Suppression of methane/air explosion by water mist with potassium halide additives driven by CO2☆
2019-02-09WeiTanDongLiyanLiuGuoruiZhuNanJiang
Wei Tan,Dong Lü,,Liyan Liu,*,Guorui Zhu,Nan Jiang
1School of Chemical Engineering and Technology,Tianjin University,Tianjin 300354,China
2 Tianjin Fire Research Institute of MEM,Tianjin 300381,China
Keywords:Methane Explosion suppression Water mist Halide CO2
ABSTRACT To enhance the explosion suppression effects of water mist,various potassium halide additives were tested in a confnied vessel filled with a 10%mixture of methane/air.Air and CO2(0.7 MPa)were used as driver gases.The results revealed that halide additives exhibit considerable suppression effects on explosion overpressure.A 30%KI mist decreased the explosion overpressure by 27.46%compared with the suppression by pure water mist under the same conditions.When CO2is used as the driver gas,it will dissolve in water under high pressure.The synergistic effect of a CO2solution with an effective additive afforded significant suppression.Under the same conditions,the overpressures suppressed by a mist of 30%KI+0.7 MPa CO2solution decreased by 33.53%compared with those suppressed by pure water mist driven by air.The synergistic suppression effect is much better than that of a 0.7 MPa CO2solution mist or 30%KI mist alone.The multicomponent additives can be considered when suppressing methane/air explosions with pressure-formed water mist.
1.Introduction
Many studies on the use of ultrasonically formed water fog with and without additives to suppress gas explosions have been reported to date.Zhang et al.[1]used water fog to suppress methane explosions in a closed vessel.P.G.Holborn[2,3]used water fog to mitigate lean hydrogen deflagrations and modeled this mitigation process in a nuclear waste silo ullage.Some studies have suggested that the use of additives in water fog can enhance its explosion suppression ability.Cao et al.[4,5]used“ultrafine water mist”(formed by an ultrasonic atomization system;average diameters <8.3 μm)of a NaCl solution to suppress a methane explosion.Some studies enhanced the suppression effect of water fog with additives by other methods.For example,Yu et al.[6]changed the character of NaCl water fog with an electric charge to enhance its suppression ability.
In practice,fire rescue departments prefer to use common water mist formed by high pressure,not water fog formed by ultrasonic methods,for rescue in most flammable gas leakage accidents.Water fogs formed by an ultrasonic atomization system often have sizes of approximately 10 μm [7]and slow initial speeds,which is unfavorable when aiming from a long distance.Water mist formed by high pressure is a more reasonable alternative in emergencies involving flammable gas leakage accidents,because it can provide a sufficient flow rate to dilute the explosive gas,cool the heat source[8],and suppress the explosion from an adequate protection distance.Water mist spray systems cannot respond quickly enough to a gas explosion shock wave,and so users often spray it in advance to decrease the potential consequences.For example,the Lanzhou fire branch used water spray lances to produce water mist pressurized by a water pump to contain natural gas leaking from the main pipe of the natural gas network of the city[9]in the Chengguan district of Lanzhou,China.However,one issue cannot be ignored:the water mist may increase the explosion overpressure,due to its high momentum.Van Wingerden et al.reported that water mist larger than 50 μm can enhance turbulence[10].This will premix the flammable gas with air and enhance the flame turbulence,leading to an increase in flame propagation speed,and finally increase the explosion overpressure.To improve this shortcoming,the explosion suppression effects of additives were researched in this work.
Some chloride salts can ionize at high temperatures and subsequently take part in free radical chain reactions to inhibit an explosion[6].Furthermore,other work has suggested that Cl−in the droplets can also destroy a portion of these free radicals in the chain reaction[4].Many potassium salt additives have been used as the active substance during the process of fire extinguishing by water mist;they show a capacity to capture free radicals formed in flames[11-13].In this work,the explosion suppression effect of potassium halide additives was investigated.
