Effect of sodium content on the interaction between Ni and support and catalytic performance for syngas methanation over Ni/Zr-Yb-O catalysts☆
2019-02-09
Department of Chemical and Biochemical Engineering,National Engineering Laboratory for Green Chemical Productions of Alcohols-Ethers-Esters,College of Chemistry and Chemical Engineering,Xiamen University,Xiamen 361005,China
Keywords:CO methanation Ni/Zr-Yb-O catalyst Sodium content Syngas Stability
ABSTRACT In this paper,Ni/Zr-Yb-O catalysts with different sodium contents are prepared by a co-precipitation method,using aqueous Na2CO3solution as a precipitant,and the effect of sodium on the catalyst structure and catalytic performance for syngas methanation is extensively investigated using five Ni/Zr-Yb-O catalysts,containing 0,0.5,1.5,4.5 and 13.5 wt%Na+,those are denoted as Cat-1,Cat-2,Cat-3,Cat-4 and Cat-5 respectively.It is found that the interaction between Ni and support determines the catalytic performance of Ni/Zr-Yb-O and the residual sodium content negatively affects the interaction between Ni and support.Cat-1 exhibits an excellent catalytic performance.During a long run time of 380 h,no deactivation is observed and both CO conversion and CH4selectivity maintain a level above 90%.However,Cat-3 and Cat-5 suffer rapid deactivation under the same reaction condition.The characterization results indicate the strong interaction between Ni and support enables Cat-1 to possess well dispersed Ni species,resistance to sintering and carbon deposition and thus the excellent catalytic performance.However,the presence of sodium ions over Ni/Zr-Yb-O degrades the interaction between Ni and support and the catalytic performance,especially for the stability.The relative weak interaction between Ni and support results in severe sintering of both ZrO2and Ni under the reaction condition,carbon deposition and the poor catalytic performance.
1.Introduction
In recent years,production of synthetic natural gas(SNG)via methanation using synthesis gas(syngas)from coal or biomass has attracted more and more attention in academia and industry[1,2],especially in China.Among the studied active metals,such as Fe,Co[3],Ru[4,5],Pt,and Ni[6-11],for methanation,Ni-based catalysts exhibited superiority owing to low cost,good availability and high activity comparable to nob-metal catalysts.However,Ni based catalysts suffered from deactivation due to thermal degradation(nickel sintering)and/or carbon deposition[12-16].
Many strategies,including utilization of novel preparation methods,introduction of promoters and selection of proper supports,have been proposed to increase the activity and stability of Ni based catalysts for syngas methanation.Most of the reported works about new preparation methods focused on increasing Ni dispersion and/or the interaction between the active metal and support.
The Ni-Al2O3catalysts prepared by a solution combustion method showed good thermal stability at high temperature as the Ni particles could be scattered and spatially isolated by Al2O3[17].Using SBA-16 as the support is another strategy as the presence of citric acid could anchor small nickel particles (3-5 nm)into the pores(6-7 nm)of SBA-16 during impregnation process.Such a process led to the confinement of nickel particles.Such a confinement effectively increased Ni dispersion and the interaction between Ni and silica,enabling the prepared catalyst to possess high resistance to sintering and thus excellent catalytic performance for CO methanation[18].But still,these methods cannot fulfill the requirements in industries to some extent.
Yan et al.reported that the method of plasma decomposition could enhance the interaction between Ni and the SiO2thus resulted in a high Ni dispersion as well as Ni sintering resistance[19].In addition,the plasma decomposition effectively suppressed the carbon deposition by inhibiting the formation of inactive carbon species.As a result,the catalyst prepared by plasma exhibited significantly improved activity with enhanced stability.The plasma assisted catalyst preparation methods could be perhaps effective in obtaining methanation catalysts with good activity and stability.However,such a method is difficult to scale up at least recently.
