Preparation of hollow B-SiO2@TiO2composites and their photocatalytic performances for degradation of ammonia-nitrogen and green algae in aqueous solution☆
2019-02-09
Faculty of Chemistry and Chemical Engineering,Jiangsu University,Zhenjiang 212013,China
Keywords:B-SiO2@TiO2composites Photocatalysis Ammonia-nitrogen Green algae
ABSTRACT Hollow B-SiO2@TiO2composites were prepared by the wet chemical deposition method starting from TiCl4and hollow B-SiO2microspheres.TiO2layers composed of anatase TiO2nanoparticles were coated on the surfaces of the hollow B-SiO2microspheres probably through the formation of Ti--O--Si and Ti--O--B bonds.A great number of--OH groups were also present at the TiO2coating layers.The presence of Ti--O--Si bonds and Ti--O--B bonds resulted in the formation of defects in the TiO2coating layers,which decreased the band gap of the TiO2coating layers to ca.3.0 eV and endowed the TiO2coating layers with visible light absorption performance.The buoyancy hollow B-SiO2@TiO2composites exhibited high photocatalytic activities for the degradation of ammonia-nitrogen and green algae.The conversion of ammonia-nitrogen reached 65%when the degradation of ammonia-nitrogen(43 mg·L-1 at pH value of 8)was catalyzed by the B-SiO2@TiO2(100:10)composite under the simulated solar light irradiation at 35 °C for 660 min.The green algae(5 mg·L-1)were almost completely degraded over the B-SiO@TiO2(100:20)photocatalyst under the visible light irradiation at 35°C for 510 min.
1.Introduction
Ammonia-nitrogen pollutants,free ammonia(NH3)and/or ammonium ion()in aqueous solution from municipal and industrial wastes and excessive nitrogenous fertilizers are toxic to the aquatic organisms,such as fishes,crustaceans,molluscs,and benthic organisms[1-7].Ammonia-nitrogen pollution has caused serious eutrophication in the surface water of the Chinese large rivers and lakes,such as Huaihe,Yellow river,and Tai lake[6-9].Even in the underground water,it was found that the nitrate pollution exceeded the US EPA's maximum level of 10 mg·L-1at 90%in 25 of 36 shallow aquifers and 10 of 37 deep or karst aquifers[10].
The eutrophication causes the blooms of algae and aquatic plants and subsequently endangers aquatic life and water quality all over the world[11-15].Microcystin-LR as one of cyclic heptapeptides produced from the algae is toxic to animals and human beings.The World Health Organization(WHO)suggests that the maximum concentration of microcystin-LR in drinking water is 1.0 μg·L-1[16].
To eliminate ammonia-nitrogen and algae from polluted water,researchers have made a great effort by using many methods.Ammonia-nitrogen could be effectively adsorbed on chitosan/nanoclay composite[17],chitosan-g-poly(acrylic acid)/attapulgite composite[18],Ca-bentonite[19],and Na-zeolites[1,20]with the adsorption capacities of 19.3-46.9 mg·g-1.
Photocatalytic degradation of pollutants has attracted a great attention of researchers because the reactive oxygen species,such as superoxide radicals(·O2-)and hydroxyl radicals(·OH),have strong oxidative ability and take a crucial role in many photodegradation reactions[21-23].Ammonia-nitrogen could be also degraded by the photocatalysis method.When the hierarchical CuAl layered double hydroxide was used as the photocatalyst for the photocatalytic oxidation of ammonium ion in an aqueous solution with H2O2at 15-45°C under visible light irradiation,the ammonia-nitrogen removal efficiency reached 99.7%[24].When the abatement of ammonia aqueous solution was photocatalyzed by the TiO2(P25)catalyst,ammonia could be effectively converted to nitrogen species,such aswith the selectivity of 99%[3].The toxic intermediatecould be rapidly oxidized to[25,26].
Algae are conventionally degraded by using algae repellents,such as chlorine and hydrogen peroxide.The chemical algae repellents could be toxic to aquatic organisms.It has been recently reported that algae(microcystin-RL)could be degraded with the degradation degree around 90%over the environmentally friendly photocatalysts,such as TiO2nanoparticles[13,15],ZnO nanoparticles[15],and TiO2nanotubes[27]under UV light irradiation,and graphite/TiO2/C3N4composite[28,29],Ag3PO4/NG/polyimide[30],and floating graphite-supported TiO2[31]under visible light irradiation.During the photocatalytic degradation processes,it is reported that hydroxyl radical(·OH),superoxide anion radical(),hydroperoxyl radical(HO2·),and singlet oxygen(O2)could effectively attack and cleave the 6-decadienoic acid chain of microcystin-RL to form non-toxic components,resulting in the reduction of microcystin-RL toxicity[13,30].
Although anatase TiO2nanoparticles are commonly used as the effective photocatalysts for the degradation of ammonia-nitrogen and algae,phase pure anatase TiO2nanoparticles only absorb the light with wavelengths less than 385 nm due to their wide band gap of 3.2 eV,limiting the use of solar light as the power source.Furthermore,the solid nanoparticle photocatalysts could sink without mechanical stirring due to their larger densities than water.For the practical application in the remediation of polluted river and lake,the photocatalysts should have buoyancy performance.
Recently,it has been reported that the heterojunction photocatalysts exhibited excellent photocatalytic activity than the single ones[32-34].The photocatalytic degradation of ammonia-nitrogen and algae over nanosized TiO2-containing heterojunction photocatalyst is worthy of investigation.
In our present work,the buoyancy hollow B-SiO2microspheresupported TiO2nanoparticle composites(B-SiO2@TiO2)were prepared by the wet chemical precipitation method using the hollow boron silicate glass microspheres as the floating supports and titanium tetrachloride(TiCl4)as the TiO2source.The chemical structures and morphologies of the as-prepared composites were characterized by XRD,SEM,TEM,XPS,FTIR,and UV-Visible diffuse reflectance spectroscopy techniques.The interaction between the hollow boron silicate glass microspheres and TiO2nanoparticles was analyzed.The degradation of ammonia-nitrogen and green algae in an aqueous solution was catalyzed by the hollow B-SiO2@TiO2composites under simulated solar light and visible light irradiation,respectively.
2.Experimental
2.1.Materials
Ammonia(28 wt%)aqueous solution,titanium tetrachloride(TiCl4,99 wt%),hydrochloric acid(38 wt%),salicylic acid(C7H6O3,99.5 wt%),sodium hypochlorite aqueous solution(NaClO,active chlorine of 5.2 wt%,NaOH of 7.5 wt%),potassium sodium tartrate tetrahydrate(C4H4KNaO6·4H2O,99.0 wt%),sodium pentacyanonitrosylferrate(III)dihydrate(Na2(Fe(CN)5NO·2H2O),99.0 wt%),TiO2nanoparticles(P25),and anhydrous ethanol were purchased from Sinopharm Chemical Reagent Co.,Ltd.,China.Hollow boron silicate glass microspheres(B-SiO2,VS5500,3M)were purchased from Shanghai Xianglan Chemicals Co.,Ltd.,China.Ion-exchanged water was used for all the experiments.
2.2.Preparation of hollow B-SiO2@TiO2composites
The hollow B-SiO2@TiO2composites were prepared by depositing TiO2on the surfaces of hollow boron silicate glass microspheres.The preparation procedures were stated as follows.Firstly,100 g of the hollow boron silicate glass microspheres was added into 500 ml of ionexchanged water in a 2 L beaker and the suspension was heated to 85°C in a water bath.TiCl4aqueous solution(42 ml,1.5 mol·L-1)was added dropwise into the suspension in 2 h.During the TiCl4addition process,the pH value of the suspension was adjusted at 2 by adding a 5 wt%NaOH aqueous solution.After aging for 3 h,the as-prepared hollow B-SiO2@TiO2composites were filtrated and washed with ionexchanged water until the pH value of the filtrate was ca.7.And then the samples were dried at 120°C for 24 h.The mass ratio of hollow BSiO2microspheres to TiO2was 100:5 and the composite sample was denoted as B-SiO2@TiO2(100:5).
When the volumes of the TiCl4aqueous solution were 84 and 168 ml,the mass ratios of hollow B-SiO2microspheres to TiO2were 100:10 and 100:20,respectively.The as-prepared composite samples were denoted as B-SiO2@TiO2(100:10)and B-SiO2@TiO2(100:20).
The anatase TiO2sample was also prepared according to the procedures as described above without the addition of the hollow B-SiO2microspheres.
2.3.Degradation of ammonia-nitrogen and green algae
The photocatalytic degradation of ammonia-nitrogen in an aqueous solution was carried out in a 500 ml quartz glass reactor over the asprepared B-SiO2@TiO2photocatalysts under a simulated solar light source irradiation(Xenon lamp,100 W,4%UV light;Visible light at ca.420 nm).Before the photocatalytic reaction,the ammonia-nitrogen adsorption without light irradiation was carried out for 30 min.The typical photocatalytic reaction procedures were stated as follows.An ammonia-nitrogen aqueous solution with an initial ammonia concentration of 50 mg·L-1was prepared by diluting a concentrated ammonia aqueous solution(28 wt%)with ion-exchanged water.The photocatalytic degradation of ammonia-nitrogen was carried out by adding 0.1 g of B-SiO2@TiO2photocatalyst in 200 ml of the ammonianitrogen aqueous solution under stirring and simulated solar light irradiation at 25 °C,pH=8.During the photocatalytic reaction process,2.0 ml of reaction suspension was taken out at different reaction time periods for analysis.
Ammonia-nitrogen was analyzed according to the method of salicylic acid visible absorption spectroscopy(HJ536-2009)[35].The analysis method was briefly illustrated as follows.The reaction suspension was filtrated using a filter with the average pore size of 2 μm and 1 ml of filtrate was diluted to 50 ml.Five milliliters of the diluted solution were added into a 10 ml colorimetric tube.One milliliter of chromogenic solution,2 drops of sodium pentacyanonitrosylferrate(III)(0.1 g in 10 ml H2O)aqueous solution,and 2 drops of sodium hypochlorite aqueous solution(active chlorine of 3.5 g·L-1)were added into the above-mentioned solution.The aqueous solution was diluted to 10 ml by adding ion-exchanged water.The absorbance at 697 nm was recorded on a UV-visible spectrophotometer.The concentration of the ammonia-nitrogen was calculated according to the standard sample.The chromogenic solution was prepared by dissolving 50 g of salicylic acid,80 g of NaOH,and 50 g of potassium sodium tartrate in 800 ml ion-exchanged water and diluted to 1000 ml.
The ammonia-nitrogen conversion was calculated according to Eq.(1).

