Fe3O4nanoparticles impregnated eggshell as a novel catalyst for enhanced biodiesel production
2019-02-09ChChingakhamAshaDavidSajith
Ch.Chingakham,Asha David,V.Sajith,*
1 School of Nano Science and Technology,National Institute of Technology Calicut,India
2 Malabar Christian College,Calicut,India
Keywords:Catalyst Biodiesel Nanoparticles Fe3O4Impregnation Transesterification
ABSTRACT Biodiesel is a green fuel which can replace diesel while addressing various issues such as scarcity of hydrocarbon fuels and environmental pollution to an extent.The high production cost of biodiesel and the recovery of the catalyst after the transesterification process are the major challenges to be addressed in biodiesel production.In the present work,a cheap and promising solid base oxide catalyst was synthesized from chicken eggshell by calcination at 900°C forming catalyst eggshells(CES)and was impregnated with the nanomagnetic material(Fe3O4)to obtain Fe3O4loaded catalytic eggshell(CES-Fe3O4).Fe3O4nanomaterials were synthesized by co-precipitation method and were loaded in catalytic eggshell by sonication,for better recovery of the catalyst after transesterification process.CES-Fe3O4material was characterized by Thermogravimetric analysis,X-ray diffraction,Fourier transform infrared spectroscopy,a vibrating-sample magnetometer,Brunauer-Emmett-Teller,Dynamic light scattering,and Scanning electron microscopy.Biodiesel was synthesized by transesterification of Pongamia pinnata raw oil with 1:12 oil to methanol molar ratio and 2 wt%catalyst loading for 2 h at a temperature of 65°C and yields were compared.The reusability of the catalyst was studied by the transesterification of the raw oil and its catalytic activity was found to be retained up to 7 cycles with a yield of 98%.
1.Introduction
The demand rate of non-renewable energy sources like petroleum,coals,natural gases etc.is increasing day by day which leads to scarcity of these fuels[1].Apart from ever-growing prices of petroleum-based fuels,the harmful exhaust emissions from automobiles directly affecting human health and the environment are of great concern [2].Hence research is now being directed in the quest of alternative,renewable and eco-friendly fuels for satisfying the energy demand.Biodiesel has drawn unique consideration as a potential alternative fuel for current oil-based non-renewable energy sources [3].Biodiesel is a renewable alternative to diesel,which mainly comprises mono-alkyl esters of unsaturated fatty acid and also has comparable physical properties of diesel,with unique advantages of being renewable and biodegradable with nontoxic emissions [4-7].Biodiesel is generally produced by transesterification of vegetable oils or animal fats using short-chain alcohols like methanol and ethanol in the presence of catalysts.In the initial step of transesterification,triglyceride gets converted to diglyceride,followed by the conversion of subsequent higher glyceride to lower glyceride and then to glycerol,yielding a methyl ester molecule from each glyceride [8].Different types of alcohols such as methanol,ethanol,propanol,and butanol are being used for the biodiesel synthesis.During the reaction,when methanol is utilized as a reactant,unsaturated fatty acid methyl ester blend(FAME)is obtained whereas,in the presence of ethanol unsaturated fatty acid ethyl esters blend(FAEE)is obtained[9-12].
The catalyst used for the biodiesel synthesis can be broadly classified into homogeneous and heterogeneous catalysts.The normal catalyst for the transesterification procedure of vegetable oils is a homogeneous base catalyst,for example,sodium hydroxide(NaOH),potassium hydroxide(KOH)and sodium methoxide(NaOCH3).The main problems with the use of a homogeneous catalyst are the lack of reusability and formation of soap via saponification reaction,which consumes the catalyst,thus reducing the biodiesel yield while making the purification steps more complicated[13].Homogeneous acid catalysts like sulfuric acid lead to serious environmental and corrosion problems [14-17].Consequently,the development of strong heterogeneous catalysts has recently gained much attention owing to their advantage of reusability,without any environmental impacts.Solid catalysts are generally recovered by centrifuging or filtering through the membrane but consume time and energy.Separation of the catalyst with the aid of magnetic field helps to recover the catalyst and increase the number of cycles for transesterification and also has much potential for industrial applications[18-24].
