Preparation of solid acid catalyst /TiO2/γ-Al2O3for esterification:A study on catalytic reaction mechanism and kinetics
2019-02-09
School of Chemical Engineering,Sichuan University,Chengdu 610065,China
Keywords:Solid acid catalyst Nano-TiO2Esterification Kinetics
ABSTRACT In this work,a series of/TiO2/γ-Al2O3solid acid catalysts were synthesized by impregnation method,in which nano-TiO2was prepared by sol-gel method,and then the nano-TiO2sol was loaded on porous γ-Al2O3supporter through impregnation.The structure and property of catalyst were characterized by XRD,N2-BET,SEM,TEM,XPS,NH3-TPD,Pyridine-IR and FT-IR.In addition,the catalyst of chelate bidentate coordination acid center model was established.The catalytic performance test was carried out in the esterifciation of n-butyl alcohol with lauric acid and the catalyst showed excellent activity.The experimental results showed that the medium strength acid sites were more dominant active sites than the strong and weak acid sites for the rapid esterifciation reaction.Its kinetic behaviors and activation energy were studied for the esterification under the catalytic reaction condition.
1.Introduction
Acid catalysts are indispensable for a great deal of reactions in industrial production,in which the traditional liquid acid catalysts own high catalytic performance have a considerable proportion.But the defect of corrosion equipment,refractory acid,short life and high pollution[1-3]restrict their development,it is thus imperative to develop some new-type green catalysts.As a promising alternative to traditional liquid acid catalysts,solid acid catalysts possess several important advantages,such as no-corrosion,easy separation operation,environment-friendly and well reusability[1,4].Nowadays,solid acid catalysts have been widely applied in many organic reactions of dehydration,esterification,polymerization,isomerization and acylation[5],among them,the/MxOycatalysts,especially/TiO2catalysts have attracted highly attention,because TiO2as a transition metal oxide owns unique excellent properties,such as high melting point,strong heat resistance,good toxicity resistance,wide adjustable range.In addition,TiO2owns acidity,easy to form Lewis acid sites with other substances.At the same time,the TiO2can improve the dispersion of active component.Thereby,the/TiO2catalysts have been widely applied in esterificatione[6],poxidation[7],photocatalysis[8]and acylation [9]etc.However,pure/TiO2catalysts usually have relative low specific surface area and poor stability,in addition,it is easy to lose sulfur components and carbon deposition to deactivate[2,5].Literature[1]reported that it was an effective way to optimize catalytic performance of/MxOycatalysts by supporting it on the porous and high surface area materials.
In this work,porous spherical γ-Al2O3supporter with high specific surface was used to modify the catalytic activity and stability ofTiO2catalysts.For the preparation of/TiO2/γ-Al2O3catalysts,at present,the preparation of TiO2is mostly based on inorganic titanium salts and organic titanium salts as precursors,such as Ti(SO4)2[4],TiCl2[5],titanium tetra-isopropoxide [8],tetrabutyl titanate [10].In present work,TiO2was prepared by sol-gel method using tetrabutyl titanate as precursor.The TiO2sol can be loaded on the porous spherical γ-Al2O3supporter[3,10].This TiO2catalysts have widely applied due to cost reduction,small mass transfer resistance,good mechanical property,large specific surface area and good dispersibility[11].In addition,γ-Al2O3is a common material,making it easy to access and relatively cheap.
In our work,the esterification about n-butyl alcohol with lauric acid was used as a template reaction to evaluate the catalytic performance of prepared catalysts.N-butyl laurate is an important commercial ester,which is widely used in various cosmetics,textile and rubber industries.Moreover,it is also used as an edible flavor to be added to various wines,enhancing the quality of liquor.
2.Experimental
2.1.Materials and chemicals
Tetrabutyl titanate(≥98.5%),lauric acid(≥98.5%),ethanol absolute(≥99%),1-butanol(≥98.5%)and nitric acid(65.0%-68.0%)were provided by Chengdu Kelong Chemical Co.,Ltd.(Chengdu,China).All chemicals are analytical pure reagents and used without further purification.The water in the whole experiment was deionized water.The porous spherical γ-Al2O3supporter with a high specific surface area whose diameter is 1-2 mm was provided by Zhengzhou Jintai desiccant(Zhengzhou,China).
2.2.Catalyst preparation
TiO2sol was prepared by sol-gel method according to earlier reports[3,10].This method was a versatile method to prepare the nano metal oxide[10].Briefly,25 g tetrabutyl titanate was added to 50 g anhydrous ethanol dropwise under adequately stirring,forming solution A(in 250 ml round bottomed with four-necked flask);10 g nitric acid(65.0%-68.0%)and 50 g anhydrous ethanol were mixed into 32.5 g deionized water under stirring,forming solution B.Then solution B was added into solution A with a dropping funnel under stirring at room temperature,keep stirring for further 40 min to obtain TiO2sol.
2.3.Characterization of catalyst
The X-ray diffraction (XRD)was conducted on AXIS Ultra DLD diffractometer(Kratoscompany,Britain)using Cu-Ka irradiation.The scanning electricity and voltage were 40 mA and 40 kV,respectively.The range of collecting angle 2θ is 10°-80°.
N2adsorption-desorption isotherms at 77 K was measured at Micromeritic ASAP2460 analyzer.The pore size distribution and pore volume were obtained by the Barrett-Joyner-Halenda(BJH)method,and the specific surface area was obtained by Brunauer-Emmett-Teller(BET)method.
Field-emission scanning electron microscopy(FESEM)with energy dispersive spectrometry(EDS)measurements were performed with a JSM-7500F(JEOL,Japan)and Transmission electron microscopy(TEM)were examined using a ZEISS Libra 200 FE with an accelerating voltage 200 kV.
NH3temperature-programmed desorption (NH3-TPD)was performed by a Chembet Pulsar TPR/TPD.The sample(0.15 g)was heated from room temperature to 500°C at a rate of 20°C⋅min−1under a He flow (30 ml·min−1),and it has been pretreated for 1 h under these conditions to remove the impurities absorbed on the surface of the sample.Then the sample was cooled to 80°C and a mixture gas flow(30 ml·min−1)of 5%NH3/He was introduced and absorbed by the sample for 1 h,and the sample has been swept by a He flow(30 ml·min−1)for 1 h to remove the physical absorption of NH3.Finally the sample was heated from 80°C to 900°C at a rate of 10°C·min−1under a He flow (30 ml·min−1)to the desorption of ammonia adsorbed on the sample.
In situ FT-IR spectroscopy of pyridine absorption was performed on a Nicolet 380 spectrometer equipped with an in situ quartz detector set.The sample was put on the sample pool,then pretreated at 350°C in a vacuum for 2 h.When the sample pool was cooled to 25°C,pyridine molecules were absorbed by the sample in the sample pool.The absorbed pyridine of sample has been degassed at room temperature under vacuum for 30 min.Finally,the related information was recorded by the spectrum.
X-ray photoelectron spectroscopy (XPS)was performed on an Escalab 250Xi using Al Ka line as the emission source (hv=1484.6 eV).The electron binding energy values were calibrated referenced to the C 1s(285 eV).
The Fourier transform infrared spectroscopy (FT-IR)spectra of the catalysts were performed on IR Affinity-1over the range of 400-4000 cm−1.
2.4.Catalytic activity test
The catalytic performance test was carried on the esterification of butyl alcohol with lauric acid.This esterification[15-17]was performed in a three-necked flask equipped with a thermometer,a reflux condenser tube,water separator and magnetic stirrer.Reaction conditions were as follows:the molar ratio of lauric acid to n-butanol was 1:3;the range of reaction temperatures was 127-130 °C;the amount of the catalyst was 4 wt% of the lauric acid;and the reaction time was 6 h.The esterification rate of lauric acid was determined by the acid value of lauric acid before and after reaction,which was titrated by KOH-ethanol standard solution,based on national standards GB/T 5530-2005[3,18].

