Recently,SDD was extensively investigated by many researchers.Zhao et al.[6]used ferrate(VI)solution as an absorbent for SDD experiments in the CFB reactor.It was found that,under optimal operating conditions,the removal efficiencies were 96.1%for SO2and 67.2%for NO,respectively.Although an excellent desulfurization efficiency was achieved,its denitrification efficiency was far below the current requirements of environmental protection.Hu et al.[7]used sodium humate solution as an absorbent for SDD experiments in a bubbling reactor.The removal efficiencies of SO2and NO2are 98%and 95%,respectively.However,it's difficult for sodium humate to oxidize NO which was the main nitrogen oxide in the flue gas.Therefore,in the practical application,an additional process is needed to remove NO in the flue gas.Pourmohammadbagher et al.[8],Fang et al.[9]and Zhao et al.[10,11]used sodium chlorite(NaClO2)as an oxidant for the SDD of the flue gas.The experimental results show that the NaClO2solution has a positive effect on SDD.But under acidic conditions,NaClO2will generate chlorine dioxide(ClO2)and chlorine(Cl2),which cause the secondary pollution of the atmosphere.

Furthermore,Zhao et al.[13]investigated the removals of SO2and NO by a complex oxidant composed of hydrogen peroxide(H2O2)and sodium persulfate(Na2S2O8),the flue gas reacts with gas-phase H2O2and Na2S2O8firstly,and subsequently with the alkali solution.Zhang et al.[14]employed the same complex oxidant for SDD in a bubbling reactor.According to results of the two reactions for SDD,the bubbling reactor is better than that of the steam evaporation,but the desired removal conversions are not achieved for both of them.Sun et al.[15]connected two bubbling reactors in series,into which a nitric acid(HNO3)solution and a sodium hydroxide(NaOH)solution were added,respectively.Under the optimal operating conditions,the removal conversions of SO2and NO were 100%and 81%,respectively.Although the removal efficiency was desired,the concentration of nitric acid required for the reaction is too high to protect the equipment to a large extent,which increases the investment of the equipment.It can be seen from the above that the use of NaClO2alone had better removal conversions for SDD in the flue gas,but it can cause the secondary pollution.In order to further improve the removal efficiency for the flue gas and reduce the formation of Cl2and ClO2,some researchers prepared a few composite absorbents containing NaClO2and carried out their SDD experiments.Hao et al.[16],Wang and Zhong[17]and Zhao et al.[18]added Na2S2O8,NaClO and H2O2into the NaClO2solution,respectively.From the experimental results,although the removal efficiencies of the SDD have been improved,the secondary pollution has not been solved,so it is necessary to find a new oxidant.Du et al.[19]used a FeIIEDTA solvent in a double-stirred reactor to remove NO and obtained the corresponding kinetic formula.Although there is a good denitrification efficiency,the optimum pH value is 1.5-2.5,so there is a very high requirement for corrosion resistance of the equipment in its actual production.Zhao et al.[20]used KMnO4solution for SDD experiments in a CFB system.Under the optimal conditions,the conversions of SDD are 94.5%and 64.2%for SO2 and NO,respectively.Although the desulfurization effect was satisfactory,the removal efficiency of NO was not up to the standard.In addition,some scholars used ozone[12,21,22],hydroxyl radicals(·OH)[23],etc.for SDD in flue gas.Currently,due to the equipment and operating costs,these technologies are only at the laboratory stage and have not been used in industrial applications.Han et al.[24]used NaClO solution for NO oxidation-absorption experiments in a countercurrent-spraying reactor.According to the experimental results,the average removal conversion of NO was 74%.It can be seen that the NaClO solution still maintains a high removal efficiency of NO after a long-time operation,so it has a great potential in industrial applications.Li et al.[25]prepared an absorbent with KMnO4and NaClO for the simultaneous absorption of SO2and NO in a spray tower.Under its optimal operating conditions,the removal conversions of SO2and NO were 98.8%and 70.9%,respectively.Although the removal efficiency is still not satisfactory,it is proved that the composite absorption liquid composed of KMnO4and NaClO can be applied to the SDD,which gave a great inspiration to this paper.In addition,a lot of oxidants were used for the removal of SO2and NO,such as Fenton-like reagent[26],ethylenediamine[27],calcium hypochlorite(Ca(ClO)2)[28],and triethylenetetraminecobalt[29].Among the oxidants mentioned above,NaClO possesses the most prospect in industrial application due to its low cost,strong oxidizing and being easy to store and transport[24].However,only a few scholars have studied the use of NaClO solution for the SDD.
In this paper,the effect of NaClO/KMnO4solution on the SDD was firstly investigated in the homemade bubbling reactor.And then,the initial pH value and temperature of the absorption liquid,concentrations of KMnO4and NaClO as well as gas flow were determined.Finally,the reaction mechanism was proposed.
2.Experimental
2.1.Experimental apparatus