A wide range of heterogeneous catalysts has been investigated by various researchers.Among them,calcium oxide(CaO)and hydroxyapatite from solid waste like waste eggshells,shells and waste animal bones have received much interest due to their mild reaction condition,relatively cheap price and lower impact on the environment[4,6-8,10,12,15,22,24].Eggshell shows high thermal stability,relatively low density and phase continuity as compared to calcium carbonate(CaCO3).In addition,the porous structure of eggshell provides high surface area as compared to artificial materials.The waste eggshell based catalysts demonstrated reasonable performance even though high catalyst loading and high methanol/oil ratio are required.The eggshell based catalyst can be made reusable for transesterification by loading it with Fe3O4nanoparticles,making it magnetic in nature[7].Monica et al.have reported that supporting materials such as Fe3O4for the catalyst CaO reduces the problem of the formation of high viscous glycerin due to the presence of lattice oxygen on the surface of the catalyst.The addition of Fe3O4simplifies the process of separation with the application of an external magnetic field[25].Iron oxide nanoparticles such as magnetite(Fe3O4)have drawn much attention in recent years due to their potential applications in magnetic media,catalysis,color imaging,biomedicine,etc.Fe3O4has been widely used as a catalyst in the field of photocatalysis,biocatalysis and phase transfer catalysis [7,16,17,19].Han et al.reported the use of KF/CaO-Fe3O4as a catalyst for the transesterification.The loading of Fe3O4nanoparticles in eggshell based catalyst not only helps in the recovery of the catalyst but also enhances the surface area and hence the biodiesel yield[19].Xie and Ma have reported Fe3O4as a biocatalyst by treating magnetic Fe3O4nanoparticles with(3-aminopropyl)triethoxysilane for the conversion of soybean oil [3].Chakraborty et al.used hydroxyapatite impregnated with Antimony(III)chloride as a heterogeneous catalyst,synthesized by the calcination of pork bone at 500°C[12].The impregnation of the supporting catalyst was done by magnetic stirring.Nisar et al.synthesized heterogeneous catalyst by the calcination of animal bone at a temperature of 1100°C.[8].Wei et al.and Savaliya et al.synthesized calcium oxide as the catalyst for the transesterification by the calcination of eggshell at a temperature in the range 900-1000°C[4,6].
Recent studies show the potential of eggshell based heterogeneous catalyst for transesterification,owing to its low production cost and high basicity.In this present work,we report a novel method of using waste chicken eggshells loaded with Fe3O4nanoparticles as a reusable heterogeneous catalyst for the transesterification.Fe3O4nanomaterials were synthesized by co-precipitation method and were loaded in catalytic eggshell by sonication.Biodiesel was synthesized by conventional transesterification method with methanol recovery setup.The effect of parameters such as molar ratio,time,temperature and loading of catalyst was studied.The synthesized methyl ester was analyzed using gas chromatography-mass spectrometry [GC-MS]to evaluate the yield and purity.XRD data was refined by using Rietveld refinement software and quantified the amount of metal hydroxide Ca(OH)2and metal carbonate CaCO3conversion during the formation CES-Fe3O4,which has not been reported earlier.
2.Materials and Methodology
2.1.Synthesis of ferric oxide(Fe3O4)nanoparticles
Ferric oxide nanoparticles were synthesized by co-precipitation method.1 mol·L−1of ferrous sulfate(FeSO4·7H2O)and mol·L−1of ferric sulfate heptahydrate(Fe2(SO4)3·7H2O)were mixed in 60 ml of deionized(DI)water and sonicated for 30 min while maintaining the temperature at 65°C.Ammonia(NH3)solution was added drop by drop to the solution till the pH reaches 12,finally forming a black precipitate.Ferric oxide nanoparticles were removed by applying an external magnetic field and were washed several times with DI water until the pH drops to 7.The material was dried at 70°C for 12 h to obtain ferric oxide nanoparticles.
2.2.Preparation of CES-Fe3O4based heterogeneous catalyst
Eggshells of chicken were collected from different hotels.The eggshells were washed few times with DI water to remove the impurities and dried at 100 °C in an oven.The dried eggshell was crushed and grounded to fine powder,followed by calcination in a furnace under atmospheric conditions at different temperatures(500-900°C)for 4 h[4].CaCO3is converted to CaO by calcination and the eggshell powder obtained after the calcination was stored in a desiccator for 24 h,to avoid catalytic poisoning due to contact with air[6].For the preparation of Fe3O4loaded eggshell based catalyst,3 g of CaO and 1-5 wt%of Fe3O4nanoparticles were taken in 30 ml and 20 ml of DI water,respectively and mixed by means of a magnetic stirrer followed by ultrasonication for 10 h,thus loading the Fe3O4nanoparticles in catalyst eggshells.The solution was dried for 12 h in a hot air oven followed by calcination for 4 h at 600 °C.The sample was grounded into a fine powder and stored in a vacuum desiccator.