where V0and V1were the dissipative volume of KOH-ethanol standard solution before reaction and after reaction,respectively.Herein,A0and A1represent the acid value of lauric acid before and after reaction,respectively;N represents the concentration of KOH-ethanol standard solution;M represents the relative molecular mass of KOH and W represents the mass of sample.
3.Results and Discussion
3.1.Characterization of the catalysts
Fig.1 shows the XRD patterns of A,SA-1,TA and STA-1 catalysts.The diffraction peaks at 19.1°,36.2°,44.0°,58.2°and 64.2°corresponding to the diffraction of(102),(111),(211),(313)and(020)planes,respectively,are the characteristic peaks of γ-Al2O3monoclinic spinel(JCPDS# 35-0121).From the Fig.1,the diffraction peaks of γ-Al2O3loaded by sulfuric acid become wider and weaker,indicating that the crystallization degree of γ-Al2O3decreases.The reason probably is that sulfate ions will prevent the growth of alumina crystallites.Some possible reasons for no obvious TiO2diffraction peaks [19-23]:(1)nano-TiO2are very small and highly dispersed on the inner and outer surface of the porous γ-Al2O3;(2)the presence of bulk γ-Al2O3in the samples can prevent the growth of TiO2crystallites;(3)the nano-TiO2possibly present in an amorphous state.

