Flow-mode synthesis of biodiesel under simultaneous microwave-magnetic irradiation
2019-02-09BehzadKhedriMostafaMostafaeiSeyedMohammadSafieddinArdebili
Behzad Khedri,Mostafa Mostafaei, *,Seyed Mohammad Safieddin Ardebili
1 Mechanics of Biosystems Engineering Department,Razi University,Kermanshah,Iran
2 Biosystems Engineering Department,Shahid Chamran University of Ahvaz,Ahvaz,Iran
Keywords:Biodiesel Microwave Magnetic field Transesterification Waste cooking oil
ABSTRACT In this study,aiming at optimization of a novel continuous biodiesel production system was developed by combining technologies based on microwaves and magnetic fields.Factors affecting microwave-assisted biodiesel(alkyl esters)production reaction were analyzed in this investigation.Studied factors included magnetic field intensity(0,0.225 and 0.450 T),microwave power(400,821,and 1181 W),percentages of KOH and NaOH catalysts at constant concentrations of 1 wt%(0,50%and 100%),and percentages of ethanol and methanol at a constant molar ratio of 6:1(0,50%and 100%).Response Surface Methodology(RSM)was used to optimize the reaction conditions.RSM-based analysis indicated that,all independent parameters had significant effects on the reaction efficiency.Results of the investigations reveal that the largest effects on the conversion efficiency were due to type of alcohol and magnetic field intensity.The optimized conditions were found to be a magnetic field intensity of 0.331 T,a microwave power of 677.77 W,catalyst percentages of 30.35%and 69.65%for KOH and NaOH,respectively,and alcohol percentages of 80.47%and 19.53%for methanol and ethanol,respectively.Under the optimal conditions,yield of the reaction was 96.2%.
1.Introduction
Energy plays an indelible role in bringing man to development.In the 21st century,the world needs increasingly more energy to expand industries,transportation systems,and achieve domestic and international goals.With increasing demand for energy and dependence on limited resources,energy has become a major challenge in the present century[1,2].Many researchers has addressed the problems caused by fossil fuels and promoted the use of renewable fuels such as biodiesel[3].According to American Society for Testing and Materials(ASTM),biodiesel is a fuel composed of mono-alkyls of long-chain fatty acid esters[4,5],which can be obtained from different renewable raw materials such as edible and non-edible oils and animal fats through transesterification reaction[6-9].The transesterification reaction can be enhanced by various stimulation techniques such as ultrasonication[10-12],microwave irradiation[13-15],or combinations of different technologies[16-18]to obtain higher conversion rates.
Conventional heating methods for biodiesel production are powerintensive.The main problem with such methods is that,those apply heat only to the surface of the precursors;while microwave systems can directly transfer thermal power to the reactants,reducing the reaction time[19,20].In a research work,biodiesel was produced from waste oil using microwave and two types of high-and low-free fatty acid oils at different power levels(300-700 W).The highest conversion yields were achieved with the oil with low free fatty acid at 500 W(98.2%)and the oil with high free fatty acid at 600 W(92.8%).A further increase in the microwave power to 700 W resulted in lower reaction yield with both types of the oil.[21].Another study investigated the application of NaOH in continuous microwave-assisted biodiesel production from palm oil.Methanol was applied as alcohol and the four different microwave power levels were tested(200,400,600 and 800 W).Optimal parameters were found to be catalyst concentration of 1 wt%,alcohol to oil molar ratio of 12,microwave power of 400 W,and reaction temperature of 27°C.This set of conditions resulted in a very good FAME conversion of 99.4%[22].It can be seen that,an increase in microwave power can enhance biodiesel production up to a certain level,beyond which any further increase in microwave power may damage the molecular structure of triglyceride[23],lowering the reaction yield.In another research,biodiesel production was examined under microwave irradiation for 40-180 s.The results showed a conversion efficiency of more than 99%in 40 s.Increasing the radiation time to 180 s resulted in lower production rate[24],possibly due to hydrolysis of esters and formation of more free fatty acids for saponification reaction[25].Other studies have looked forward to reduce production time and improve production efficiency.Accordingly,biodiesel has been produced from cotton seeds by a combination of stirring and microwave irradiation.Response Surface Methodology(RSM)has been used to determine optimized conditions in terms of alcohol to oil molar ratio of 17:1,temperature of 70°C,stirring rate of 380 rpm,reaction time of 12 min,and microwave power of 270 W.This set of conditions resulted in the biodiesel production efficiency of 99.5%[26].The use of combined methods such as microwave irradiation and ultrasonication in either sequential[13,27,28]or simultaneous[18,29,30]arrangements has been very much considered to solve the associated problems with mass and heat transfer.In a study,ultrasonication,microwave irradiation,and normal heating techniques were used for biodiesel production.The obtained reaction times using microwave irradiation(35 min),ultrasonication(2 h)and conventional heating(8 h)indicated superiority of the microwave-assisted method[31].
Analyzing the effect of ultrasonic energy on the transesterification reaction showed that application of ultrasonic irradiation will result in emulsification and effective mass transfer,leading to a ten-fold increase in the rate of ester formation in comparison with the conventional agitator.This improvement can be attributed to the physical and chemical effects of ultrasound to the reaction.Formation of a very small emulsion between the oil and the alcohol,proportional to the micro-turbulence created by the cavity bubbles,gave rise to a large common surface which increased the reaction speed[32].
Researches are looking forward to improve biodiesel production methods,especially the transesterification process,so as to achieve maximum possible biodiesel conversion rate at the lowest cost and time.One of these methods that has been well discussed in recent years is microwave irradiation and combination of this technology with other methods to improve the conversion rate of biodiesel production.
Faris et al.conducted a study to investigate the effect of the magnetic field on engine combustion and emissions.They reported a reduction in fuel consumption and diesel emission.In addition,they found that exposure to a magnetic field could change the fuel properties.They also noted that,the effect of external magnetic field is related to microscopic structure,which includes both the displacements and polarization of the biofuel molecules[33].
However,some research works demonstrated the significant impact of the external magnetic field on the chemical reactions.Kipriyanov and Purtov reported that the external magnetic field might affect the rate of the reactions with paramagnetic reactants.More specifically,their finding ensures that the magnetic field can change the system properties and theoretically described the magnetic field influence on the steady state of a non-equilibrium chemical reaction[34].Some studies showed that the static magnetic field can influence the rate constants of chemical reactions or hydrocarbon oxidation[33,34].