Therefore,the introduction of promoters is perhaps the main stream to improve the activity and stability in reported literature.La2O3can restrain the growth of NiO nanoparticles,improve the dispersion of NiO and strengthen the interaction between NiO and SiC,which made Ni-La/SiC more active and stable for CO2methanation than Ni/SiC [20].Iron could be the other promoter on the purpose of stabilizing Ni.Addition of Fe can effectively improve the activity of Ni/-Al2O3for CO methanation,in which the enhanced catalytic performance of bimetallic Ni-Fe catalyst was attributed to the formation of Ni-Fe alloy[21].
Catalytic supports strongly affect the catalytic performance of catalyst.Takenaka et al.found the CO conversions at 523 K were higher in the order of Ni/MgO <Ni/Al2O3<Ni/SiO2<Ni/TiO2<Ni/ZrO2[22].Compared with the traditional Al2O3support,barium hexaaluminate(BHA)and perovskite oxide CaTiO3(CTO)were more suitable as supports for Ni based catalysts in methanation due to their resistant to carbon deposition[23].Although extensive studies have been carried out for syngas methanation,Ni based catalysts with high resistance to sintering and carbon deposition are still highly demanded in industries.
Generally,the industrial syngas methanation is carried out firstly under high temperature such as 650°C to take the advantage of reaction heat and obtain high pressure steam.The reactors operated at lower temperature,due to the thermodynamic limit,will be followed aiming to completely convert CO and CO2into methane.Based on such requirements in the application,the suitable methanation catalysts are those with high activity at low temperature and high stability at high temperature.In our previous work,highly efficient Ni-ZrO2catalysts doped by Yb2O3denoted as Ni/Zr-Yb-O for co-methanation of CO and CO2have been prepared by a co-precipitation method using aqueous K2CO3solution as a precipitant[24].The Ni/Zr-Yb-O catalyst exhibited high activity and extremely high thermal stability.Compared with K2CO3,Na2CO3may be more suitable for preparing Ni/Zr-Yb-O catalyst in terms of its low cost,which becomes a severe factor in large scale catalyst synthesis.Unlike K ions,Na ions however were easier incorporated into the internal crystal structure of ZrO2due to the smaller cation radius[25].The effect of Na incorporation on the structure and catalytic performance of Ni/Zr-Yb-O catalyst needs to be investigated extensively.In addition,it should be pointed out that the sodium ions could be incorporated into the NiO lattice,thereby lowering the activation energy for sintering which increases the risk of catalyst deactivation greatly[26].
In this work,the composition of Ni/Zr-Yb-O catalysts for syngas methanation was prepared by a co-precipitation method,using aqueous Na2CO3solution as a precipitant.The effect of catalyst impurities by sodium on the properties,structure and catalytic performance of Ni/Zr-Yb-O catalysts for methanation was extensively investigated and discussed.Characterizations including X-ray diffraction(XRD),N2adsorption,H2temperature programmed reduction(H2-TPR),X-ray fluorescence spectrometer(XRF),transmission electron microscopy(TEM),High-sensitivity low-energy ions scattering(HS-LEIS)and thermogravimetric analysis (TGA)were performed to reveal the relationship between the catalytic performance and physicochemical properties.
2.Experimental
2.1.Preparation of the catalyst
2.1.1.Chemicals
(Ni(NO3)2·6H2O),(Zr(NO3)4·5H2O),Yb(NO3)3·6H2O and Na2CO3were purchased from Sinopharm Chemical Reagent Co.Ltd.(Shanghai,China).Water used in all experiments was deionized water produced by Millipore Milli Q system.All reagents were used as received.