2.3.Characterization
The calcined egg shells were characterized by Thermogravimetric analysis(TGA),X-ray diffraction(XRD),Scanning electron microscopy(SEM),Transmission electron microscopy(TEM),Fourier transform infrared spectroscopy(FT-IR),Brunauer-Emmett-Teller(BET),and a vibrating sample magnetometer (VSM).TGA (Perkin Elmer)experiments were carried out under nitrogen atmosphere in the temperature range 100 to 1000°C with a heating rate of 10°C·min−1.Xray diffraction patterns were obtained using an X-ray diffractometer(Rigaku Miniflex 600)and Rietveld refinement method was used for refining the XRD data.The refinement of XRD data was done under slow scanning of 0.02 degree stepping and Fullprof suite program was used for the refinement process.Scanning electron microscopy (Hitachi,SU6600)and Transmission electron microscopy (Philips Tecnai G2 F20)analysis of the catalyst was done to obtain the size and morphology.FT-IR spectroscopy was performed in the wavelength range 400-4000 cm−1.The magnetization curve properties of the samples were studied using a vibrating sample magnetometer(VSM,Lakeshore VSM 7410)at room temperature.The specific surface area of the prepared catalyst samples was characterized by a Surface area analyzer(Belsorp max,Microtrac Belcorp).The average size of the materials was measured by means of DLS (Malvern Nano ZS).The fatty acid profile of Pongamia oil,both before and after transesterification was obtained using gas chromatography-mass spectrometry (GC-MS)(JEOL GC MATE II).
2.4.Transesterification
Pongamia pinnata oil was washed with hot water and filtered thoroughly to remove the undissolved materials.The oil was dried in a dry oven for 24 h.The acid value of the oil was 6.3 mg NaOH·g−1with a free fatty acid content of 3.15 wt%.As the free fatty acid content is high,esterification was done before the transesterification reaction in a three-necked flask.In the esterification reaction,100 ml of P.pinnata oil was dried in an oven at 110°C for 12 h.The oil was blended with 5(v/w)% of sulfuric acid and the molar ratio of oil and methanol was fixed as 1:6[15].The reaction was performed for 1 h at 65°C and the speed of the mechanical stirrer was set at 600 rpm.The reaction mixture was transferred to a separating funnel on completion of the reaction.Esterified oil was used for transesterification and CES-Fe3O4powder was used as a heterogeneous catalyst while optimizing the parameters.
The fatty acid composition of the biodiesel synthesized from raw P.pinnata was determined by using a gas chromatograph with a mass spectrometer(JEOL GC MATE II).The retention time and the percentage of relative abundance of fatty acid were obtained from the GC-MS data.The area of the peaks in the GC-MS data was used for the estimation of FAME conversion[8,12,15,22],as follows:

where ∑A denotes the total peak area,ASIdenotes the peak area of methyl heptadeconoate(internal standard),CSIis the concentration of methyl heptadecanoate(mg·ml−1),VSIis the volume of internal standard solution used(ml)and w is the mass of biodiesel sample(mg).
The percentage yield is obtained from FAME as follows:

where WBiodieselis the weight of biodiesel produced and WRawoilis the weight of raw oil(P.pinnata)used.
3.Results and Discussions
3.1.Catalyst characterization
The thermal stability of the calcined eggshell was studied by thermogravimetric analysis and is shown in Fig.1(a).TGA results show weight loss in the temperature range of 300°C-400°C and above 800°C,the weight of the sample remains constant.The eggshell mainly contains CaCO3and transformation of the CaCO3to CaO occurs at a temperature more than 800°C[4,6].Hence calcination temperature of eggshell was fixed at 900°C to obtain CaO catalyst from the waste eggshell.