Nitrogen combined with ultrafine water mist/fog has been used to suppress gas explosions.Ingram et al.[14]used“fine water mist”(with a droplet diameter <2.5 μm)containing sodium hydroxide additives to suppress an explosion of a hydrogen/oxygen/nitrogen mixture.Holborn et al.[15,16]used“ultrafine water mist”(with a diameter <10 μm)with nitrogen to suppress a gas explosion.Some researchers suppressed methane explosions by CO2/N2[17]and CO2/ABC powder[18,19].As an inert gas to combustion,CO2readily dissolves in water under pressure.Compared to water and nitrogen,when pressurized CO2is used as the driver gas for water mist,it dissolves in water and forms a H2CO3/CO2homogeneous solution,which can be easily sprayed into dangerous areas.When the H2CO3/CO2water solution is sprayed out from a nozzle,CO2will escape from the water mist due to the loss in pressure,especially at high temperatures,thereby making the explosion environment cool and inert.In this work,the synergistic effect of pressurized H2CO3/CO2water solutions with KI was investigated.
2.Experimental Setup and Procedures
2.1.Experimental setup
To test the suppression effects of different additives,a series of methane/air explosions were suppressed by water mist with and without additives in a stainless steel container filled with a 10% methane/air mixture.Meanwhile,to test the effect of the additives selected by the small-scale experiment,enlarged tentative trials with a volume of 1 m×1 m×1.6 m were conduct as a preliminary study,which is presented in the supplementary material.
A container,15 cm×15 cm×90 cm,with front and rear openings sealed with quartz glass,was made as the explosion vessel.An electric igniter supplied by an ignition transformer with an output voltage of 2× 7.5 kV (double electrodes,maximum discharge voltage=7.5 kV)and an RMS current of 20 mA was fixed to the bottom of the explosion vessel.A high frequency pressure sensor of −0.1-2 MPa and a common water spray nozzle were fixed mid-length and at the top of the explosion vessel,respectively.A high-pressure water tank comprising a water inlet and pressure gauge was fixed on a steel ladder at a height of 2.5 m.An air compressor was employed to maintain the water tank at high pressure.A high-pressure CO2bottle was connected to the water tank to provide high pressure H2CO3/CO2water solutions to test the synergistic suppression effect of the additives.A vacuum pump was fixed to the bottom of the explosion vessel.A methane bottle was connected to the explosion vessel through reduction valves.A highspeed camera with a frame rate of 1000 FPS was set in front of the explosion vessel to record the process.The sketch of the experimental apparatus is presented in Fig.1.
2.2.Experimental procedures
The tank was first filled with 2000 ml water(or water with additives).Compressed air(or CO2)was then used to pressurize the water tank to 0.7 MPa.A 10%methane/air mixture was achieved in the explosion vessel by the partial pressure method.Vacuum the explosion vessel to −0.025 MPa by the air extractor,then open the ball valves of the methane bottle and turn on the pressure reducing valve until the pressure of the explosion vessel is −0.015 MPa.Then,turn on the air inlet valve to let air into the explosion vessel slowly.When the indication of the vacuum gauge becomes zero,turn off the air inlet valves.Finally,stand for 5 min before ignition.The ignition,water mist,and data collection were controlled by software programed with C++6.0.
KCl,KBr,and KI were tested as halide additives.Due to their toxicity,fluorides were not included in the study.CO2(0.7 MPa)was used to produce water mist instead of air as another driver gas.Compared to air,CO2can easily dissolve in water,forming an additive+CO2multicomponent solution.Thereby,the synergistic effect of a CO2solution with effective additive was analyzed.Enough CO2should be added to maintain a pressure of 0.7 MPa for more than 60 min.To compare the suppression effects of the different additives,the overpressures without any suppression and with pure water mist(without additives)were tested as baselines.
The temperatures during the experiments varied from 20-25 °C.Each test was repeated five times under identical conditions in this work.
2.3.Trial design
To assess the suppression effect of the mists with additives,explosions without any suppression and with pure water mist suppression were selected as baselines.To ensure the even distribution and higher turbulence of the mixture,more closely simulating the practical scenario,the premix was sprayed with water mist for 3 s before stopping the spray and igniting the mixture.
The trials for alternative baselines are listed in Table 1.
The three scenarios are illustrated in Fig.2.
The tests of the explosion overpressures suppressed by water mist with additives were also conducted according to Scenario 3.
Suppression effects of mists with additives were compared under the same conditions by testing the maximum explosion overpressures.Considering the saturation concentrations of the additives,30% and 15%concentrations were chosen for the samples whose saturation concentrations ≥30%at 0°C.The saturation concentration of KCl is 21.94%at 0°C,and thus 20%and 15%were chosen.The additives and their concentrations are shown in Table 2.