where XANis the ammonia-nitrogen conversion,C0(mg·L-1)is the ammonia-nitrogen concentration after adsorption in the dark for 30 min,and Ct(mg·L-1)is the ammonia-nitrogen concentration at the reaction time t.
The effect of pH value and reaction temperature on the photocatalytic degradation of ammonia-nitrogen over the B-SiO2@TiO2photocatalysts was also investigated.
The experimental procedures of green algae degradation were illustrated as follows.Green algae were collected from the Yudai river located at Jiangsu University,China.The green algae were separated from the polluted river water sample by filtration.The ground green algae were dispersed in ion-exchanged water.The green algae degradation was photocatalyzed by the B-SiO2@TiO2photocatalysts under visible light irradiation(Xenon lamp,100 W;Visible light at ca.420 nm)in a 500 ml quartz glass reactor.According to the method reported in reference[36],the content of algae in aqueous solution was expressed as the concentration of chlorophyll a,around 5 mg·L-1.The volume of the reaction solution was 250 ml.The concentration of chlorophyll a in the reaction solution at different reaction time was analyzed by the method reported in reference[36].The analysis procedures were briefly stated as follows.
Reaction suspension of ca.10 ml was taken out at different reaction time periods and centrifugated at 8000 r⋅min-1for 10 min.The precipitate was added into 5 ml of ethanol(90%)and ground for 5 min.The mixture was added into a capacity tube and the volume was set at 10 ml by adding ethanol(90%)solution.The sample was kept in a refrigerator at 4°C for 12 h.After the extraction of the remained green algae,the extraction solution was separated by centrifugation at 8000 r⋅min-1for 10 min.The concentration of chlorophyll a in the extraction solution was analyzed by UV-visible light absorption spectroscopy.The concentration of chlorophyll a(Chl.a)was calculated according to the absorbance at the wavelength of 665 nm(Eq.2).Ethanol solution(90%)was taken as the reference sample.