FTIR spectrum in Fig.1(b)of eggshell shows that most of the peaks are in the range 1444 cm−1,874 cm−1,549 cm−1and 461 cm−1which corresponds to the existence of asymmetric stretch,out of the plane bend and in-plane bend of the CES-Fe3O4,respectively[26-28].CaO catalyst was synthesized from chicken eggshell and its absorption band peak is less than 1064 cm−1.The eggshells on calcination,lose carbonate due to the decomposition of CaCO3to CaO,which results in the decrease in the mass of the functional group attached to the CO3−ions accordingly,diminishing the intensity of CaCO3peaks.A new peak around 3638 cm−1corresponding to OH-stretching vibration and bending hydroxyl group was also observed in the FTIR spectra of the calcined eggshells,which corresponds to the OH−groups in calcium hydroxide,Ca(OH)2[10,16,26].
Fig.2(a)shows the magnetic hysteresis of the Fe3O4particles and magnetic composite catalysts measured at room temperature by VSM.Fe3O4particle exhibits superparamagnetic behavior and the saturation magnetization is around 29.852 emu[7,17,19].The coercivity,sensitivity,and retentivity were found to be 16.599 G,−2.6000 emu and 542.80 emu,respectively.CES-Fe3O4particles exhibit comparatively low superparamagnetic behavior with saturation magnetization around 19.465 emu [19].The coercivity,sensitivity,and retentivity of CESFe3O4particles were found to be 14.621 G,−2.6000 emu and 318.77 emu respectively.However,after the tenth cycle of transesterification with CES-Fe3O4particles as a catalyst,it exhibits a low superparamagnetic behavior with a magnetization around 1.2390 emu as seen in Fig.2(a).The corresponding coercivity,sensitivity,and retentivity were found to be 18.7686 G,−2.6000 emu and 542.80 emu,respectively.The magnetic composite catalyst can be recovered with the aid of a magnetic field [29].The surface area and pore size of CES and CES-Fe3O4based heterogeneous catalysts were measured by sorption BET method.Fig.2(b)shows the graphic isotherms of adsorption-desorption of CES and CES-Fe3O4based catalysts.The surface area for CES and CES-Fe3O4materials was found to be 7.026 m2·g−1and 25.618 m2·g−1,respectively and the pore volume estimated for these samples was 0.0106 cm3·g−1and 0.0956 cm3·g−1respectively.Pore distribution was found to be high for CES-Fe3O4based catalyst,with a pore size of 14.929 nm,which is classified as mesoporous type[14].The isotherm graph of CES and CES-Fe3O4shows that it belongs to Type IV of the isotherm,attributing to monolayer-multilayer adsorption[30-32].The area of the hysteresis loop formed between the adsorption and desorption curve in Fig.2(b)indicates that the catalytic behavior of the CES-Fe3O4catalyst is better as compared to CES catalyst due to increase in the surface area owing to the formation of flakes and rough sheets as shown in the TEM image Fig.4(b).
The average size of the CES and CES-Fe3O4samples measured by DLS was found to be 2612 nm and 1629 nm respectively,as shown in Fig.2(c).The smaller size was observed for CES-Fe3O4sample,due to continuous sonication for 10 h during the preparation of nanocomposite catalyst.The single peak in the size distribution of CES-Fe3O4sample confirms the presence of uniform size particles whereas the two peaks for the CES sample is due to the presence of different sized particles.Fig.3(a)shows the SEM images of the raw eggshell which has an irregular crystal structure.Even though eggshell has an irregular crystal structure,flower petal-like structures are formed after calcination as shown in Fig.3(b).The TEM image of Fe3O4loaded calcined eggshell after sonication for 10 h is shown in Fig.4(b)which confirms the presence of spherical Fe3O4nanoparticles in the structure of calcined eggshell.SEM image of Fe3O4loaded calcined eggshell shows a rough flake structure in Fig.4(a).Due to the continuous sonication for 10 h,the petal structures were converted to a flake structure.Fig.4(c)shows the polycrystalline diffraction patterns in the Selected Area Electron Diffraction(SAED)pattern of CES-Fe3O4nanoparticles.The identified diffraction planes correspond to(201),(100),(102),(101),(001),(110)and (111)indicating that particles are in crystalline phase[3,7,16,17,19].These results are consistent with those obtained from XRD analysis,seen in Fig.4(e).Fig.4(d)shows the image of catalyst CES-Fe3O4recovery by using an external magnet.In addition,TEM image reveals that the Fe3O4sample displays a well-defined crystal lattice stripe with a spacing of 0.282 nm and 0.492 nm corresponding to the(220)and(001)planes respectively,as shown in Fig.4(f)[3].The energy dispersive spectroscopy results(Fig.4(g))confirm the presence of Iron(Fe),Oxygen(O),and Calcium(Ca)elements in the prepared sample and Carbon(C)which could be due to the formation of CaCO3.