Fig.1.Sketch of the explosion experimental setup.

Table 1 Three suppression scenarios for alternative baselines
To research the synergistic effects of effective additives and CO2solution,a solution of 0.7 MPa CO2combined with the most effective salt was tested in this work.
Each trial was repeated 5 times under identical conditions.
The water mist diameter and the flow rate of the test nozzle were tested by the CNCF(China National Center for Quality Supervision and Test of Fixed Fire-fighting System and Fire-resisting Building Components).The water mist diameters are listed in Table 3.The flow rate of the nozzle is 1.31 L·min−1under 0.7 MPa.
3.Results and Discussion
Overpressure was selected as the parameter to indicate the explosion intensity in this work.Two indexes,Pi/P0and Pi/Pw,were proposed to compare the suppression effects,where Piwas an explosion overpressure suppressed by any additive,P0was the overpressure without any suppression,and Pwwas the overpressure suppressed by pure water mist.According to Section 2.3,two scenarios(Scenario 1 and Scenario 2)could be selected as the candidate for use as P0.The selection of P0is discussed in the following section.
3.1.P0and Pwdetermination
The values of P0and Pware listed in Table 4,accompanied by their respective mean overpressures.
Compared with scenario 1 and scenario 2,scenario 2 provided 3 s spraying before igniting,which makes a similar early stage condition as real rescue and scenario 3.In a real rescue scenario with leaking flammable gas,firemen will spray water mist(or that with additives)continuously in advance and wait for the leaked gas to dissipate or enter dangerous areas to save lives.At that point,a potential explosion may occur.Therefore,scenario 2 is closer to scenario 3.Thus,the mean overpressure in scenario 2(6.16×105Pa)was chosen as P0,and the mean overpressure in scenario 3(6.92×105Pa)was chosen as Pw.
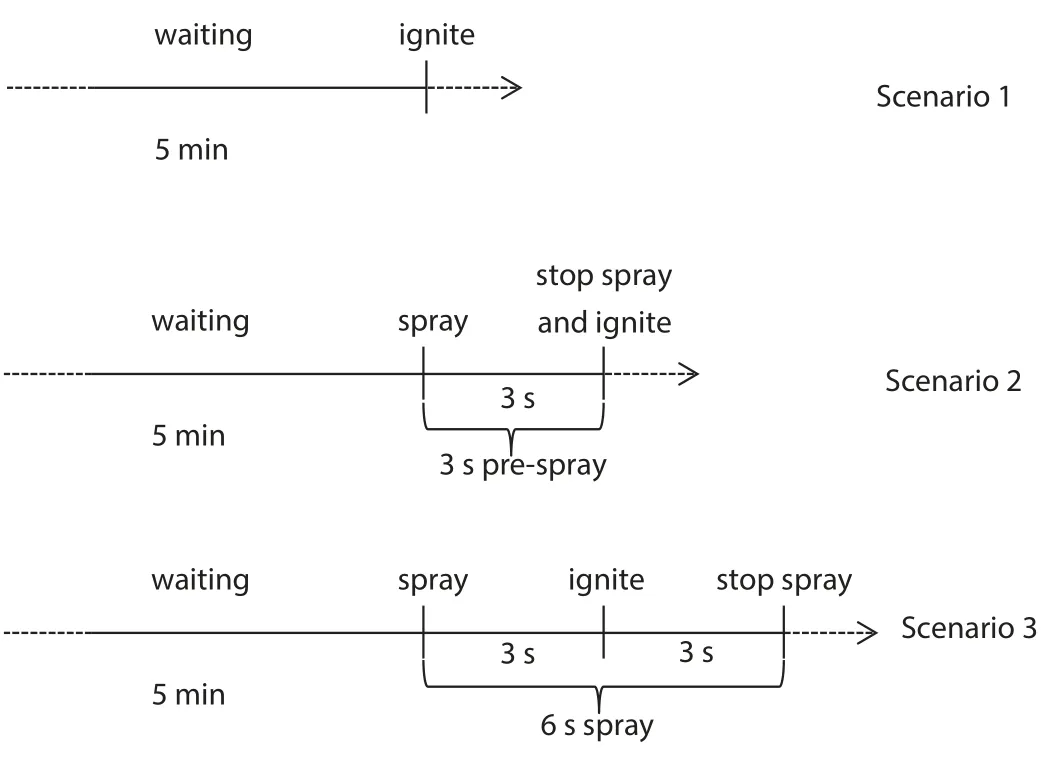
Fig.2.Three suppression scenarios of spraying water mist.