Fig.1.Influence of microwave field on heating of materials[19].
As a matter of fact,microwave technique will result in a local and prompt heating of the reactant materials through dipolar polarization,ionic conduction and interfacial polarization(Fig.1).Since the reactive substances used to produce biodiesel have polar properties,along with the results reported on the effect of magnetism on the chemical reactions,a comprehensive study was conducted to investigate the influence of magnetism on the transesterification reaction.The magnetic treatment of the biofuels is a novel method to improve the production and application of biofuel process.Therefore,the aim of this investigation is to provide a new system for the production of biodiesel with the help of simultaneous microwave irradiation and magnetic field to improve the production conditions and reaction efficiency.Also,RSM was utilized to investigate effects of different factors such as magnetic field intensity,microwave power,percent ratio of ethanol to methanol as alcohols and,percent ratio of KOH to NaOH as catalysts on the transesterification reaction and optimize continuous process by finding optimal set of conditions for the production of biodiesel with the mentioned system.
2.Materials and Methods
2.1.Materials
Waste cooking oil was taken from the restaurant of Razi University,Kermanshah,Iran.To prepare the waste oil before starting the biodiesel production process,two important factors,namely lack of water and absence of excess material(such as food pieces or any other substance)should be monitored.Impurities in the waste oil were removed by filtration.The sedimentation method was employed to separate the wateroil molecules.For this purpose,the oil was heated to 60°C for 15 min and then transferred to a decanter for 24 h.The water molecules will be separated from the oil due to the difference in their densities.Characteristics of the waste oil are given in Table 1.Since the combination of two types of NaOH and KOH catalysts,as well as two types of ethanol and methanol alcohol together has not been investigated so far,the alcohol used in this study was a mixture of ethanol and methanol at three ethanol percentages of 0,50%and 100%;the purity of each alcohol was beyond 99%.It should be mentioned that 0% of alcohols blend means that methanol was only used.Molar ratio of alcohol to oil was set to 6:1,based on results of other research works[35,36].NaOH and KOH catalysts were used at three KOH percentages of 0,50%and 100%at a fixed catalyst concentration of 1 wt%,based on results of other research works[21,37].The 0% of catalyst mixture means that only NaOH was used.
2.2.Equipment
A schematic of the system used in this study for producing biodiesel is presented in Fig.2.Hybrid magnetic and microwave technique for continuous biodiesel production has been recorded an Iranian patent No.92588,dated 21.6.2017.A domestic microwave oven(Panasonic NN-ST342WW)was used at three electric power consumption levelsof 400,821 and 1181 W.Permanent neodymium magnets(N42;220×50×50 mm;1.28 T at the surface)were used to establish a constant magnetic field at three intensities:0,0.225 and 0.450 T.A frame was created for the magnets to provide different intensities of magnetic field,wherein one of the magnets was fixed and the other one could have its position adjusted using a mechanism of nut-screw(Fig.3).Uniform magnetic field was measured by using a Tesla meter(LUTRON MG-3002).