Fig.2.(a)VSM image of Fe3O4and CES-Fe3O4.(b)Curves of adsorption-desorption isotherm of CES and CES-Fe3O4.(c)Size distribution of CES and CES-Fe3O4.
Fig.5(a)shows the transformation of the catalyst into new phases[3,6,7,19].The peaks corresponding to angles 17.3°,29.2°,47.4°,72°,84.1°,33.6°,51.3°,54.6°,62.3°,and 64.2°is clearly visible from Fig.5(a).The sonication of CaO catalyst in Fe3O4solution results in the conversion of CaO to Ca(OH)2and also to CaCO3phase loaded with Fe3O4.CaO catalyst being unstable and hygroscopic in nature tends to convert to a stable form.The formation of Ca(OH)2helps to improve the yield,even though the drawback for the formation of hydroxide compound has been reported[19].Han et al.have reported the benefit of formation of hydroxide which increases the biodiesel yield.The water molecules adsorbed initially on the metal oxide catalyst surface forms metal hydroxide(OH−)which helps to accept one proton from methanol to generate methoxide(CH3O−)[19].The high conversion of oil to methyl ester observed in the present work could be due to the formation of excess metal hydroxide Ca(OH)2of 94%,as per the Rietveld refinement data.
The XRD pattern shows the confirmation of CES-Fe3O4and was further studied by using the Rietveld refinement method to determine the formation of Ca(OH)2and CaCO3.The detailed structural properties of prepared Ca(OH)2were obtained through Rietveld refinement of the XRD pattern,as shown in Fig.5(b).The refined XRD data confirms that the prepared sample contains 94% of Ca(OH)2phase and 6% of CaCO3phase.Further,structural details of individual phases were also extracted from the refinement output.The presence of a higher percentage of hydroxide phase in the material results in the better conversion of oil to methyl ester.

Fig.3.SEM image of(a)raw eggshell;(b)CES after calcination at 900°C.

Fig.4.(a)SEM image of CES-Fe3O4.(b)TEM image of CES-Fe3O4.(c)Pattern of CES-Fe3O4nanoparticles.(d)Recovery of catalyst CES-Fe3O4by using an external magnet.(e)XRD data of CES-Fe3O4.(f)Surface morphology and SAED.(g)EDS spectra of CES-Fe3O4.
3.2.Transesterification reaction
The transesterification of P.pinnata oil was done with CES-Fe3O4as a catalyst.Various parameters affecting the transesterification of the biodiesel includes(i)reaction temperature,(ii)reaction time,(iii)catalyst loading and(iv)oil to methanol ratio.The parameters were optimized to obtain the maximum yield of biodiesel based on one-factor approach and the details are presented here.
3.2.1.Temperature
The effect of reaction temperature on transesterification of P.pinnata oil was studied and results are shown in Fig.6(a).FAME yield was found to be increased with increase in temperature,and achieved a maximum value at 65°C,followed by a decrease in the yield[4].The increase in the yield with the temperature could be due to the high mobility of molecules with increase in temperature resulting in high reaction rate[33].However as the reaction temperature goes beyond 70°C,which is the boiling temperature of methanol,methanol evaporates decreasing the biodiesel yield.Hence the reaction temperature was fixed at 65°C.
3.2.2.Reaction time
The effect of reaction temperature on the biodiesel yield was studied and the results are shown in Fig.6(b).The biodiesel yield was found to increase with an increase in the reaction time,with a maximum yield of 98%attained for a reaction time of 120 min,followed by a decrease in yield with time[6].The reaction could have achieved equilibrium at the end of 120 min and afterward the shifting of the reaction occurs in the reverse direction,leading to the soap formation[34].Hence the reaction time was fixed at 120 min.
3.2.3.Catalyst loading

Fig.5.(a)XRD pattern of the CES-Fe3O4with confirmation phases.(b)Refinement data of CES-Fe3O4.

Fig.6.Variation of biodiesel yield with(a)reaction temperature,(b)reaction time,(c)loading of catalyst and(d)oil to molar ratio.