2.1.2.Material synthesis
The procedure was similar with the one reported in our previous work[24].The aqueous solution containing the desired (Ni(NO3)2·6H2O),(Zr(NO3)4·5H2O),Yb(NO3)3·6H2O and the aqueous solution containing the desired Na2CO3were simultaneously added drop by drop into a Pyrex flask under vigorous stirring,where the temperature was kept at 80°C and the pH at~7.5.After completing precipitation process,the suspension was continuously stirred for 30 min at 80°C and then cooled down to room temperature.After filtering,the filtered cake was washed with deionized water.The catalysts,with different sodium contents were obtained by controlling both the times of washing and the amount of deionized water.The washed solid was then dried at 120°C for 12 h and calcined at 400°C for 4 h,yielding the precursor of Ni/Zr-Yb-O catalyst(in oxidation state).Five catalysts,containing 0,0.5,1.5,4.5 and 13.5 wt%Na+,were obtained denoted as Cat-1,Cat-2,Cat-3,Cat-4 and Cat-5,respectively.All samples of catalyst precursor were pressed,crushed,and sieved to a size of 40-80 mesh for evaluation of syngas methanation.
2.2.Catalyst characterization
The sodium content was measured over an S8 Tiger apparatus(Bruker,Germany)with a high-end wavelength dispersive X-ray fluorescence(XRF)spectrometer.The specific surface area of the catalyst was determined by N2adsorption (the BET method)using a Micromeritics Tristar 3000 instrument.The Powder X-ray diffraction(XRD)was obtained over a Rigaku Ultima IV with Cu Ka radiation at 40 kV and 25 mA.Transmission electron microscopy (TEM)images were obtained on a Tecnai F30 electron microscope(Phillips Analytical)operated with an acceleration voltage of 300 kV.The HS-LEIS analysis was performed on an Ion-TOF Qtac100 low energy ion scattering analyzer and Ne+ions with a kinetic energy of 5 keV were applied.H2-temperature-programmed reduction(H2-TPR)was carried out under a 5%H2/Ar flow with a rate of 40 ml·min−1,and the temperature was raised to 800°C at a ramp of 10°C·min−1.Thermogravimetric analysis(TGA)was used to measure the amounts of carbon deposited on the spent catalysts and was performed on a TG209F1 instrument under an air flow from room temperature to 700°C.Chemisorption experiments with H2were conducted over AutoChem 2920 to determine active surface area,metal dispersion and nickel crystallite size.
2.3.Catalytic activity measurements
Performance of the catalysts for CO methanation was evaluated in a fixed-bed continuous-flow reactor equipped with an online gas chromatograph(GC).Prior to the reaction,the sample(0.2 g catalyst+0.8 g quartz sand)was reduced in situ under purified H2stream at 550°C for 2 h.The reaction of CO methanation was conducted at a stationary state under the reaction conditions of 0.1 MPa,200-800°C,V(H2)/V(CO)/V(N2)=72/24/4,and GHSV=10000 ml·g−1·h−1,where N2was used only as an internal reference gas for the convenience GC analysis.Exit gas from the reactor was flowing while maintaining at 130°C to the sampling valve of the GC(Model GC-950 by Shanghai Haixin GC Instruments,Inc.)for online analysis.The GC was equipped with dual detectors(TCD and FID)and dual columns filled with carbon molecular sieve(TDX-201)and Porapak Q-S(USA),respectively.The former column(2.0 m length)was used for the analysis of N2(as an internal reference gas),CO and CO2,and the latter(2.0 m length)for hydrocarbons and other C-containing hydrogenated products.Conversion of CO(denoted as X(CO))was determined using the internal reference(N2).The carbonbased selectivity for the carbon-containing products,including methane and other C-containing hydrogenated products(denoted as S(CH4),etc.in later text)was calculated by an internal normalization method.
3.Results and Discussion
3.1.Catalytic performance of Ni/Zr-Yb-O for syngas methanation
Firstly,the effect of sodium ion content on the catalytic performance of Ni/Zr-Yb-O for syngas methanation was studied and the results are shown in Fig.1.The CH4selectivity exhibited a similar pattern with the CO conversion and thus is not illustrated in Fig.1.