Fig.1.Schematic diagram of the experimental system.(1)N2cylinder,(2)SO2cylinder,(3)NO cylinder,(4)globe valve,(5)mass flow controller,(6)gas mixer,(7)homemade bubbling reactor,(8)gas distributor,(9)constant temperature water bath,(10)flue gas analyzer,(11)vent.
The experimental device and connection as shown in Fig.1,include a simulated flue gas system,a reaction system,a temperature control system and a flue gas analysis system.The simulated flue gas system includes a pure nitrogen(N2)gas cylinder(Changchun Juyang Gas CO.,LTD,purity 99.9%),a pure SO2gas cylinder(Dalian Special Gases CO.,LTD,purity 99.9%),a pure NO gas cylinder(Dalian Special Gases CO.,LTD,purity 99.9%),a gas mixer and four mass flow controllers.The reaction system is a laboratory-scale bubbling reactor with a height of 150 mm and a volume of 1 L,which holds 700 ml CLA.The temperature control system adjusts the temperature of the reaction solution through a water bath(HH-S1,Yuhua Instrument CO.,LTD,Gongyi,China).The analysis system of flue gas is a M-9000 combustion analyzer(Shanghai Encel Instruments CO.,LTD).Determination of the anion composition of the CLA after the reaction is tested by an ion chromatograph(ICS-1000,DIONEX CHINA LIMITED).The different kinds of reacted ions in the CLA were analyzed by titration and ion chromatography,and the test methods are shown in Table 1.

Table 1 Ion concentration and species detection method
2.2.Reagents
All reagents used in this experiment are analytical reagent(AR),including NaClO(≥10%[available chlorine],Fuchen Chemical Reagents CO.,LTD,Tianjin,China),KMnO4(≥99.5%,Xilong Scientific CO.,LTD,Shantou,China),NaOH(≥96.0%,Beijing Chemical Works,Beijing,China),sulfuric acid(H2SO4)(95.0%-98.0%,Beijing Chemical Works,Beijing,China),dipotassium hydrogen phosphate(K2HPO4)(≥99.0%,Aladdin Industrial Corporation,America),4-Amino-N,Ndiethylaniline monohydrochloride[PDP](≥98.0%,MERYER CO.,LTD,China),ammonium iron(II)sulfate hexahydrate((NH4)2Fe(SO4)2·6H2O)(≥99.5%,MERYER CO.,LTD,China),thioacetamide[CH3CSNH2](≥99%,MERYER CO.,LTD,China),phosphoric acid(H3PO4)(≥85.0%,Beijing Chemical Works,Beijing,China),ammonium persulfate[(NH4)2S2O8](≥98.0%,Beijing Chemical Works,Beijing,China)and silver nitrate[AgNO3](≥99.5%,Beijing Chemical Works,Beijing,China).
2.3.Experimental methods
The simulated flue gas used in this experiment contained N2,SO2and NO.The three gases entered the mixer from their cylinders and were metered through the mass flow controllers.In the mixer,SO2and NO were diluted to the demanded concentrations by N2,and the simulated flue gas was formed.According to the literature[10],the concentrations of SO2(CS)and NO(CN)were set to 1500 ppm and 1000 ppm in the simulated flue gas on our study.The gas in the mixer entered the combustion analyzer by bypass to determine the percentages of each component in the simulated flue gas prior to the reaction,which was controlled by adjusting their globe valves so that the concentrations of SO2and NO reach the set values.Then the simulated flue gas in the mixer entered the bubbling reactor and reacted with the prepared CLA,and the SO2and NO's contents in the flue gas after reaction were measured at intervals.In addition,the temperature of CLA in the bubbling reactor was adjusted by a constanttemperature water bath.The initial pH of CLA was adjusted by NaOH solution(1 mol·L-1)and H2SO4solution(1 mol·L-1),whose pH was measured with a pH meter(PHS-3G,Shanghai Yidian Scientific Instrument CO.,LTD.Shanghai,China).The removal conversions of SO2and NO can be calculated by the following formula:

3.Results and Discussion
3.1.Effect of NaClO concentration on SDD
Fig.2 shows the effect of different concentrations of NaClO aqueous solution on SDD.It can be seen that under the same conditions the removal efficiency of NO increases with the concentration of NaClO in solution.When the concentration of NaClO reaches 40 mmol·L-1,the changing tendency of removal efficiency for NO is gentle with the further increase of NaClO concentration.From the economic point of view and considering the effect of KMnO4addition on the SDD's efficiency of NaClO solution,the concentration of NaClO solution in this experiment was chosen to be 40 mmol·L-1.

Fig.2.Effect of concentrations of NaClO on removals of SO2and NO.(CS=4290 mg·m-3,CN=1340 mg·m-3,CK=0 mmol·L-1,Vg=120 L·h-1,Ts=25°C,pH=12.)
3.2.Effect of gas flow on SDD

Fig.3.Effect of gas flow on removals of SO2and NO.(CS=4290 mg·m-3,CN=1340 mg·m-3,CNa=40 mmol·L-1,CK=0 mmol·L-1,Ts=25°C,pH=12.)
Fig.3 shows the effect of NaClO solution on the SDD at different gas velocities.With the increase of gas velocity,the removal efficiency of SO2remains unchanged with the value of 100%,but the removal efficiency of NO is linearly decreased.This is because the gas-liquid contacting time decreases with increasing the gas flow rate,and NO is hardly soluble in water[6].The NO in the simulated flue gas is not sufficiently reacted with the NaClO in the CLA,and is taken out by the flue gas.The SO2is soluble in water,and thus the increase in gas velocity has a negligible effect on the removal of SO2.In order to better understand the effect of KMnO4added to NaClO solution on the SDD,the gas flow selected in this paper is 120 L·h-1.When the volume of SDD is 0.7 L the reaction time(space time)is 0.7 L·(120 L·h-1)-1=0.00583 h=21 s.Li et al.[25]used a spray tower with a diameter of 380 mm and a height of 830 mm for SDD research.And the space time was calculated to be 376.5 s according to the gas velocity(15 L·min-1),which is much higher than that in this work(21 s).In consideration of space time,the research in this paper is more feasible in industry.
3.3.Effect of KMnO4concentration on SDD

Fig.4.Effect of concentrations of KMnO4on removals of SO2and NO.(CS=4290 mg·m-3,CN=1340 mg·m-3,CNa=40 mmol·L-1,Vg=120 L·h-1,Ts=25°C,pH=12.)
Fig.4 shows the effect of the CLA on the SDD after adding different amounts of KMnO4into NaClO solution,in which the concentration of NaClO was 40 mmol·L-1.As the concentration of KMnO4in the CLA increases,the efficiency of denitrification increases compared with the use of the NaClO solution alone.This shows that the CLA prepared by NaClO and KMnO4has a good synergistic effect on SDD.However,when the concentration of KMnO4in the CLA exceeds 7.5 mmol·L-1,the effect of KMnO4concentration on denitrification becomes negligible.Therefore,the optimum concentrations of NaClO and KMnO4in the CLA were 40 mmol·L-1and 7.5 mmol·L-1,respectively.
3.4.Effect of initial pH on SDD
Fig.5 shows the effect of different initial pH values on the SDD.Because the removal efficiency of SO2was always 100%regardless of the initial pH value,the removal efficiency curve of SO2was not shown in the figure.The initial pH values of the CLA were 2,4,6,8,10 and 12(as shown in Fig.5).The initial pH of the CLA was adjusted by using a 1.0 mol·L-1NaOH solution and a 1.0 mol·L-1H2SO4solution.It can be seen from the figure that the denitrification efficiency was more than 90%when pH>8 within 50 min,but as the reaction time increases over 50 min,the denitrification efficiency gradually decreases.This phenomenon is more obvious when the initial pH value was 2 and 4.At the same time,when the pH was less than 7 for the CLA,there was an irritating gas generated.It is known from the literature[17]that under acidic conditions,the hypochlorite(ClO-)will decompose to produce Cl2.