2.4.Characterization of B-SiO2@TiO2samples
The X-ray diffraction(XRD)patterns of the hollow B-SiO2@TiO2samples were obtained on an X-ray diffractometer(D8 super speed Bruke-AEX)using CuKαradiation(λ=0.154056 nm)and scanning from 10° to 80°(2θ).The morphologies of the SiO2@TiO2samples were observed on a scanning electron microscope(SEM,HiTachi S-4800)and a transmission electron microscope(TEM,JEM-2100).The Fourier transform infrared(FT-IR)spectra of the samples were obtained on an IR spectrometer(Nicolet Nexus470)using the KBr disc technique and scanning from 400 cm-1to 4000 cm-1.The surface chemical structures of the samples were determined by X-ray photoelectron spectroscopy(Thermo ESCALAB 250 Xi).The UV-visible diffuse reflectance spectra of the samples were obtained on a UV-Vis-Nir spectrophotometer(UV-3600 Plus).The specific surface areas of the samples were measured by the N2adsorption/desorption technique at-196°C and calculated by the Brunauer-Emmett-Teller(BET)method on a surface area analyzer(Nova 2000e).
3.Results and Discussion
3.1.Characterization of hollow B-SiO2@TiO2samples
The XRD patterns of the hollow B-SiO2microspheres showed that the hollow microspheres consisted of amorphous SiO2with a wide peak at around 23° and B2O3with two diffraction peaks at 14.0° and 28.2°(JCPDS 06-0297)(Fig.1).For the B-SiO2@TiO2samples,XRD peaks appearing at(2θ)25.2°,37.9°,48.4°,54.7°,and 63.1° were observed,which were ascribed to those of the standard anatase TiO2(JCPDS 21-1272).The XRD analysis revealed that the titanium component in the samples was in the anatase TiO2phase.And the content of anatase TiO2increased upon increasing the TiO2loading,which was certified by the increase in its XRD peak intensity.
The SEM images show that the hollow B-SiO2microspheres and the B-SiO2@TiO2samples had sphere shapes(Fig.2).The surfaces of the hollow B-SiO2microspheres were smooth.The B-SiO2@TiO2samples had rough surfaces.No TiO2nanoparticles were found apart from the B-SiO2@TiO2samples,indicating that the TiO2nanoparticles were anchored at the surfaces of the hollow B-SiO2microspheres.The average particle sizes of the hollow B-SiO2microspheres and the B-SiO2@TiO2samples with the B-SiO2/TiO2mass ratios of 100:5,100:10,and 100:20 were 23.5,24.1,24.4,and 24.8 μm,respectively.According to the average diameters of the hollow B-SiO2microspheres and the B-SiO2@TiO2samples,the thicknesses of the TiO2layers on the hollow B-SiO2spheres were 0.3,0.45,and 0.65 μm.