Fig.1.Effect of sodium content on the catalytic performance of Ni/Zr-Yb-O for syngas methanation.
Five catalysts,containing 0,0.5,1.5,4.5 and 13.5 wt%Na+,were obtained by controlling both the times of washing and the amount of deionized water,they were denoted as Cat-1,Cat-2,Cat-3,Cat-4 and Cat-5,respectively.The XRF characterization(shown in Table 1)indicated that the sodium ion contents of the five catalysts were“undetectable”,0.3 wt%,1.6 wt%,4.4 wt%and 13.2 wt%,respectively.The sodium ion content strongly affected the activity.The higher sodium ion content the catalyst contained,the poorer activity and stability the catalyst exhibited.Cat-5 exhibited very poor activity and CO conversion was always lower than the other four catalysts at various temperatures.At temperatures lower than 300°C,only very little CO can be converted.With temperature increasing,CO conversion increased and reached the highest value of 90.6%at 390°C,but still lower than the other four catalysts as they can achieve over 97% under the same conditions.Cat-4,Cat-3,Cat-2 and Cat-1 showed relatively high CO conversions at temperatures lower than 390°C.With further increasing temperature,the CO conversion of the four catalysts decreased due to the exothermic nature of CO methanation reaction.However,the activity of Cat-4 dropped more than Cat-3,Cat-2 and Cat-1.Table 1 also presented the Ni contents of the Cat-1,Cat-2,Cat-3,Cat-4 and Cat-5.It can be seen that the presence of Na ions decreased the Ni content.Cat-5 exhibited the lowest Ni loading.This may be one possible reason for the very poor catalytic performance.

Table 1 Physicochemical parameters for Ni/Zr-Yb-O
The alteration of CO conversion at 390°C as a function of time on stream over Cat-5,Cat-3 and Cat-1 catalysts is shown in Fig.2.The CH4selectivity exhibited a similar pattern with the CO conversion and thus is not plotted in the figure.As can be seen,the sodium ion content affected not only activity but also stability.Cat-5 gave the lowest initial CO conversion and suffered from a rapid deactivation.During 13 h on stream,the CO conversion decreased from 90.5% to 4.3% and then retaining at this level.Cat-3 also deactivated in an observable way,as CO conversion decreased from 96.9%to 64.4%after a 20 h reaction although slower than Cat-5.In contrast to Cat-5 and Cat-3,Cat-1 exhibited an excellent stability and indicated high conversion of CO(>97%)without any loss in activity even after the 20 h catalytic reaction.The stability of Cat-1 was further investigated and the results are shown in Fig.3.It can be seen that Cat-1 possessed an excellent stability as the CO conversion remained on the level above 99.0%during a 380 h continuous test.No activity decrease was observed even if space velocity was increased from 10000 to 40000 ml·g−1·h−1,although CH4selectivity decreased to some extent(still >92%)for high space velocity.