Table 2 Testing additives and concentrations
The mean overpressure in scenario 2 was higher than that in scenario 1,possibly because the 3-s prespray in scenario 2 mixed the methane/air mixture more homogeneously.Furthermore,the mean overpressure in scenario 3(suppressed by pure water mist)was higher than those without any suppression(Scenarios 1 and 2).These adverse results may occur in realistic rescues.If we use pure water mist to suppress a possible explosion,the suppression process may enhance the explosion overpressure.This destructive outcome occurs because the water mists flow at high speeds,and their large particle size promotes the mixing of the flammable gas with air,enhancing the turbulence and flame propagation speed during explosion.The results for explosions suppressed by mist with additives are listed in the following sections.
3.2.Suppression effects of halide additives
3.2.1.Results of overpressures suppressed by halide additives
The explosion overpressure results suppressed by halide additives are listed in Table 5.
Mean overpressures of halide additives at different concentrations with error bars are presented in Fig.3.
The halide additives effectively suppressed methane explosions,and the peak overpressure decreased as the atomic number of the halogen increased(at the same concentration).Therefore,the suppression ability of the halide additives was of the order KI >KBr >KCl.Additionally,the suppression abilities of these additives increase with an increase in their concentration.
3.2.2.Analysis of suppression mechanism
3.2.2.1.Radicals'effect on suppression.The OH,O,and H radicals are the main active species in the chain reaction of methane explosions[20].If the radicals are captured in the explosion process,the chain reactions will be interrupted and the explosion could be suppressed to some degree.
Cl will affect the chain reaction of the methane explosion.The primary equations of the reaction mechanism of Cl have been researched in some references[4].Br and I may have a similar reaction mechanism.Thus,the halide atoms'reaction mechanisms may consist of the following reactions.

Table 3 Diameters of water mist from the spray nozzle under 0.7 MPa

Table 4 Overpressures in three explosion scenarios for baseline selection

In addition,the concentrations of H and OH can be diluted with the catalysis of X.

Furthermore,K can combine with OH as a chemical inhibitor of the methane explosion.

According to Cao[4],by Reaction(1),this mechanism shows that Cl atoms combine into Cl2molecules at high temperatures.However,Cl formed by KCl at high temperatures should be a negative ion,which cannot form Cl2(Reaction (1))because of the charge conservation rule.Similarly,K in Reaction(5),which first formed from KCl,will be a positive ion.The KX solid particles formed at high temperatures may react with H and OH radicals formed in the chain branching reactions in explosions.The Cl and K radicals may form from these mechanisms as follows:


Table 5 Overpressures suppressed by water mist with halide additives
①Pwis the mean explosion overpressure suppressed by water mist,6.92×105Pa.
② P0is the mean explosion overpressure without any suppression,6.16×105Pa.
③The saturation concentration of KCl is 21.94%at 0°C.