Fig.1.X-ray diffraction patterns of(a)the hollow B-SiO2microspheres and the hollow BSiO2@TiO2samples with different B-SiO2/TiO2mass ratios of(b)100:5,(c)100:10,and(d)100:20.●,anatase TiO2;▼,B2O3.
To determine the thicknesses of the TiO2coating layers,the B-SiO2@TiO2samples were also observed by TEM(Fig.3).The TEM images clearly show that the anatase TiO2coating layers were anchored at the surfaces of the hollow B-SiO2microspheres.The anatase TiO2coating layers were composed of nanosized TiO2particles with the average particle size of ca.3 nm.The thicknesses of the TiO2coating layers increased from 0.33 to 0.47,and 0.64 μm upon changing the B-SiO2/TiO2mass ratios from 100:5 to 100:10,and 100:20.The thicknesses of the TiO2coating layers observed by TEM images were consistent with those calculated according to the SEM images.
The specific surface areas of the B-SiO2@TiO2samples with the mass ratios of 100:5 to 100:10,and 100:20 were 24,25,and 34 m2·g-1,respectively.The surface areas of the B-SiO2/TiO2samples increased with the increase in TiO2loadings.
The surface chemical states of the B-SiO2@TiO2samples were analyzed by XPS.The X-ray photoelectron spectra of O 1s,Si 2p,Ti 2p,and B 1s of the anatase TiO2,hollow B-SiO2microspheres,and hollow BSiO2@TiO2samples are shown in Fig.4.The O 1s binding energies of the TiO2and B-SiO2components in the B-SiO2@TiO2samples were around 530 eV and 532.6 eV,which were larger than those of the anatase TiO2and hollow B-SiO2microsphere(529.8 eV and 532.2 eV),respectively.The binding energies of Ti 2p3/2and Ti 2p1/2of the B-SiO2@TiO2samples were 458.5 and 464.3 eV,respectively.However,the binding energies of Ti 2p3/2and Ti 2p1/2of the anatase TiO2sample were 458.4 and 464.2 eV.The peaks of Ti 2p3/2and Ti 2p1/2of the B-SiO2@TiO2samples slightly shifted to higher binding energies than those of the anatase TiO2sample.
It was interesting to find that the binding energies of Si 2p and B 1s of the B-SiO2@TiO2samples were around 103.2 and 193.0 eV,respectively,which were obviously larger than those of the hollow BSiO2microspheres(102.8 and 192.4 eV).The XPS analysis indicated that coating TiO2layers on the B-SiO2microsphere surfaces affected the chemical states of silicon and boron atoms.It could be explained as that there were chemical interactions between the TiO2coating layers and the B-SiO2microspheres.As compared to the sole anatase TiO2and B-SiO2microspheres,the shifts of the O 1s,Ti 2p,Si 2p,and B 1s of the B-SiO2@TiO2samples were probably caused by the formation of the Ti--O--Si and Ti--O--B bonds at the interfaces[37,38].