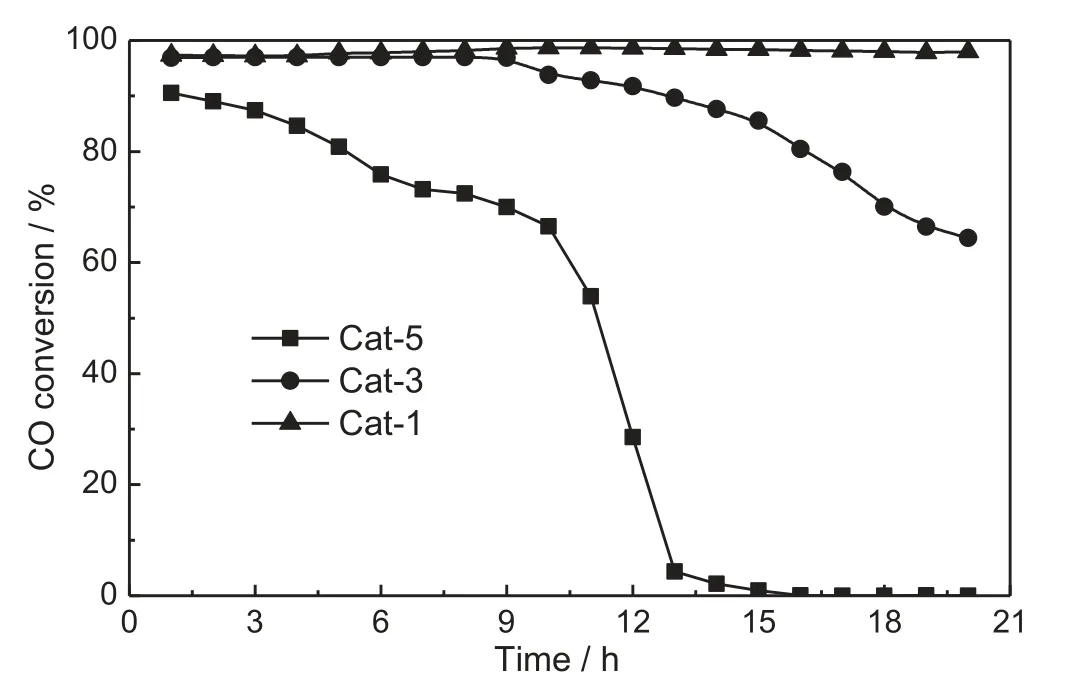
Fig.2.Effect of sodium content on the stability of Ni/Zr-Yb-O for syngas methanation.

Fig.3.The stability of Ni/Zr-Yb-O(Cat-1)for syngas methanation.
3.2.Physicochemical Properties Characterization
The X-ray diffraction(XRD)results of Ni/Zr-Yb-O with various sodium ion contents are shown in Fig.4.As can be seen,the sodium ion content strongly affected the crystalline structure of catalyst.The presence of excessive sodium ions favored the formation of crystal ZrO2.The support of Cat-1 and Cat-2 was mainly present in the form of amorphous ZrO2while typical diffraction peaks due to the tetragonal ZrO2were observed in Cat-5,Cat-4 and Cat-3 at 2θ=30.1°,50.1°and 60.1°[24].This was further confirmed by the results of N2adsorption,demonstrated in Table 1.Cat-5,Cat-4 and Cat-3 exhibited much lower surface area(<75 m2·g−1)and pore volume than Cat-2 and Cat-1(>110 m2·g−1)probably due to the formation of crystal ZrO2.Although NiO was detected over all the five catalysts,Cat-1 and Cat-2 showed weaker and broader NiO XRD peaks than Cat-5,Cat-4 and Cat-3,indicating that NiO species were dispersed better in Cat-2 and Cat-1[27].For the Cat-5 sample,the XRD peaks attributing to NaNO3were detected because of the relatively high sodium ion content.Noted that no XRD peaks attributed to Yb species were observed,although Yb loading was around 9.5%for the fived catalysts,as shown in Table 1.The possible reason was that Yb might be dispersed very well.These results indicated that Zr-Yb-O support was successfully prepared as reported in our previous paper.
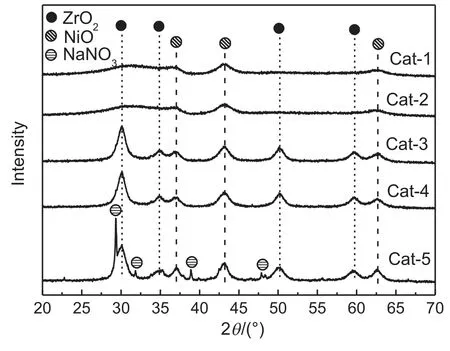
Fig.4.XRD patterns of Ni/Zr-Yb-O catalysts with different sodium contents.