Fig.3.Mean explosion overpressure suppressed by halide additives.Note:Pwis 6.92×105 Pa,P0is 6.16×105 Pa.
In Reaction(9),the molar reaction enthalpy is −4.0 kcal(1 cal=4.1868 J)[21]when X refers to Cl.K radicals will still be consumed rapidly in explosion systems containing water mist to break the reaction equilibrium,which keeps Reaction(9)to the right.The molar reaction enthalpy of Reaction (10)is approximately 20 kcal,9.5 kcal or−3 kcal when X refers to Cl,Br or I,respectively.According to the reaction enthalpy,Reaction (10)will be slower than Reaction (9).Thus,Reaction(9)will be the major process by which KX react with radicals.Thus,Reaction(5)should be continued.That may explain the amount of white fog(HX)generated while suppressing explosions by KX,especially when X refers to I,as the photos recorded by high-speed camera with frame rate of 1000 FPS show in Fig.4.

Fig.4.White fog generated in methane/air explosion suppressed by 30%KI mist.Note:the pictures are with time intervals of 10 ms.(The photographing frequency was 1000 FPS,and every tenth picture was selectEd.)

Fig.5.NH4H2PO4mist makes explosion more severe.Note:the pictures are with time intervals of 10 ms.(The photographing frequency was 1000 FPS,and every tenth picture was selectEd.)

Fig.6.Comparison of the flame propagation velocity(time interval=10 ms)of methane/air explosion suppressed by different mists.Note:the pictures are with time intervals of 10 ms.(The photographing frequency was 1000 FPS,and every tenth picture was selectEd.)
3.2.2.2.H+'s effect on suppression.Friedman and Levy[22]revealed that OH−works on flame inhibition,as shown in Reactions(6)and(7).According to this principle,the decomposition capacity order of KX should be KI >KBr >KCl;the KI solution can form KOH relatively more easily.Furthermore,acid materials will be disadvantageous to suppression.For an example,NH4H2PO4is a component of typical dry powder extinguishing agents,but its water solution is acidic.The mean explosion overpressure suppressed by 15% NH4H2PO4is 7.43×105Pa,increased by 7.37% and 20.62%,respectively,compared with simple water mist suppression and no suppression.
Compared with the faint light of the explosion suppressed by water mist,the light of the explosion suppressed by 15%NH4H2PO4is very dazzling,as shown in Fig.5.
3.2.2.3.Volume and electron affinity.A different explanation was proposed by V.Babushok et al.[23]:an ideal inhibition cycle,in which an inhibitor(Inh)catches a free radical,and then the bimolecular complex with the radical catches a second radical to scavenge the radicals,as is

R1and R2are free radicals generated from the explosion process.In Reactions(11)and(12),Reaction(12)should be faster than Reaction(11),because free radicals are very unstable and they tend to be a normal molecule.Thus,Reaction(11)should be control the reaction rate.In V.Babushok's opinion,if InhR1's activation energy for the reverse process is sufficiently high,and the molecule,InhR1,is sufficiently large,then Reaction (11)could happen at high pressures.KI has a largervolume than KBr and KCl;furthermore,the electron affinity of I is lower than that of Br and Cl,and thus,the unpaired free radical groups can share an electron with I−to combine into a new molecule of InhR1relatively more easily than Br−and Cl−.Thus,the suppression ability of the halide additives was in the order of KI >KBr >KCl.