Fig.2.SEM images and particle size distributions of(a1,a2)the hollow B-SiO2microspheres and the B-SiO2@TiO2samples with the B-SiO2/TiO2mass ratios of(b1,b2)100:5,(c1,c2)100:10,and(d1,d2)100:20.The inserted images are the magnified ones.
The chemical structures of the B-SiO2@TiO2samples were also analyzed by FT-IR(Fig.5).For the hollow B-SiO2microspheres,The IR bands at 1070 and 799 cm-1were the asymmetric and symmetric stretching vibrations of Si--O--Si bonds.For the B-SiO2@TiO2samples,the bands appearing at 1000 cm-1and 800 cm-1could be ascribed to the stretching vibrations of Si--O--Si and Ti--O--Ti bonds[37].Two new bands appearing at 3410 and 1640 cm-1were also observed,which could be ascribed to the vibrations of hydroxyl groups.The FTIR analysis revealed that--OH groups were present in the TiO2coating layers of the B-SiO2@TiO2samples.

Fig.3.TEM images of the B-SiO2@TiO2samples with the B-SiO2/TiO2weight ratios of(a)100:5,(b)100:10,and(c)100:20.
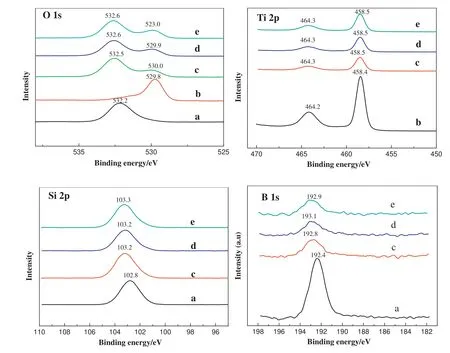
Fig.4.XPS spectra of O 1s,Ti 2p,Si 2p,and B1s of(a)the hollow B-SiO2,(b)the anatase TiO2,and the B-SiO2@TiO2samples with the B-SiO2/TiO2mass ratios of(c)100:5,(d)100:10,and(e)100:20.