Table 2 presents the active surface area,metal dispersion and nickel crystallite size determined by H2-chemisorption.It can be seen that the sodium ion content strongly affected the active surface area,metal dispersion and nickel crystallite size.Cat-1 exhibited the best metal dispersion(18.1%),the highest active surface area(41.3 m2·g−1)and the smallest metal particle(5.6 nm)while Cat-5 presented the poorest metal dispersion(4.0%),the lowest active surface area(7.5 m2·g−1)and the biggest metal particle(25.2 nm).This suggested that the presence of excessive Na was detrimental to the nickel dispersion.The possible reason was that the presence of excessive Na resulted in crystal NiO(as shown in Fig.4)which led to the formation of big Ni metal particle after reduction.

Table 2 The active surface area,metal dispersion and nickel crystallite size of Ni/Zr-Yb-O
To further explore the effect of Na+on the catalyst structure,Cat-1 and Cat-4 calcined at various temperatures were characterized by XRD and the results are showed in Fig.5.For Cat-4,a wide peak at around 43.4°,attributed to NiO,was observed over the catalyst calcined at 300°C,on the other hand no peak of NiO was detected over Cat-1 calcined at the same temperature.Incorporation of Na into NiO lattice increased the risk of NiO sintering as reported in literature[26],and the presence of Na+starting to affect the formation of crystal NiO at a much lower temperature was observed in this work.When temperature was increased from 300 to 400 °C,the XRD peaks of NiO became sharper and four peaks due to ZrO2were also observed over Cat-4-400,indicating that crystal ZrO2was also formed.This was in accordance with the results of the literature[25],where it was reported that Na was incorporate into the ZrO2structure to stabilize the ZrO2.For Cat-1,only a wide peak of NiO,very similar with that of Cat-4-300,was observed even after calcining at 400 °C.The effect of sodium ions on the phase structure was more significant when catalysts were calcined at 600°C.Cat-4-600 exhibited sharp XRD peaks for both NiO and ZrO2.Compared with Cat-4-600,the XRD peaks of NiO and ZrO2over Cat-1-600 were still weak and broad,although these peaks were increased to some extent after calcination at 600°C,especially for the ZrO2.Clearly,the presence of sodium ions favored the formation of both crystal NiO and ZrO2during the calcination process.

Fig.5.XRD patterns of Cat-1 and Cat-4 calcined at various temperatures.
The H2-TPR was performed to investigate the reducibility of catalysts and the interaction between the Ni and support and the results are showed in Fig.6,the H2-TPR curve for pure NiO is also provided for the sake of comparison.Cat-5 presented two reduction peaks,the big one at around 400°C and the other at around 535°C.The former was attributed the reduction of bulk NiO while the latter to the NiO interacted with the support[18,19,28].This observation indicated the NiO over Cat-5 was mainly presented in the form of bulk NiO.With the decrease of sodium ion content,the interaction between the NiO and support increased.Therefore,the reduction peak at low temperature decreased while the reduction peak at high temperature increased and shifted to higher temperatures.For Cat-2 and Cat-1,only high temperature reduction peak was observed.These results confirmed the fact that the sodium ions weakened the interaction between NiO and Zr-Yb-O support and led to the production of bulk NiO with large particles.The XRD results indicated that sodium ions led to the formation of both crystal NiO and ZrO2.Therefore,it is reasonable to deduce that the presence of sodium results in the formation of crystal NiO and ZrO2and thus weaken the interaction between NiO and Zr-Yb-O support.

Fig.6.H2-TPR patterns of Ni/Zr-Yb-O catalysts with different sodium contents.
3.3.The migration of Ni from bulk to surface
High-sensitivity low-energy ion scattering(HS-LEIS)was carried out to further clarify the effect of sodium on the structure of the catalyst.