Fig.5.Effect of initial pH on removals of SO2and NO.(CS=4290 mg·m-3,CN=1340 mg·m-3,CNa=40 mmol·L-1,CK=7.5 mmol·L,Vg=120 L·h-1,Ts=25°C.)
Therefore,the ClO-in the CLA is consumed under the acidic conditions because of reaction(3),and is not involved in the oxidation of SO2and NO in the flue gas.This is the reason why the denitrification efficiency of the CLA is lowered when the pH is low.The pH value of the newly configured CLA was close to 12.From the experimental results,the CLA has a very good SDD efficiency when the pH ranged from alkaline to weak acid,so it is not necessary to adjust the initial pH value during the utilization.
3.5.Effect of temperature on SDD

Fig.6.Effect of temperature on removals of SO2and NO.(CS=4290 mg·m-3,CN=1340 mg·m-3,CNa=40 mmol·L-1,CK=7.5 mmol·L-1,Vg=120 L·h-1,pH=12.)
This experiment is a gas-liquid two-phase reaction,and temperature has an important effect on both physical diffusion and chemical absorption.On the one hand,the rise of temperature facilitates the diffusion of ions in the solution,which accelerates the removal of SO2and NO in the solution.On the other hand,the increase in temperature reduces the solubility of SO2and NO in the liquid,especially for NO,which increases the gas-liquid mass transfer resistance,and has a negative effect on the removal of SO2and NO in flue gas.As shown in Fig.6,the removal conversions of SO2and NO both decrease with increasing the CLA temperature,together with a larger decrease of removal conversion for NO.It shows that the solubility of NO is less than SO2in water and can be affected greatly by temperature,so the technical difficulty of SDD through liquid phase lies in the removal of NO.The experimental results show that the optimum reaction temperature for SDD of the CLA was 25°C.
4.Parallel Experiments
According to the above experimental results,the optimum reaction conditions of the CLA were determined for SDD in the homemade bubbling reactor.The flow rate of flue gas was 120 L·h-1,the concentration of NaClO was 40 mmol·L-1,the concentration of KMnO4was 7.5 mmol·L-1,the reaction temperature was 25°C and the initial pH was 12.

Fig.7.Parallel experiments on removals of SO2and NO.(CS=4290 mg·m-3,CN=1340 mg·m-3,CNa=40 mmol·L-1,CK=7.5 mmol·L-1,Vg=120 L·h-1,Ts=25°C,pH=12.)

Table 2 Results of parallel experiments
Parallel experiments were carried out under the optimal operating conditions,and the experimental results are shown in Fig.7 and Table 2.The average removal conversions of SO2and NO are 100% and above 94%,respectively,and the standard deviations were 0 for SO2and 3.2 for NO,respectively.The repeatability of the experimental results is good,and the performance of experiment apparatus is stable.
The results studied in this work are better than those reported in the literature[25],the 98.8%for SO2and 70.9%for NO.The main reason for the different SDD efficiency is the different reactor forms.In the gas-liquid contact,the bubbling reactor can disperse the gas into the continuous liquid phase,and the spray reactor can disperse the liquid into the continuous gas phase.Therefore,the gas-liquid contact of bubbling reactor is better than that of spray reactor for SO2and NO[30].