Fig.1.XRD patterns of A,SA-1,TA,and STA-1.
The N2absorption-desorption isotherms of STA-1 and A samples are depicted in Fig.2(a).It can be seen from the figure that two samples show a type IV isotherms with a type of H2-type hysteresis loop according to IUPAC standard,indicating the existence of mesoporous structure in the catalysts.The hysteresis loop of STA-1 is narrower to A,owing to the reduction of STA-1 pore tortuosity[24].Corresponding pore size distribution curves are shown in Fig.2(b),it can be observed that average pore diameter of the A is smaller than that of the STA-1,which may be attributed to the reason that the sintering process was superior recrystallization process during the high temperature calcination,leading to the STA-1 catalyst of the pore size lightly increased and the specific surface area decreased.Another reason for the decrease of specific surface area is likely due to that some pores are plugged up in the impregnation process[19].At the same time,a slight increase in pore volume,probably indicating that the presence of sulfate ion and TiO2form the porous network during calcination[25].However,the STA-1 maintains relatively high specific surface area due to the high specific surface area of γ-Al2O3,which can provide enough space to active components and diffusing reactants and products.The concrete textural properties of STA-1 and A sample are displayed in Table 1.
The SEM images of STA-1 sample are displayed in Fig.3,and magnified imaging(Fig.3b)reveals that the surface of catalyst is composed of aggregated nanoparticles accompany with interparticle pores.Thepicture shows that the sulfate ion and TiO2have a good dispersity on the γ-Al2O3,in accordance with the results of the XRD.This rimous texture is beneficial to the infiltration of reactant liquid,accordingly improving the utilization of active mass.Furthermore,the SEM-EDS result in Fig.3(c)confirms the existence of the light amount of Ti species in the STA-1 sample.