Fig.7 illustrated the results of Cat-4 and Cat-2 calcined at 400°C for 4 and 8 h.It can be seen that Na was detected over both Cat-4 and Cat-2,and Cat-4 exhibited stronger Na signal than Cat-2,revealing more Na on the surface of Cat-4.When calcination time was increased for 4 to 8 h,Na signals of the both samples were increased,especially for the Cat-4.This observation implied that the surface of the two catalysts calcined for 8 h contained more Na than the corresponding ones calcined for 4 h.These results indicated that,for Cat-4,Na can be incorporated in the bulk of the catalyst and migrated from bulk to the surface during the calcination process since HS-LEIS only provides the surface information.Na incorporation made Na perhaps occupy the sites where NiO and ZrO2can interact strongly.Additionally,when sodium was migrated from bulk to the surface,cation vacancy was left and the M--O(Zr--O and Ni--O)bonds may be broken.The bulk-phase and structure of catalyst will be restructured during the calcination process,which may also contribute to the formation of crystal NiO and ZrO2.The breaking of M--O,especially for Zr--O--Ni,will further weaken the interaction between NiO and ZrO2.

Fig.7.The LEIS results of Cat-2 and Cat-4.a and b Cat-4 calcined for 4 and 8 h,c and d Cat-2 calcined for 4 and 8 h.
3.4.The resistance to sintering and carbon deposition
Fig.8 presents the XRD results of Cat-4 and Ca-1 tested at temperatures in the range of 250 to 650°C.In order to confirm the transformation of catalyst structure after the reaction,the XRD patterns of the fresh reduced catalysts are also shown in Fig.8.Typical XRD peaks of metallic nickel were observed over the reduced Cat-4 and Cat-1[29],indicating that NiO was reduced to Ni metal during the reduction process.Compared to Cat-4,the XRD peaks of metallic nickel over Cat-1 were much weaker and broader.This demonstrated that the Ni metal over Cat-1 was dispersed better than that over Cat-4 [19].As showed in Fig.6,the main reduction peak of fresh Cat-1 was located at 550°C.Although all of the NiO should be generally reduced to metallic nickel after reduction at 550°C for 2 h in H2stream,NiO was still detected over the reduced Cat-1.These NiO nanoparticles were possibly from the reoxidation of well dispersed Ni metal,considering that the reduced samples exposed in air before XRD testing and smaller metal particle is more easily oxidized.Compared with the reduced samples,the two tested catalysts presented stronger and sharper ZrO2and metallic Ni XRD peaks.The possible reason for such observation is that both the support and active metal sintered and aggregated into large particles during the process of catalytic performance test due to the strongly exothermic nature of CO methanation and at the high reaction temperature(250-650°C).Especially for Cat-4,the presence of Na facilitated the ZrO2aggregation,which in turn weakened the metal-support interaction and subsequently resulted in Ni sintering.It should be noted that the Ni metal over the tested Cat-1 exhibited weaker and broader XRD peaks than the reduced Cat-4.This indicated that Ni metal over Cat-1 possessed a better ability against sintering and was still dispersed well due to the strong interaction between Ni species and ZrO2support,even if it underwent the catalytic performance testing at temperatures in the range from 250 to 650°C.Carbon species was detected over the used Cat-4.This suggested that carbon accumulation occurred over Cat-4 during the process of catalytic performance testing.The carbon formation rate increases with increasing metal particle size[19,30,31].The large Ni particle was one of the reasons for Cat-4 having poor coke resistance capacity.No carbon species was observed over the used Cat-1 probably due to the good dispersion of Ni metal.

Fig.8.XRD patterns of the reduced and used Ni/Zr-Yb-O catalysts.a the reduced Cat-4;b the used Cat-4;c the reduced Cat-1;d the used Cat-1.