Table 1 Characteristics of waste oil used in this research

Fig.2.A schematic of the continuous biodiesel production setup with simultaneous microwave irradiation and magnetic field application.
2.3.Biodiesel production
Biodiesel was produced by using the apparatus developed in this study.Given that the mixture of methoxide and oil is two-phased and immiscible,in the beginning of each test,reactants were stirred for 10 min by a magnetic stirrer at 400 r⋅min-1at 60°C.The stirring rate and temperature have been introduced as optimal values in other research works[26,38,39].Subsequently,the reactants were transferred to a decantation tank whose decantation valve was set;the materials were then transferred to a microwave-assisted reactor.Flow rate of the feed material into the microwave was constant and in such a way that the residence time of the material inside the microwave-assisted reactor was 1.5 min(since most of research works have reported optimal reaction time as 1 min[40,41]and 2 min or close to 2 min[17,35],even though other values have also been reported as optimum time[42,43]).Therefore,an average reaction time was selected in this study.Then biodiesel production was undertaken under different microwave irradiation power.
2.4.Gas chromatography(GC)analysis
Purity of the produced biodiesel by the combination of microwave and magnetic field was evaluated using GC analysis.At the end of each experiment,a 15-ml sample of biodiesel was taken and stored at-5°C until GC analysis.Experimental tubes containing the biodiesel were centrifuged at 4000 r·min-1for 5 min.Standardization process and GC tests were carried out according to our pervious papers[3,9,44].Column temperature was first set to 60°C for 2 min.It was then raised to 210 °C at 10 °C·min-1and then further increased to 235 °C at 5°C·min-1.The temperature was kept constant for 7 min.Total required time for the analysis was 29 min.Nitrogen served as carrier gas,with its pressure and flow-rate fixed at 25.93 psi and 108 ml·min-1,respectively.Purity of the produced biodiesel and percent conversion of oil to biodiesel were calculated from Eqs.(1)and(2),respectively[45,46]:

Fig.3.Uniform magnetic field established in this research.
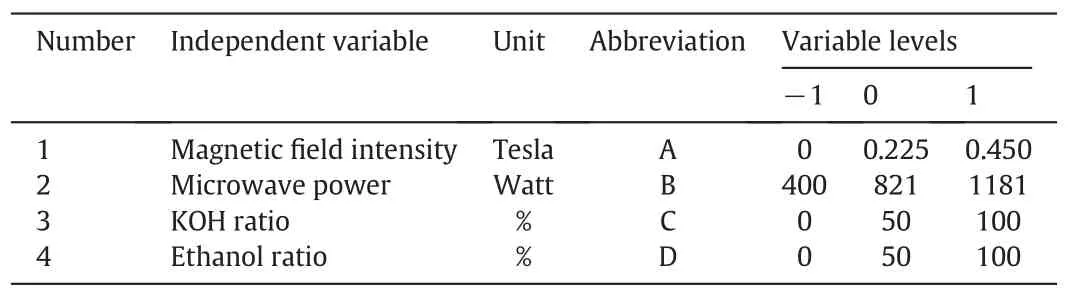
Table 2 The coded and actual levels of independent variables

where,C,ΣA,AIS,MISand M are purities of produced biodiesel,sum of all areas under the corresponding peaks to C6 to C24 fatty acids,area under the peak of internal standard,mass of internal standard,and mass of biodiesel sample,respectively.Eq.(2)was used to calculate the reaction efficiency.

where,WAlkylester,WWCO,MAlkylester,and MWCOare mass of produced biodiesel,mass of waste cooking oil,average molar mass of producedalkyl esters(biodiesel),and molar mass of the waste cooking oil,respectively.