Table 3 Results of titration experiments
5.Reaction Mechanism
The experimental results show that the CLA has a good effect on the removals of SO2and NO.And its absorption capacity is much higher than that of the two liquids used alone.The species and contents of ions in the absorption liquid before and after the reaction were analyzed by ion chromatography and titration.The results were shown in Figs.8 and 9 and Table 3.The main ions contained in the solution after reaction wereand Mn2+,and the treated flue gas detected by the combustion analyzer contains only a small amount of NO and does not contain SO2.This shows that SO2and NO in the simulated flue gas are oxidized by the oxidants in CLA,thereby achieving the purpose of SDD.

Fig.8.Chromatogram for .
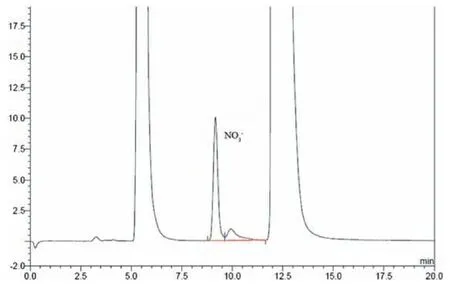
Fig.9.Chromatogram for .
Reactants,the reaction products and results,the reaction process for SDD in CLA are as follows:
(a)Since SO2is easily soluble in water,first the SO2in the flue gas is dissolved in water,then reacts with ClO-andin the CLA whose initial pH is close to 12,which is alkaline.The reaction equations are as follows[17,24,31]:

However,unlike SO2,NO is hardly soluble in water,and mass transfer resistance between gas and liquid phases is large.Therefore,the oxidation reaction of NO mainly occurs on the gas-liquid phase interface,and the oxidized product can be absorbed by the liquid phase[15,17,31].

(b)With the process of reaction,the pH of CLA gradually decreases.When the pH is near neutral,the solution becomes cloudy.This is because manganese dioxide(MnO2)is formed when permanganate reacts with SO2and NO.Moreover,the solubility of MnO2in water is very low,so the solution becomes cloudy,which agrees with literature[25,31].

(c)As the reaction proceeds,the CLA was changed from turbid to a colorless and transparent liquid,and a gas with pungent odor was generated.At this time,the pH of the CLA was acidic.The production of irritating gases is due to the decomposition of ClO-under acidic conditions to produce Cl2.The solution changed from turbid to transparent because ClO-reacts with MnO2under the acidic conditions to form divalent manganese ions.Since the concentration of Mn2+in the solution is less than those of other ions,the solution appears colorless,which agrees with literature[20,24,29-32].