The five catalysts tested at temperatures in the range from 250 to 650°C were further characterized by TEM and the results including statistical TEM particle size distribution are showed in Fig.9.For the used Cat-5,large particles with the mean size of~70 nm were observed,indicating that severe aggregation occurred during the process of catalytic performance testing.With the sodium content decreasing,the size of catalyst particle became smaller and more uniform.For the used Cat-2 and Cat-1,all the observed catalyst particles were around 20 nm and Ni was well dispersed over the support.Additionally,the carbon deposition became less significant with the decrease of sodium content.Much more tubular carbon was observed over the used Cat-5 while no carbon over the used Cat-1 was observed.This was confirmed by the TG results showed in Fig.10.The total carbon deposition over the used Cat-5,Cat-4 and Cat-3 was around 3.3 wt%,2.8 wt%and 2.1 wt%,respectively.No observable carbon was detected over Cat-2 and Cat-1.The mass increase at 300-500 °C was due to the oxidation of metal nickel.Clearly,higher sodium content resulted in severe metal aggregation and thus carbon deposition.

Fig.9.TEM images and particle size distribution of the used Ni/Zr-Yb-O catalysts.a the used Cat-5;b the used Cat-4;c the used Cat-3;d the used Cat-2;e the used Cat-1.
Five Ni/Zr-Yb-O catalysts,containing 0 wt%,0.5 wt%,1.5 wt%,4.5 wt%and 13.5 wt%Na+have been prepared by a co-precipitation method,using aqueous Na2CO3solution as a precipitant,and the effect of sodium content on the catalyst structure and catalytic performance for syngas methanation was extensively studied.It was found that sodium content strongly affects the interaction between Ni and support and thus the catalytic performance.The Ni/Zr-Yb-O catalysts containing low sodium content,such as Cat-1,exhibited excellent catalytic performance while the ones containing high sodium,such as Cat-5,showed low activity and suffered rapid deactivation meaning poor stability.Generally,the better dispersion(active metal)the catalyst possesses,the higher CO methanation activity it exhibits [1,2,32].The results of XRD for the fresh and reduced catalyst revealed that the Ni was well dispersed over Cat-1.The small and uniform Ni metal particles resulted in the high catalytic activity.Additionally,the small Ni metal particles enhanced coke resistance,which is another reason for the high activity[1,2].The H2-TPR results confirmed the strong interaction between Ni and support.The strong interaction enabled Cat-1 catalyst to resist sintering(both Ni metal and ZrO2support)and carbon deposition[18,19],suggested by the results of XRD and TEM of the used catalysts.The resistance against sintering and carbon deposition ensured the excellent stability.However,the presence of excessive sodium was detrimental to the catalytic performance.The incorporation of sodium into the catalyst bulk and the migration from the bulk to the surface weakened the interaction between Ni and support due to the formation of crystal NiO and ZrO2.The weak metal support interaction induced large Ni metal particles and aggregation of Ni metal and ZrO2support during the catalytic reaction process,which increased the possibility of carbon deposition and thus lowered the activity and stability.
4.Conclusions
The effect of sodium content on the catalytic performance and interaction between Ni and support was extensively studied for syngas methanation over Ni/Zr-Yb-O catalyst.The results indicate that the strong interaction between Ni and support,well dispersed Ni species,resistance to sintering and carbon deposition contribute to the high activity and stability.The residue sodium content should be strictly controlled,since excessive sodium lowers the catalytic performance and results in severe carbon deposition due to the formation of crystal NiO and ZrO2and weakens the interaction between Ni and ZrO2support.

Fig.10.The TG results of the used Ni/Zr-Yb-O catalysts with various sodium contents.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scaling of the bubble/slug length of Taylor flow in a meandering microchannel☆
- Analyzing of mixing performance determination factors for the structure of radial multiple jets-in-crossflow☆
- Particle-resolved simulation of packed beds by non-body conforming locally refined orthogonal hexahedral mesh☆
- Visual study on the characteristics of liquid and droplet in a novel rotor-stator reactor☆
- Molecular dynamics simulation of supercritical CO2microemulsion with ionic liquid domains:Structures and properties☆
- Modeling bubble column reactor with the volume of fluid approach:Comparison of surface tension models☆