Table 3 Experimental design based on the Box-Behnken(BB)method
2.5.Experimental design
Response Surface Methodology(RSM)is a combination of mathematical and statistical approaches based on multiple regression analysis used to quantify relationships between one or more measured responses and vital input factors.In this study,the Box-Behnken(BB)design was used for evaluating and predicting effects of independent variables(magnetic field intensity,microwave power,KOH percentage and ethanol percentage)on the yield of transesterification reaction.Coded and actual levels of the independent variables are given in Table 2.
For four independent variables,the Box-Behnken(BB)experimental design predicts 29 experiments with 5 repeats at the center of the experimental design to determine associated experimental error.The pure error sum of squares(SS)measures the effect of repeatability error.This term is calculated with 5 repeated central experiments by the following equation:
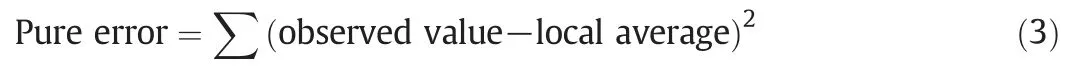
Modeling and analysis of the experimental data were performed utilizing Design-Expert version 10.0.0 software.
3.Results and Discussion
All experiments and their conditions are reported in Table 3.As suggested by Design-Expert software and taking into account the capabilities of quadratic function,it was selected with a high value of R-squared(0.957)to predict reaction efficiency(Eq.(4)).

In the above equation,the positive(negative)sign indicates positive(negative)effect of the respective parameter on the reaction efficiency.
In Table 4,results of the analysis of variance(ANOVA)of independent parameters on the dependent variable are presented.Accordingly,a p-value of less than 0.0001 indicates significance of the model results,while p-values greater than 0.05 are indicative of insignificance of the model[47,48].

Table 4 Results of ANOVA of independent parameters on the transesterification reaction efficiency

Fig.4.The perturbation plot of all the independent factors.
As shown in Table 4,all independent parameters had significant effects on the transesterification reaction efficiency.In the meantime,ethanol percentage,magnetic field intensity,KOH catalyst percentage and microwave power had the most significant effects on the reaction efficiency(mentioned in the order of decreasing);ethanol percentage and microwave power had the highest and lowest effects on the reaction efficiency,respectively.The perturbation plot(Fig.4)was employed to compare the effects of all the factors at a particular point in the design space.The response was plotted by changing only one factor over its range while holding all the other factors constant.The perturbation plot is a good visual tool for showing the relative size of the effects.A steep slope or curvature in the ethanol factor percentage indicates the sensitivity of the response to this factor.At the midpoint(coded 0)of all the factors,a relatively flat line for KOH catalyst percentage and microwave power curves reflects the insensitivity of the process to variations in those particular factors.
3.1.Effect of magnetic field
Effect of magnetic field on the transesterification reaction efficiency was evaluated in this study for the first time.Other studies have indicated that microwave electromagnetic waves have non-thermal effects on reaction efficiency[49].Therefore,it can be said that,due to the nature of the magnetic field which affects ions and polar molecules engaged in the reaction,the field tends to enhance biodiesel production.Increasing the intensity of uniform magnetic field from 0 to 0.450 T enhanced the reaction efficiency(Fig.4).
As shown in Fig.5,the reaction efficiency increased by increasing magnetic field intensity and decreasing microwave power,so that the lowest microwave power and the highest magnetic field intensity caused a yield more than 90%.Interactions of the magnetic field intensity and KOH percentage are shown in Fig.6.
As observed,NaOH catalyst had the greatest effects on efficiency with increasing the magnetic field,and higher efficiency was observed at higher percentages of KOH.The greater the difference in electronegativity between atoms of a compound,the more polarized will be the resultant bond[50].Since sodium and potassium are alkaline metals in a group of the periodic table,sodium has a higher electronegativity and thus,it has a greater polarity.This issues that use of pure NaOH was more affected from magnetic field than to pure KOH,and increased biodiesel production and improved the transesterification reaction efficiency.