Table 1 The textural properties of STA-1 and A samples
The Fig.4 illustrates the TEM and HRTEM images of STA-1 sample.We can note from TEM images that the catalyst exhibits porous structure with interlaced nanosheet,moreover the surface and pores of the catalyst were filled with nanoparticles,the average size of the nanoparticles is about 10 nm.HRTEM studies further uncovering the crystalline nature of the catalyst.We can observe bulk γ-Al2O3crystals and tiny TiO2crystallites from Fig.4(d),as evidenced by the interplanar spacing of 0.242 and 0.218 nm that correspond to the(111)plane of γ-Al2O3and (111)plane of rutile TiO2.The amorphous phase in the surface marked with red oval is highly possible to be amorphous TiO2.
XPS characterizations were performed to explore electronic properties and surface chemical compositions of as prepared samples,and the results are shown in Fig.5.From Fig.5(a),the characteristic peaks of important elements(O,Al,S and Ti)in/TiO2/γ-Al2O3catalysts could be observed,indicating that TiO2andare successfully loaded onto the γ-Al2O3supporter by impregnation.The spectrum of O1s is shown in Fig.5(b),it can be fitted to three peaks:the lower energy peak located at 530.8 eV,which can be ascribed to lattice oxygen in metal oxide;the higher binding energy peak located at 533.3 eV,which can be attributed to hydroxyl groups in H2O;and the biggest peak located at 532.3 eV,which is assigned to the oxygen of sulfate group [20].The spectrum of Ti 2p is depicted in Fig.5(c).Two main peaks can be observed at 459.6 eV and 465.8 eV,corresponding to Ti2p1/2and Ti2p3/2,respectively,indicating the forming of TiO2.These two peaks arise from the energy level splitting caused by the spin-orbit coupling of electrons.Obviously,Ti2p peak intensity is quite weak,which are mainly due to either the quantity of Ti is too small or TiO2strongly reacts with[26].It is consistent with XRD and SEM-EDS results.The spectrum of S2p is shown in Fig.5(d).The peak at 170 eV is ascribed to the S6+high oxidation state[10].Its position moves to higher binding energy slightly,because the S incombine with the greater electronegativity of oxygen in TiO2.It is consistent with the bridged/chelating bidentate complex structure of catalyst [27].The spectrum of Al 2p shown in Fig.5(e),the peak can be observed at 75.2 eV position attributed to Al3+,illustrating the forming of Al2O3.The XPS data can provide the surface S/Ti atomic ratio of STA-1 is 8,indicating that the loading of sulfate ion on the catalyst surface is more than that of titanium dioxide,which maybe help to form a strong Lewis acid sites.The surface O,Al,Ti,S atomic ratio of the catalyst samples are 58.28%,31.29%,0.35%,and 2.77%,respectively.

Fig.2.N2-BET and pore size distributions patterns of A and STA-1.

Fig.3.The(a)low resolution and(b)high resolution SEM,and(c)SEM-EDS images of STA-1 sample.

Fig.4.The(a,b,c)TEM and(d)HRTEM images of STA-1 sample.

Fig.5.XPS spectra of the(a)STA-1 catalysts and high-resolution spectra of(b)O 1s,(b)Ti 2p,(d)S 2p and(e)Al 2p.

Fig.6.FT-IR spectrum of pyridine absorbed onto STA-1.
In situ FT-IR spectroscopy of pyridine adsorption on the samples was carried out to explore the acid sites(Brønsted and Lewis)of catalyst,and the results are shown in Fig.6.The characteristic peak at 1555 cm−1can be attributed to the absorption of pyridine at Brønsted acid sites to form pyridine ammonium ion.The characteristic peak at 1440 cm−1is assigned to the strong complex formed by the absorption of pyridine at Lewis acid sites [28,29].The characteristic peak at 1500 cm−1is due to the absorption of pyridine at both Lewis and Brønsted acid sites [30].Generally,the relative content of Lewis and Brønsted acid sites can be obtained from the peak area intensity of corresponding peaks.We can observe that the area of Lewis acid sites is larger than that of Brønsted acid sites.Therefore,we can know that the Lewis acid sites dominate in STA-1 catalysts.However,when there is H2O molecules,Lewis acid sites can be converted to Brønsted acid sites[28].Acid sites make reactant molecules better used to improve the reaction reactivity.

Fig.7.The NH3-TPD profiles of STA-0.5,STA-1 and STA-1.5.
NH3-TPD experiments were performed to investigate the change of acid amount and acid sites strength in as prepared samples with the different impregnated concentrations of sulfuric acid and the results are exhibited in Fig.7.It shows that medium strength acid sites are dominated in the three samples.In addition to having a medium acid site,STA-1.5 also has a strong absorption peak of strong acid sites.According to the area of the peak,we probably know the total acid amount of each sample.The total acid amount of STA-0.5,STA-1and STA-1.5 is 1.048 mmol·g−1,1.192 mmol·g−1and 1.356 mmol·g−1,respectively.The results indicate that acid amount and acid sites strength increased with the increase of sulfuric acid concentration.The increases of total acid amount is likely due to more interactionwith TiO2.The increases of the acid strength can be probably attributed to stronger electron offset around the Ti atom due to stronger electron withdrawing effect of.Therefore,the vacancies around the titanium atoms can receive and absorb electrons to form Lewis acid sites.This may suggest that two active components of TiO2and SO42−interact to form the catalyst acid sites[2].This result is consistent with the catalyst model of the bidentate coordination structure.