Fig.5.Fourier transform infrared(FT-IR)spectra of(a)the hollow B-SiO2microspheres and the B-SiO2@TiO2samples with the B-SiO2/TiO2mass ratios of(b)100:5,(c)100:10,and(d)100:20.
The UV-visible diffuse reflectance spectra of the TiO2nanoparticles(P25)and B-SiO2@TiO2samples are shown in Fig.6.According to the UV-visible diffuse reflectance spectra,it was found that the band gaps of the TiO2nanoparticles(P25)and B-SiO2@TiO2samples with the BSiO2/TiO2mass ratios of 100:5,100:10,and 100:20 were 3.2,3.06,3.0,and 3.02 eV,respectively,indicating that the B-SiO2@TiO2samples could absorb visible light.It was suggested that the interaction between the TiO2nanoparticles and the B-SiO2microspheres constructed a lot of defects in the TiO2coating layers,endowing the TiO2coating layers with the visible light absorption performance.
3.2.Photocatalytic degradation of ammonia-nitrogen
The photocatalytic degradation of ammonia-nitrogen over the BSiO2@TiO2catalysts with different TiO2contents was carried out in 200 ml of ammonia aqueous solution at the pH value of 8 and 25°C under visible light irradiation or simulated solar light irradiation(Fig.7).It was found that under the visible light irradiation,the conversions of ammonia-nitrogen at 660 min were 10.4%,21.1%,and 16%,respectively.The B-SiO2@TiO2composites exhibited photocatalytic activity for the degradation of ammonia-nitrogen under visible light irradiation.Under the simulated solar light irradiation,the conversions of ammonia-nitrogen at 660 min increased to 33.8%,62.2%,and 48.3%,respectively.The simulated solar light irradiation promoted the degradation rate of ammonia-nitrogen.The photocatalytic activity of the B-SiO2@TiO2catalyst with the B-SiO2/TiO2mass ratio of 100:10 was larger than that with the B-SiO2/TiO2mass ratio of 100:20,even the latter had a larger surface area.It could be explained as that the B-SiO2@TiO2(100:10)exposed more defects,giving a higher photocatalytic activity for the ammonia-nitrogen degradation.
The effect of reaction temperature on the ammonia-nitrogen photocatalytic degradation was carried out on the B-SiO2@TiO2(100:10)catalyst(Fig.8).The conversion of ammonia-nitrogen increased upon raising the reaction temperature.At the reaction temperature of 35°C for 660 min,a maximum ammonia-nitrogen conversion of 65%was obtained.It could be explained as that high reaction temperature probably increased the diffusion rate of reactant in aqueous solution,resulting in a higher degradation rate.
The effect of pH value on ammonia-nitrogen degradation was investigated by adjusting the pH values of the reaction solution to 9 and 10 with a trace amount of NaOH aqueous solution(Fig.9).With the increase in pH value,the degradation rate of ammonia-nitrogen increased.At the highest pH value of 10,the maximum ammonianitrogen conversion of 75%was obtained after reacting for 660 min.It could be explained as that at a high pH value,a large amount of OH-anions were available,which could react with the holes formed in the TiO2layers to produce more·OH radicals.The oxidative·OH radicals effectively oxidized ammonia-nitrogen to nitrate and water.

Fig.6.UV-visible diffuse reflectance spectra of the(a)TiO2nanoparticles(P25)and(b-d)B-SiO2@TiO2samples with different B-SiO2/TiO2mass ratios of(b)100:5,(c)100:10,and(d)100:20.

Fig.7.The photocatalytic degradation of ammonia-nitrogen in an aqueous solution(initial concentration of 50 mg·L-1,200 ml,pH of 8,0.1 g catalyst)over the B-SiO2@TiO2catalysts with different B-SiO2/TiO2mass ratios of(a)100:5,(b)100:10,and(c)100:20 under simulated solar light irradiation and visible light irradiation at 25°C.

Fig.8.The photocatalytic degradation of ammonia-nitrogen in an aqueous solution(initial concentration of 50 mg·L-1,200 ml,pH of 8,0.1 g catalyst)over the B-SiO2@TiO2(100:10)catalyst under simulated solar light irradiation at 15,25,and 35°C.
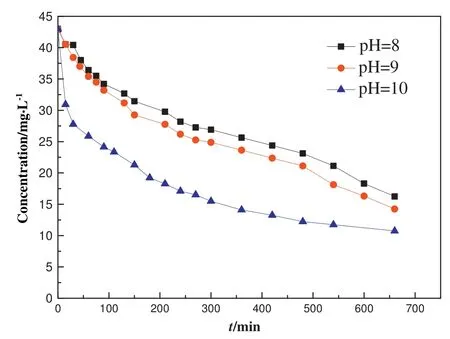
Fig.9.The photocatalytic degradation of ammonia-nitrogen in an aqueous solution(initial concentration of 50 mg·L-1,200 ml,0.1 g catalyst,pH values of 8,9,and 10)over the BSiO2@TiO2(100:10)catalyst under simulated solar light irradiation at 25°C.

Fig.10.The photocatalytic degradation of green algae in an aqueous solution(initial concentration of ca.5 mg·L-1,250 ml,0.08 g catalyst)over the B-SiO2@TiO2catalysts with the B-SiO2/TiO2mass ratios of(a)100:5,(b)100:10,and(c)100:20 under visible light irradiation at 30°C.

Fig.11.The photocatalytic degradation of green algae in an aqueous solution(initial concentration of ca.5 mg·L-1,250 ml)over the B-SiO2@TiO2(100:20)catalyst with different catalyst loadings under visible light irradiation at 30°C.

Fig.12.The photocatalytic degradation of green algae in an aqueous solution(initial concentration of ca.5 mg·L-1,250 ml,0.08 g catalyst)over the B-SiO2@TiO2(100:20)catalyst under visible light irradiation at different reaction temperatures.