Table 6 Suppression effects of a CO2/water solution and additives/CO2solutions

Fig.7.Mean explosion overpressure with error bars suppressed by CO2/H2CO3solution and that with 30%KI.Note:Pwis 6.92×105 Pa,P0is 6.16×105 Pa.
3.2.2.4.Burning velocity.Another explanation could be that a slower burning will result in a lower explosion overpressure.The flame front velocity of the explosion in KI mist was lower than that in pure water mist.It took approximately 70 to 100 ms for the front flame to burn from the bottom to the top in the confined vessel in pure water mist;however,in 30%KI mist,it took approximately 120 to 150 ms.Furthermore,in the pure water mist,the flame front has no clear boundary layer,showing a character of turbulent combustion.In 30%KI mist,the flame shows a laminar combustion with a clear boundary layer,as shown in Fig.6.
3.3.Synergistic effects of pressured CO2solution
The mean explosion overpressure suppressed by the CO2solution was determined to be 6.54×105Pa,which is lower than the value observed for the pure water mist(6.92×105Pa)but still higher than the overpressure without suppression(6.16×105Pa).Thus,the suppression effect of CO2solution is small under the testing conditions.At room temperature and a pressure of 0.7 MPa,the solubility of CO2is~5 dm3·L−1and the spray flow rate is 1.31 L·min−1.The maximum overpressure appeared at approximately 3.5 s,and therefore,only approximately 76.4 ml CO2was introduced into the explosion vessel.Assuming all the CO2in the water mist volatilized before the explosion,the CO2concentration in the explosion vessel should be~0.45%.According to Gant et al.[24],if 0.45%CO2is added to a 10%CH4/air mixture,the decrease rate of the explosion overpressure in a 20-L sphere is~2% (fixed curve).The suppression effect of the CO2/water mist under the testing conditions was worse than that of sole CO2suppression.Overall,the suppression of the CO2/water mist is still not significant under the established test conditions.
The synergistic effect of the CO2solution with 30%KI results in significant suppression effects.From Tables 3 and 4,the mean overpressures suppressed by 30%KI and 30%KI+0.7 MPa CO2are 0.502MPa and 0.460MPa,respectively.Compared to pure water mist driven by air,the overpressures suppressed by a mist of 30%KI+0.7 MPa CO2solution decreased by 33.53%under the same conditions.
The results are listed in Table 6.
Mean overpressures suppressed by CO2/water mist and CO2/30%KI mist with error bars are illustrated in Fig.7.
Median values of explosion overpressures suppressed by pure water mist,0.7 MPa CO2solution mist and 30%KI+0.7 MPa CO2solution are presented in Fig.8.
To make the suppression method more applicable,some enlarged trials were attempted in a 1 m × 1 m × 1.6 m enclosure,which are shown in the supplementary material.The explosion flame was obviously smaller and lower than that without suppression and that suppressed by water mist.The overpressures suppressed by the mists fluctuated,which may have resulted from unstable turbulent flow affected by the mist.More enlarged studies,including the nozzle arrangements,spray pressure,solution mist size,spray angles,etc.,should be studied in future works.
4.Conclusions
Halide additives in water mist were investigated to enhance the suppression of methane explosions.A 10% methane/air mixture was employed as the explosive mixture.CO2under a pressure of 0.7 MPa was used to drive the solution of the selected additives to investigate the synergistic suppression effects.
Halide additives at high concentrations exhibited a significant effect on reducing the overpressure of the methane/air explosion.The suppression effect of halide additives increased in the order of Cl−<Br−<I−.The explosion overpressures suppressed by 30%KBr and 30%KI were 0.76 times and 0.73 times that of the overpressure suppressed by pure water mist,with decrease rates of 23.84%and 27.46%,respectively.Furthermore,the overpressure decrease rates suppressed by 15% solutions of KCl,KBr,and KI were 12.43%,17.20%,and 20.81%,respectively.
Dissolving CO2into the additive solutions increased the suppression effect significantly.The explosion overpressures suppressed by 30%KI+0.7 MPa CO2were 0.66 times that of the overpressure suppressed by pure water mist,with a decrease rate of 33.53%.The suppression effects were not only much better than those of the 0.7 MPa CO2water solution but also better than that suppressed by 30%KI under the same conditions.

Fig.8.Median values of explosion overpressures suppressed by pure water mist,CO2solution mist and KI+CO2solution water mist.
For more practical applications of water mist with additives in fire rescue,larger explosions and new formulas which have lower concentrations would be the focus of future works.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2019.03.020.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scaling of the bubble/slug length of Taylor flow in a meandering microchannel☆
- Analyzing of mixing performance determination factors for the structure of radial multiple jets-in-crossflow☆
- Particle-resolved simulation of packed beds by non-body conforming locally refined orthogonal hexahedral mesh☆
- Visual study on the characteristics of liquid and droplet in a novel rotor-stator reactor☆
- Molecular dynamics simulation of supercritical CO2microemulsion with ionic liquid domains:Structures and properties☆
- Modeling bubble column reactor with the volume of fluid approach:Comparison of surface tension models☆