Through the analysis of the reaction product,the content of Mn in the solution before and after the reaction was unchanged when the CLA composed of NaClO/KMnO4was used in the SDD experiments.However,precipitation occurred during the experiment and disappeared as the reaction progressed.This is because reactions(10),(15),(16),(18),(19),(27)and(28)happened.A small amount of MnO2precipitated during the reaction,then the existence of ClO-promoted MnO2to oxidize NO in CLA.At the same time,ClO-and HClO produced by NaClO react with SO2and NO dissolved in the solution[33].From this reactions(4)-(9),(13)and(14)can be inferred.When the reaction takes place in the liquid phase,the existence forms of Cl species are ClO-and Cl-,and the existence forms of S species areas well as the existence form of N species is.In addition,as the reactions progress,the pH in solution decreases continuously,and ClO-generates Cl2when the pH value in solution is less than 7.Cl2also has a strong oxidizing property,so the remaining reactions can be determined.This is consistent with the findings of Li et al.[25].
According to the above results,the newly configured CLA has a pH close to 12,but as the reaction progresses,the pH of the solution gradually becomes weakly acidic.This requires the equipment to undergo some anti-corrosion treatment,otherwise the absorption liquid will corrode the equipment.The reacted solution is acidic and contains a large amount of acid ions.If directly discharged into the ecological environment,it will lead to soil salinization and acidification of the water source,resulting in soil compaction,crop yield reduction and even no harvest.Among them,manganese ions and their compounds can also cause damage to the nervous system of human being and animals[34].Therefore,the reacted solution must be discharged into the environment after treatment to avoid secondary pollution.The absorption liquid after reaction contains Cl-,According to the literature[35],Cl-is first removed from the spent liquor,and the sulfate and nitrate in the solution are kept,which can be reused as raw materials for fertilizer production[36,37]and which can not only avoid secondary pollution,but also improve economic benefits.Since the solution after reaction was acidic,it is necessary to add hydroxide to adjust the pH to neutral,while Mn2+reacts with hydroxide to form manganese hydroxide precipitate,and Mn2+in the waste liquid can be directly removed and recovered.
Under the optimal reaction conditions,the calculated amounts of KMnO4and NaClO consumed are 62.5 mmol and 110.8 mmol·m3of flue gas,respectively.The latest prices of the oxidants were acquired from some Chinese suppliers;NaOH,urea,NaClO and KMnO4are 3200 CNY·t-1,2000 CNY·t-1,700 CNY·t-1and 24000 CNY·t-1,respectively.Therefore,the all cost of the above oxidants required to treat 1 m3of flue gas is less than 0.3 CNY,while the traditional SCR process alone requires 0.2 CNY.From the view point of the oxidant's consumption,this experiment is not dominant in industrial applications[38].But from a process point of view,traditional SCR denitrification requires the use of large amounts of ammonia,and the most economical way to obtain ammonia is to decompose urea.This requires additional costs of equipment and floor space in industrial applications,and it is also disadvantageous to the operation and maintenance of the equipment.The catalyst is the core of the SCR technology,and its performance directly affects the denitration efficiency.In process of the industrial application,the catalyst will be deactivated due to sintering,poisoning,etc.,so the catalyst needs to be replaced and regenerated[39].This also increases the cost of SCR technology.The technical process studied in this paper was to directly use the oxidants to oxidize SO2and NO in the flue gas,without adding any auxiliary devices,and the process is simple and the cost is low.Considered equipment investment,oxidants and catalytic consumption,the cost of this technology is off by more than 30%compared with the traditional technology.
6.Conclusions
(1)The CLA prepared by NaClO/KMnO4is used for SDD experiments in a bubbling reactor,which effectively improves the efficiency of SDD compared with a single absorbent.
(2)The optimal reaction conditions are 120 L·h-1flow rate of flue gas,25 °C reaction temperature,40 mmol·L-1and 7.5 mmol·L-1in concentrations of NaClO and KMnO4,respectively,and the initial pH=12.The optimal removal conversions are 100%for SO2and 94%for NO.
(3)The CLA which has a pH close to 12 for a new-prepared solution is stable and efficient for SDD when the pH ranges from strong alkaline to weakly acidic,so there is no need to adjust its pH during application and it will have a good prospect in industry.
(4)Anion chromatography and titration were used to qualitatively and quantitatively detect the anion species in CLA afterreaction.ThemainionsinthesolutionareCl-,ClO-,and Mn2+.According to the detected concentrations,the removal conversions were theoretically calculated,which basically agree with those of flue-gas analysis.NO and SO2were completely converted into,respectively.
(5)The reaction mechanism equations of NaClO/KMnO4in SDD reaction were proposed.According to the reaction mechanism,the ClO-can increase the dissolving of MnO2sediment,which accelerates the oxidation of SO2and NO,and then raises the utilization of KMnO4.These prove that NaClO and KMnO4have a certain synergistic effect in SDD reactions.
Nomenclature
Cinthe inlet concentration of NO and SO2,mg·m-3
CKKMnO4concentration,mmol·L-1
CNinlet concentration of NO,mg·m-3
CNaNaClO concentration,mmol·L-1
Coutthe outlet concentration of NO and SO2,mg·m-3
CSinlet concentration of SO2,mg·m-3
Tssolution temperature,°C
Vggas flow rate,L·h-1
η the removal efficiency of NO and SO2
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- A review of low-temperature heat recovery technologies for industry processes☆
- Current scenario and potential of biodiesel production from waste cooking oil in Pakistan:An overview☆
- Structure and synthesis of graphene oxide☆
- Co-firing of coal and biomass in oxy-fuel fluidized bed for CO2capture:A review of recent advances
- Effects of internals on phase holdup and backmixing in a slightlyexpanded-bed reactor with gas-liquid concurrent upflow☆
- Distribution performance of gas-liquid mixture in the shell side of spiral-wound heat exchangers☆