Fig.8.The FT-IR profiles of STA-0.5,STA-1,STA-1.5 and STA-1(u).
The FT-IR spectra of STA-0.5,STA-1,STA-1.5 and STA-1(u)samples are shown in Fig.8.The main four peaks between 400 cm−1and 4000 cm−1can be found in STA-0.5,STA-1 and STA-1.5.The band at 1126 cm−1is assigned to asymmetric stretching of S=O vibration,indicating the forming of bidentate coordination chelating structures,which is due to interaction of sulfate group with metal oxide[23,27].The weak broad peak at 596 cm−1is ascribed to the stretching vibration of the covalent bond of titanium and oxygen atoms.The band intensity of the Ti--O--Ti is relatively low,suggesting that the content of titanium is relatively low.It is consistent with the analysis results of XRD,SEMEDS,NH3-TPD and XPS.The peaks at 3419 cm−1and 1635 cm−1are due to the vibrations of hydroxyl group and the adsorption of water molecule on the catalyst surface,respectively[4].It can be seen from the figure that the band intensity at 1126 cm−1and 596 cm−1slightly increase with the increasing of sulfuric acid concentration,indicating that the different impregnation sulfuric acid concentrations have an effect on the structure of catalysts.Compared with STA-1 and STA-1(u),the absorption peak intensity of STA-1(u)at 1126 cm−1of STA-1(u)is weaker than that of STA-1,which may be due to the loss active S components during the process of catalytic reaction.From the figure,it can be observed that STA-1(u)has other absorption peaks around 3000 cm−1,which is the organisms.It is likely due to that the organisms had been absorbed on the catalysts in the esterification reaction process.
Combined with the XPS,NH3-TPD,Pyridine-IR and FT-IR results,we tentatively propose the chelate bidentate coordination acid center model of as prepared catalyst,as shown in Fig.9[27,31].The electron cloud on the titanium atom is offset because of the electronwithdrawing effect of the sulfate ions,and the absence of electrons to form the Lewis acid sites.When there is water,the Lewis acid sites could convert to Brønsted acid sites.