Fig.13.Possible photocatalytic degradation mechanisms of ammonia-nitrogen and green algae over B-SiO2@TiO2catalysts under simulated solar light and visible light irradiation.
3.3.Photocatalytic degradation of green algae
The photocatalytic degradation of green algae was carried out over the B-SiO2@TiO2catalysts under visible light irradiation.The degradation extent of green algae increased with the increase in TiO2contents(Fig.10).The maximum algae conversion of 94.5%was obtained when the algae degradation reaction was catalyzed by the B-SiO2@TiO2(100:20)at 30°C for 510 min.
The effect of catalyst loading on the photocatalytic degradation of green algae over the B-SiO2@TiO2(100:20)catalyst at 30°C was investigated(Fig.11).It was found that the catalyst loading of 0.08 g in 250 ml reaction solution was favorable for the photocatalytic degradation of green algae.With further increasing the catalyst loading to 0.1 g,the green algae degradation rate was slightly enhanced.It could be explained as that the excessive catalyst loading probably inhibited the light transmission in the reaction solution.
The effect of reaction temperature on the photocatalytic degradation of green algae is shown in Fig.12.It was found that the photocatalytic degradation extent increased upon raising the reaction temperatures from 25°C to 35°C.At the reaction temperature of 35°C for 510 min,the green algae conversion reached 95.1%.The results revealed that the as-prepared hollow B-SiO2@TiO2catalysts exhibited excellent photocatalytic activity for the green algae degradation under mild reaction temperature.
3.4.Possible photocatalytic mechanisms
The photocatalytic degradation mechanisms of ammonia-nitrogen and green algae were briefly explained as follows.Under UV-visible light irradiation,the electron/hole pairs were formed in the TiO2nanoparticles.The formed electrons could be trapped by the additional energy levels,which were formed due to the presence of Ti--O--Si/Ti--O--B bonds and surface--OH groups.It is worthy of noting that in addition to the formation of electron/hole pairs under the UV light irradiation,the electron/hole pairs could be also formed under the visible light irradiation because the band gap of the B-SiO2@TiO2catalysts was around 3 eV.
During the photocatalytic degradation process,ammonia-nitrogen could be oxidized to form nitrate anion and the green algae could be oxidized to other compounds with a lower toxicity.The possible photocatalytic degradation mechanisms of ammonia-nitrogen and green algae are shown in Fig.13.On the other hand,green algae as a photosensitizer may be also degraded by the photosensitization mechanism under visible light irradiation over the TiO2nanoparticles.
4.Conclusions
The TiO2coating layers could be deposited on the surfaces of the hollow B-SiO2microspheres to prepare the B-SiO2@TiO2composite photocatalysts.The TiO2coating layers could be anchored at the hollow B-SiO2microsphere surfaces via the Ti--O--Si and Ti--O--B bonds.A large amount of hydroxyl groups was also present at the surfaces of TiO2coating layers.The presence of Ti--O--Si/Ti--O--B bonds gave additional energy levels,which could trap the electrons formed under simulated solar light and visible light irradiation.
The degradation of ammonia-nitrogen could be effectively carried out over the B-SiO2@TiO2composite photocatalysts under the simulated solar light irradiation.The maximum photocatalytic conversion of ammonia-nitrogen was 65% when the degradation of ammonianitrogen(43 mg·L-1at pH of 8)was catalyzed by the B-SiO2@TiO2(100:10)photocatalyst at 35°C for 660 min.The B-SiO2@TiO2composite photocatalysts also exhibited excellent photocatalytic activity for the degradation of green algae under visible light irradiation.The green algae were almost completely degraded when the photocatalytic degradation of algae(5 mg·L-1)was catalyzed by the B-SiO2@TiO2(100:20)at 35°C for 510 min.The as-prepared buoyancy B-SiO2@TiO2composite photocatalysts have potential application for the photocatalytic degradation of ammonia-nitrogen and green algae in polluted natural lake and river.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- A review of low-temperature heat recovery technologies for industry processes☆
- Current scenario and potential of biodiesel production from waste cooking oil in Pakistan:An overview☆
- Structure and synthesis of graphene oxide☆
- Co-firing of coal and biomass in oxy-fuel fluidized bed for CO2capture:A review of recent advances
- Effects of internals on phase holdup and backmixing in a slightlyexpanded-bed reactor with gas-liquid concurrent upflow☆
- Distribution performance of gas-liquid mixture in the shell side of spiral-wound heat exchangers☆