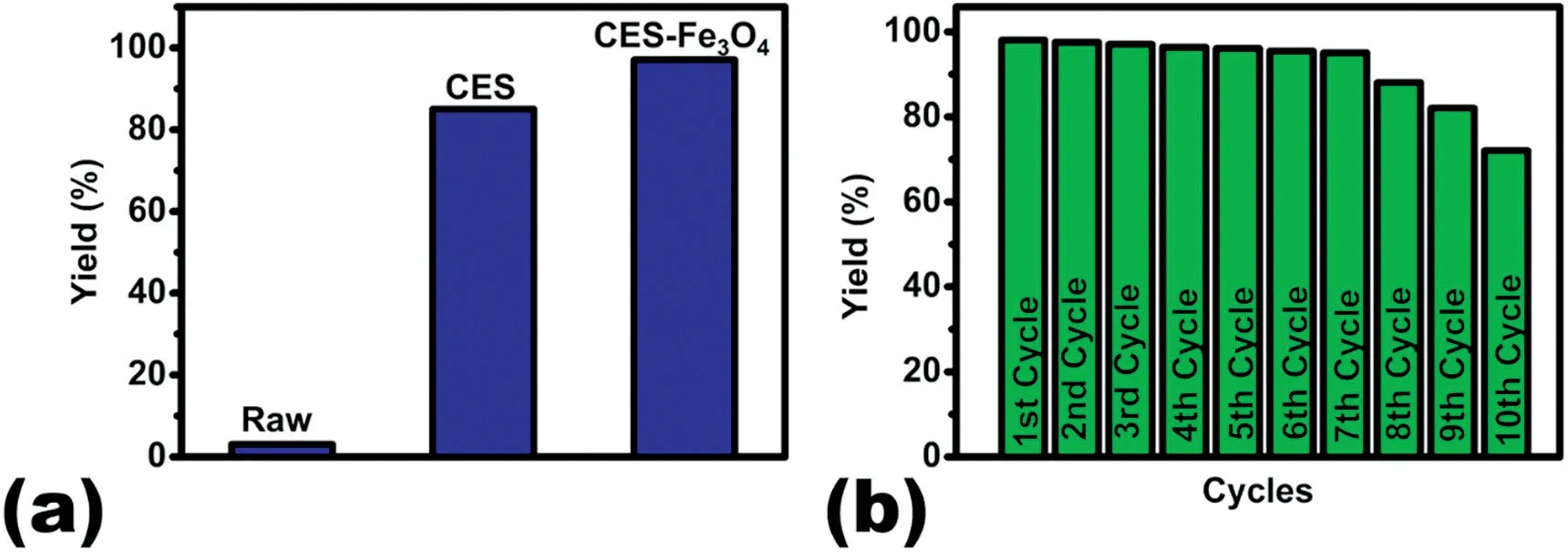
Fig.7.(a)Biodiesel yield comparison.(b)Reusability of the CES-Fe3O4.
The catalyst loading directly affects the biodiesel yield in a transesterification reaction.The high free fatty acid in the feedstock,results in the soap formation,influencing the separation process which limits the FAME yield.The variation of the yield with the catalyst loading is shown in Fig.6(c).The biodiesel yield was found to increase with an increase in the catalyst loading,up to 2 wt%loading with a biodiesel yield of 98%.However,a decrease in the yield was observed with further increase in the concentration of catalyst.An excess amount of catalyst adversely affects the mixing of methanol,oil,and catalyst leading to phase separation [35].Hence in the present study,catalytic loading was fixed as 2 wt%.
3.2.4.Oil to methanol molar ratio
Oil to methanol molar ratio directly affects the biodiesel yield.The variation of the yield with the oil:methanol ratio is shown in Fig.6(d).As the methanol to oil ratio increases from 1:3 to 1:12 the biodiesel yield was found to increase from 56 to 98%,with further decrease in the yield[27].Normally the excess methanol favors the shift in the equilibrium,to achieve high biodiesel yield[36].However,as the methanol concentration increases,the contents of catalyst and reactants are dissolved by methanol,which may inhibit the reaction thus reducing the biodiesel yield.The oil to methanol molar ratio was fixed at 1:12.

Table 1 GC-MS data of raw oil;Pongamia pinnata oil.

Table 2 GC-MS data of Pongamia pinnata oil based methyl ester
3.3.Yield comparison
The transesterification of P.pinnata oil was done by using raw animal bone,CES and CES-Fe3O4as a catalyst,with the optimized parameters and yield were compared as shown in Fig.7(a).The improved catalytic behavior of CES-Fe3O4treated sample is mainly due to the increase in surface area and pore volume due to the layered structure as mentioned earlier[26,31].The raw eggshell cannot be used as a catalyst as it does not help in the conversion of methanol to methoxide,which aids in the conversion of triglycerides to methyl ester.