Fig.5.Interaction effect of magnetic field intensity and microwave power on the reaction efficiency.
Interaction between magnetic field and the type of alcohol(ethanol percentage)is demonstrated in Fig.7.By increasing the magnetic field and decreasing ethanol(100%methanol),conversion rate increased to up to 90%.Since methanol is more polarized than ethanol[51],it is more affected by magnetic field intensity,so that reaction efficiency increased further with increasing magnetic field intensity while increasing methanol percentage.
The external force exerted by a magnetic field on the flow of hydrocarbons can polarize their molecules.For ionization of reactive substances passing through the magnetic field is based on the principle of the mutual action among the hydrocarbon molecules of oil,alcohol and catalyst[52].During the passage of hydrocarbons from the magnetic field,some of its properties,including surface tension and viscosity were affected.Also,their orientation changed to a specific isomeric form,called Ortho.The magnetic field contributed to degradation of the hydrocarbon particles and their division giving rise to enhancement of the contact surface during the reaction process[53,54].

Fig.6.Interaction effect of magnetic field intensity and KOH ratio on the reaction efficiency.

Fig.7.Interaction effect of magnetic field intensity and ethanol ratio on the reaction efficiency.
3.2.Effect of microwave power
Obtaining optimal microwave power to maximize conversion efficiency while preventing alcohol evaporation is an important issue in biodiesel production via transesterification process using microwave technology[55].
As shown in Fig.4,reaction efficiency increased with increasing microwave power to a certain level,beyond which the reaction yield decreased.According to Fig.4,maximum efficiency was obtained in the microwave power range of 700-800 W,which was in agreement with the results of the research reported by Azcan and Yilmaz(2013)on biodiesel production from waste cooking oil and microwave-assisted biodiesel production from olive oil and micronized waste oil.They introduced 800 W,as optimal power for biodiesel production[37].The interaction effect of microwave power and percentage of KOH catalyst on the reaction efficiency was increasing at first,but decreased subsequently,although the decreasing rate was lower.As is clearly shown in Fig.8,with increasing microwave power and KOH catalyst to 50%,the reaction efficiency conversion rate enhanced to up to 80%and the reaction yield decreased with further increase in microwave power and KOH percentage catalyst.
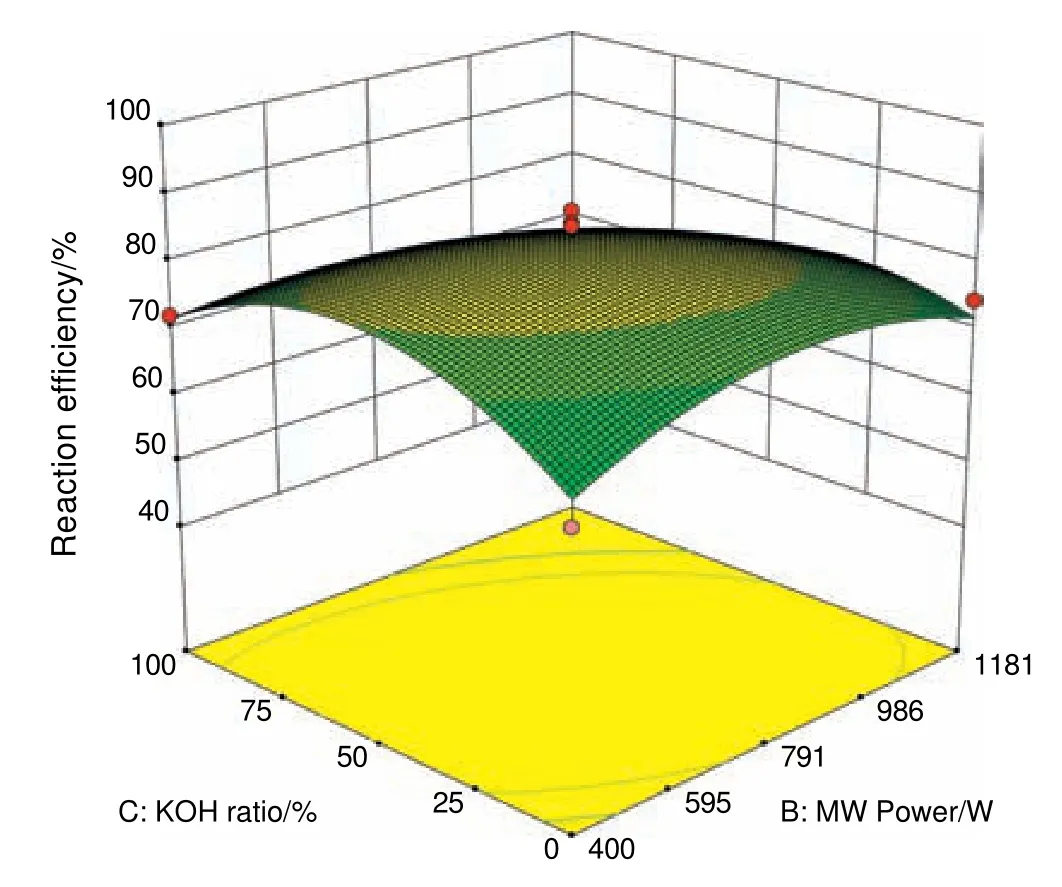
Fig.8.Interaction effect of microwave power and KOH percentage on the reaction efficiency.