Fig.9.The chelate bidentate coordination acid center model of catalyst.
3.2.Catalytic performance
The effect of different sulfuric acid concentrations of STA-0.5,STA-1,STA-1.5 and STA-1(u)catalysts on the esterification reaction were investigated,and the results are listed in Table 2.It indicates that the catalysts with different sulfuric acid concentrations have great influence on the esterification rate of lauric acid.With the sulfuric acid concentration increasing,the esterification rate of lauric acid was first increased and then decreased in the process of esterificaion reaction.We have known that the catalysts both STA-0.5 and STA-1 have only similar moderate strength acid sites from the results of NH3-TPD,but STA-1has higher catalytic activity than STA-0.5.This is probably due to the fact that STA-1 catalysts have more active sites caused by higher total acid amount,which could improve the catalytic activity for the esterification reaction.Compared STA-1 with STA-1.5,although the total acid amount of STA-1.5 is greater than STA-1,the catalytic activity of STA-1 for the esterification reaction is higher than STA-1.5.The NH3-TPD results indicated that STA-1.5 has strong acid sites besides the medium strength acid sites,therefore,it is probably that the catalytic activity for the esterification reaction is poor over the catalyst of strong acid sites.The result indicates that stronger acid sites do not necessarily produce higher catalytic activity.Because stronger acid sites are easy to lead to the side reaction and even the carbon deposition for esterification,which make the catalysts deactivated.Therefore,the study on the relationship between catalytic activity and the catalysts of acidic property have some reference value for the design of catalysts for these reactions.Compared STA-1 with STA-1(u)catalyst,it show that activity of STA-1(u)catalyst used three times drops slightly.It may be due to the deactivation of the catalyst caused by the carbon deposition and the loss of active components in the process of esterificaion reaction,which is in line with FT-IR result.The results suggest that the STA-1catalyst exhibits the superior activity for the esterification reaction,indicating that the acid amount and the strength of acid sites play vital roles in the esterification reaction.
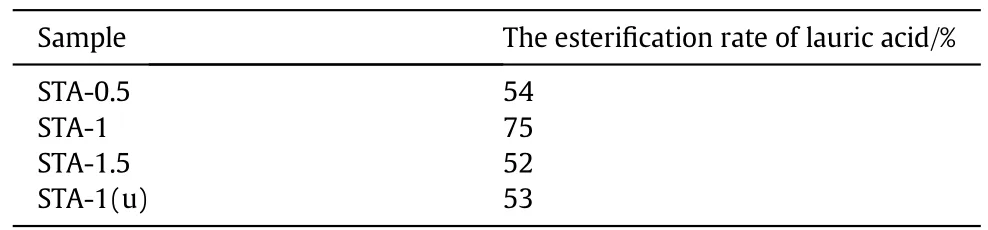
Table 2 the esterification rate of lauric acid under the action of different catalysts①
4.Dynamics Study
A simple kinetic study of the esterification reaction on n-butyl alcohol with lauric acid was performed over STA-1.A series of important parameters of esterification in kinetic study:molar ratio of acid/alcohol=1:3,amount of catalyst 4 wt%of the lauric acid,the reaction time 6 h,the study temperature of 90-120°C.Targeted Samples were analyzed at a fixed time intervals about 1 h.
4.1.Determination of rate constant and order of reaction
The kinetic model established in this paper depended on the following assumptions:
(a)The internal and external mass transfer of solid acid catalysts was negligible,owing to the esterification reaction with porous catalysts and full stirring.
(b)The high enough mole ratio of n-butyl alcohol to lauric acid was used in the esterification,so n-butyl alcohol has a large excess.Therefore,the n-butyl alcohol concentration remains constant in the process of reaction.
Under these conditions,we regard the esterification over STA-1 catalyst as an irreversible pseudo-homogeneous process[15,32],and hence to obtain the following macroscopic reaction rate equation:

Herein,CArepresents concentration of lauric acid,and k represents rate constant,Ccatand CBrepresent concentration of catalysts and n-butyl alcohol,respectively.
According to the Eqs.(1)and(2),the following logarithm equation was obtained,


Fig.10.The relationship between the concentration of lauric acid and time.

Fig.11.The relationship between ln(−dCA/dt)versus lnCA.
Fig.10 shows the change relationships between reaction time and concentrations of lauric acid in different reaction temperatures.With the progress of the reaction,the reaction rate decreased gradually.The macroscopic reaction rate constant(k1)and reaction order(n)can be calculated by plotting ln(−CdCA/dt)versus lnCAusing differential method and least square method,as shown in Fig.11.The results of the macroscopic reaction rate constant (k1)and the reaction order(n)are shown in Table 3.The change on reaction temperature has noticeable effect on the macroscopic reaction rate constant(k1).There is an increase on the macroscopic reaction rate constant(k1)at higher temperatures.This can be attributed to the increase of the movement velocity of the reactant molecules with the increase of temperature,thus increasing the frequency of collisions between reactants.

Table 3 Rate constants,the reaction order and correlation coefficient(R2)of the esterification
4.2.Determination of activation energy
Generally,the reaction rate is more affected by the temperature than the concentration.Hence,it is meaningful to study the effect of temperature on esterification about n-butyl alcohol with lauric acid over STA-1 catalyst.The macroscopic activation energy of the esterification under the catalyst was studied according to the Arrhenius law:

The transformation form of Eq.(4)is

Herein,E represents the macroscopic activation energy,and A represents pre-exponential factor.
The macroscopic activation energy of reaction and pre-exponential factor can be obtained from the Arrhenius plot(Eq.(5))lnk1versus 1/T in Fig.12.The values of the macroscopic activation energy and pre-exponential factor were calculated to be 42 kJ·mol−1and 452 mol·min−1,respectively.The value of pre-exponential factor is relatively large,indicating the high collision frequency among reactant molecules.The relevant literature [15]also reported that the control step is the chemical reaction,not mass transfer process for this esterification under the activation energy,consistent with the assumption that mass transfer was ignored.The kinetic study of the esterification can provide practical and theoretical guidance for the study of catalysts.
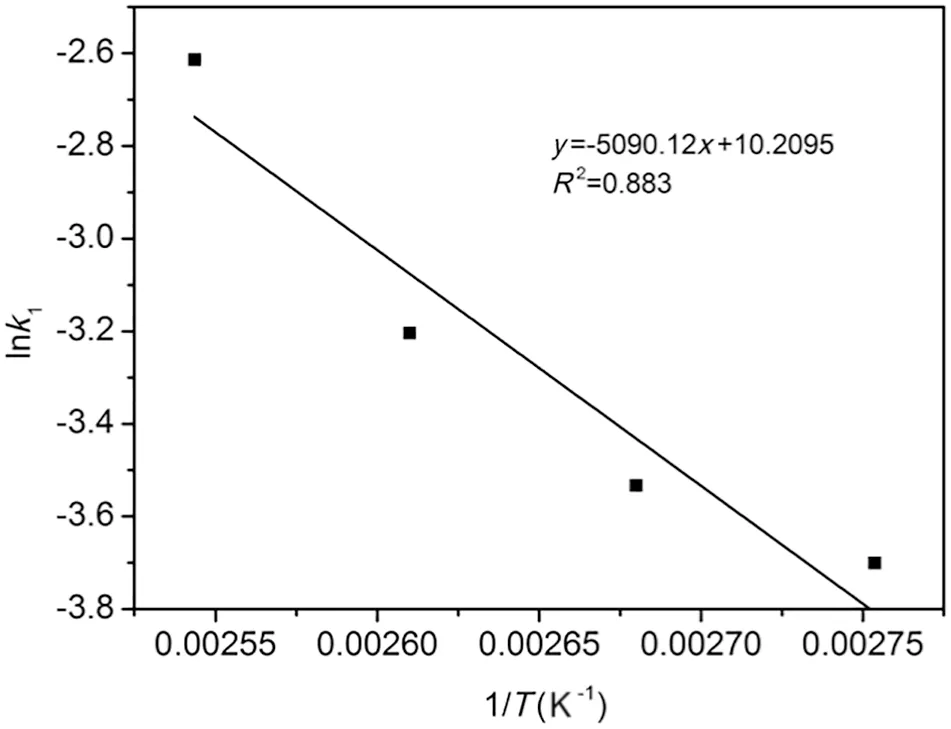
Fig.12.Arrhenius plot esterification of lauric acid with n-butanol over STA-1.
5.Conclusions
In this study,we succeeded in synthesizing novel solid acid catalysts/TiO2/γ-Al2O3,in which the nano-TiO2sol was prepared by sol-gel method.According to the above characterizations,the activity test and reaction kinetic results and discussions,the following major conclusions can be proposed:
(1)The impregnation concentration of H2SO4has a great impact on the activity of/TiO2/γ-Al2O3catalysts,and the highest activity catalyst for the esterification reaction is the 1 mol·L−1H2SO4concentration.
(2)The experiment results show that the medium strength acid sites have higher catalytic activity for the esterification reaction than the strong acid sites.
(3)The catalyst of the chelate bidentate coordination acid center model is established,in which TiO2strongly interact withto generate acid sites.
(4)A simple reaction kinetic of esterification reaction is studied.By fitting the data,the macroscopic activation energy of reaction and pre-exponential factor were found to be 42 kJ·mol−1and 452 mol·min−1,respectively.
Acknowledgements
The authors are grateful for all those who have a support for this work.The authors sincerely thank Sichuan University and laboratory for their support.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scaling of the bubble/slug length of Taylor flow in a meandering microchannel☆
- Analyzing of mixing performance determination factors for the structure of radial multiple jets-in-crossflow☆
- Particle-resolved simulation of packed beds by non-body conforming locally refined orthogonal hexahedral mesh☆
- Visual study on the characteristics of liquid and droplet in a novel rotor-stator reactor☆
- Molecular dynamics simulation of supercritical CO2microemulsion with ionic liquid domains:Structures and properties☆
- Modeling bubble column reactor with the volume of fluid approach:Comparison of surface tension models☆