Fig.8.GC-MS chromatogram of(a)raw Pongamia pinnata oil and(b)biodiesel synthesized from Pongamia pinnata oil.

Table 3 Comparison of catalytic activity with reported eggshells based catalysts
The economic feasibility of biodiesel production at large scale depends on the reusability and stability of the calcium-based catalyst.The reusability of the calcined chicken eggshell(900°C)was studied by carrying out the transesterification under the optimized condition,with an oil to methanol ratio of 1:12,a catalyst loading of 2 wt%,a reaction temperature of 65 °C and a response time of 120 min.Catalysts recovered after the transesterification reaction were washed and reused and yield obtained in each cycle was compared.Fig.7(b)shows the variation of biodiesel yield with the cycles of transesterification.The biodiesel yield of 98%was obtained initially which was found to be consistent until the 7th cycle of the transesterification reaction,followed by a decrease in the yield.The decrease in the yield is mainly due to blockage of catalyst pores which further reduces the adsorption and desorption of the reactants.
The fatty acid composition of P.pinnata oil was analyzed by using gas chromatography-mass spectrometry.Tables 1 and 2 show the GC-MS data of P.pinnata oil and P.pinnata oil biodiesel respectively,in which retention time and percentage of relative abundance of fatty acid are shown.Different peaks in the GC-MS chromatogram represent the presence of methyl esters and the maximum peak indicates the presence of Octadecenoic acid (43.94%)methyl ester and a minimum of the presence of Margaric acid(0.09%).Fig.8(b)shows that the presence of compounds with carbon atoms in the range C17-C19is maximum,while the compounds with carbon atoms in the range C15-C16is minimum.GC-MS results also establish the successful transformation of palm oil into biodiesel[15,22].From GC-MS chromatogram Fig.8(a),7 major peaks of various fatty acids were visible in the case of raw P.pinnata oil whereas 13 major peaks were visible in the case of P.pinnata oil based biodiesel.The increase in the number of peaks of methyl esters could be due to the conversion of raw P.pinnata to methyl ester(biodiesel),shown in Fig.8(b)[37].The abundance data in Fig.8(b)is higher than that in Fig.8(a),which shows good conversion of raw oil to methyl ester(biodiesel)[38].Table 3 shows the comparison of the present work with that of similar works on eggshell/shell based heterogeneous catalyst,reported earlier.Fig.9 shows the schematic diagram of preparation and working mechanism of CES-Fe3O4.
4.Conclusions
In summary,we have demonstrated a simple route for the synthesis of magnetic recoverable CES-Fe3O4cheap catalyst from waste eggshells.The solid base oxide catalyst was synthesized by calcination of chicken eggshell at 900 °C and was loaded with the nanomagnetic material(Fe3O4).Fe3O4nanomaterials were synthesized by co-precipitation method and were loaded in calcined eggshell by sonication.The calcination followed by sonication for 10 h results in the formation of flake-like structures with enhanced surface area.The surface area of calcined chicken eggshell was found to be increased from 7.026 m2·g−1to 25.618 m2·g−1with the loading of Fe3O4nanoparticles.The transesterification of P.pinnata oil was done using Fe3O4loaded calcined chicken eggshell and the parameters were optimized.The sonication of calcined chicken eggshell in Fe3O4solution results in the conversion of CaO to Ca(OH)2which aids in the enhancement of the biodiesel yield.The reusability of the catalyst was studied by performing the transesterification using the same catalyst for 10 cycles and the yield was found to be slightly decreased after the 7th cycle,which could be due to the blockage of catalyst pores,reducing adsorption and desorption of the reactants.

Fig.9.Adsorption and desorption on the active site of the heterogeneous catalyst-schematic.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scaling of the bubble/slug length of Taylor flow in a meandering microchannel☆
- Analyzing of mixing performance determination factors for the structure of radial multiple jets-in-crossflow☆
- Particle-resolved simulation of packed beds by non-body conforming locally refined orthogonal hexahedral mesh☆
- Visual study on the characteristics of liquid and droplet in a novel rotor-stator reactor☆
- Molecular dynamics simulation of supercritical CO2microemulsion with ionic liquid domains:Structures and properties☆
- Modeling bubble column reactor with the volume of fluid approach:Comparison of surface tension models☆