Fig.9.Interaction effect of microwave power and ethanol ratio on the reaction efficiency.
Interaction of microwave power and ethanol percentage is shown in Fig.9.According to Fig.9,the reaction efficiency was inversely related to microwave power and ethanol alcohol percentage.That is,the reaction yield could be increased to higher than 90% by decreasing both parameters.
Absorbability of microwaves by methanol is more than that by ethanol.As such,the transesterification reaction was further accelerated by the combination of methanol and microwave rather than ethanol and microwave[55-59].In the present study,it was also observed that,by decreasing ethanol and increasing methanol contents,the reaction efficiency increased significantly.
3.3.Effect of KOH catalyst percentage
As shown previously in Fig.4,among other percentages,the highest biodiesel production yield was obtained when KOH and NaOH catalysts were mixed at 50%and 50%,respectively.Further reduction in efficiency of the reaction was observed when percentage of KOH was increased;minimum reaction efficiency occurred with the KOH at 100%,indicating better performance of NaOH as catalyst in this reaction.Opposite results have been reported in other research works[60,61].In the present study,as shown in Fig.4,NaOH and KOH,alone ended up with production yields of 76%and about 70%,respectively.There are also no clear reasons explaining the superiority of NaOH or KOH.This can be related to effect of magnetic field on catalyst,ionization energy,basicity strength of alkali metal hydroxides and reaction conditions.NaOH on the other hand is a lighter molecule compared KOH.As the catalysts were applied at a concentration of 1 wt%,a larger number of Na+and OH-ions will be present in the reaction medium compared to KOH.Mathematically NaOH has 1.4 times more hydroxide ion(OH-)compared to KOH;so the reaction will be intensified.
Effects of interaction between the KOH catalyst and the alcohol type are shown in Fig.10.According to the figure,a decrease in ethanol percentage(i.e.an increase in methanol percentage)along with decreasing the percentage of KOH catalyst increased reaction efficiency.Accordingly,the highest reaction efficiency was observed at the lowest percentage of ethanol(100%methanol)and almost 60%KOH.In another study,biodiesel was produced from palm oil using sodium hydroxide as the catalyst under 400 W microwave power which resulted in a yield of 99.4%at a NaOH concentration of 1 wt%and the microwave irradiation duration of 1.75 min.The mentioned study indicated good biodiesel production ability of this catalyst[17].

Fig.10.Interaction effect of KOH ratio and ethanol ratio on the reaction efficiency.
3.4.Effect of ethanol percentage
This factor,which had the most effect on the reaction efficiency,is shown in Fig.4 and Table 4.The maximum rate of yield was obtained in the mixture of both alcohols.As shown in Fig.4,the reaction efficiency decreased with increasing the percentage of ethanol alcohol(or decreasing the percentage of methanol alcohol),possibly due to higher polarity of methanol which accelerates the reaction[62],and lower molecular mass of methanol than ethanol[63].When applying microwave technology,dielectric constant of the material is an important parameter to consider.Methanol alcohol has a suitable dielectric constant of about 33 which can help produce biodiesel in a reactor[64].Accordingly,reaction efficiency enhancement by increasing the methanol percentage can be a natural result,as was observed in the results of this study.
In a research on biodiesel production from waste frying oil using a mixture of methanol and ethanol,a yield of over 90% was achieved[65].Similar studies with other alcohols showed better performance of methanol for biodiesel production.In a study,methanol and ethanol were used to produce biodiesel from Karanja oil,wherein a conversion yield of 91.5%was obtained within 90.78 min with methanol under optimal percentage.The corresponding figures to ethanol were a yield of only 77.4%within 120 min,indicating better performance of methanol rather than ethanol[66].This is in agreement with the results of the present study.In Fig.4,an initial increase in efficiency is observed by increasing ethanol to methanol ratio by about 20%,possibly due to better solubility of the oil in ethanol.Any further increase in the content of ethanol ended up decreasing the reaction efficiency.This result is in agreement with those reported by other researchers[67].
3.5.Optimization
Optimizing the experimental conditions using RSM,one can achieve maximum reaction efficiency with minimizing of its standard error.In this study,optimized values of the parameters were determined using Design Expert software as follows:magnetic field intensity of 0.331 T,microwave power of 677.77 W,KOH percentage of 32.35%,NaOH percentage of 69.65%,and methanol percentage of 80.47%,and ethanol percentage of 19.53%,which ended up with a maximum efficiency of 96.2%(Fig.11).Findings of this study were in agreement with those of other studies.For example,during a study on biodiesel production,it was observed that transesterification yield increased with increasing microwave power to up to 600 W,while further increase in the power from 600 to 700 W decreased the transesterification yield[68].This confirms optimality of the value obtained in this study.
As catalysts,NaOH and KOH improved the reaction rate and enhanced final efficiency,respectively[69].Under similar conditions,however,KOH outperformed NaOH[70].Optimal catalyst mixture contained more NaOH(69.65)rather than KOH(30.35).Similar results were reported in another study investigating the performance of basic catalysts(NaOH,KOH and CH3ONa).This implies larger effect of NaOH on the progress of transesterification reaction[71].This is related to the positive effect of magnetic field on NaOH catalyst which is translated into higher reaction rate.In this study,given that reaction time was short,NaOH had a better performance and reaction efficiency could be improved by adding a small amount of KOH to NaOH(keeping constant overall catalyst concentration in the reaction).
Due to the fact that methanol is more polarized than ethanol[48];it will be more affected by magnetic field giving rise to increased reaction efficiency.Methanol also has higher microwave absorption ability.Under optimal conditions,the higher reaction efficiency could be achieved by a methanol percentage of 80.47%,and ethanol percentage of 19.53%.
4.Conclusions
In this study,a new methodology was established for continuous production of biodiesel from waste cooking oil with simultaneous use of microwave irradiation and magnetic field.Studied factors included magnetic field intensity,microwave power,percentages of KOH and NaOH catalysts and percentages of ethanol and methanol.ANOVA results showed that all of independent parameters and their interactions had significant effects on the reaction efficiency.In the proposed method,magnetic field intensity imposed a quite positive effect on the reaction yield.Increasing the magnetic field intensity significantly and linearly enhanced the reaction efficiency.A combination of ethanol and methanol improved the reaction yield compared to the case in which only one of these alcohols was employed.However,the percentage of ethanol should be adequately low to improve the solubility of methanol.Application of microwave irradiation at low power also enhanced the reaction efficiency.Moreover,mixing KOH with NaOH as catalysts significantly improved the reaction efficiency.Optimal condition for biodiesel production using simultaneous microwave irradiation and magnetic field included magnetic field intensity of 0.331 T,microwave power of 677.77 W,KOH percentage of 30.35%,NaOH percentage of 69.65%,methanol percentage of 80.47%and ethanol percentage of 19.53%,resulting in the maximum efficiency of 96.2%.The lower percentage of ethanol compared to methanol may decline the biodiesel production costs.
Acknowledgment
This study was conducted as a M.Sc.thesis at Razi University.The authors appreciate the university research deputy for its financial resources.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- A review of low-temperature heat recovery technologies for industry processes☆
- Current scenario and potential of biodiesel production from waste cooking oil in Pakistan:An overview☆
- Structure and synthesis of graphene oxide☆
- Co-firing of coal and biomass in oxy-fuel fluidized bed for CO2capture:A review of recent advances
- Effects of internals on phase holdup and backmixing in a slightlyexpanded-bed reactor with gas-liquid concurrent upflow☆
- Distribution performance of gas-liquid mixture in the shell side of spiral-wound heat exchangers☆
