Concentration-induced structural diversity and catalytic activity of BF3/n-BuOH complexes for n-decene polymerization☆
2019-02-09JunWangHongpengLiXiChenWeiguangShiNaZhangFuquanBaiXiaofengWangSihanWangXianmingXuLiboWang
Jun Wang,Hongpeng Li,Xi Chen,*,Weiguang Shi,,*,Na Zhang,Fuquan Bai,Xiaofeng Wang,Sihan Wang,Xianming Xu,Libo Wang
1Provincial Key Laboratory of Oil and Gas Chemical Technology,College of Chemistry and Chemical Engineering,Northeast Petroleum University,Daqing 163318,China
2 Daqing Chemical Research Center of Petrochemical Research Institute,Daqing 163714,China
3 Institute of Theoretical Chemistry,Jilin University,Changchun 130023,China
4 State Key Laboratory of Inorganic Synthesis and Preparative Chemistry,College of Chemistry,Jilin University,Changchun 130012,China
Keywords:Boron trifluoride n-BuOH n-decene polymerization Catalyst structures
ABSTRACT The BF3/n-BuOH complexes were investigated as active species in catalyzing n-decene polymerization reaction.The structures of BF3/n-BuOH complexes were characterized not only by modern spectrum but also by calculation at theoretical level.The results confirmed that BF3/n-BuOH complexes changed from BF3·(n-BuOH)2complexes to BF3·n-BuOH complexes with the mass fraction of BF3increasing.These two complexes have different catalytic activity,but BF3·n-BuOH was superior.The highest n-decene conversion could reach 99%and the most excellent selectivity of n-decene trimer and tetramer could reach up to 80%yield by a series of controlled conditions.This work can help to understand the catalytic active species of n-decene polymerization and provide support for industrialization of poly-alpha-olefins(PAOs).
1.Introduction
Poly-alpha-olefins(PAOs),oligomers of long-chain α-olefins,were widely used as synthetic lubricant[1].Currently,PAOs were employed in military,aerospace,automotive and other industries[2].Compared with mineral base oil,PAOs had many advantages in properties,such as,a good low temperature fluidity,corrosion resistance,high flash point,high viscosity index,low volatility,little coking and good sensibility of adding[3-5].Additionally,PAOs were usually synthesized with Lewis acid,ionic liquid,Ziegler-Natta catalyst or solid acid [6-8]as catalyst and C6-C12olefin as raw materials.Among the catalyst,BF3catalyst applying BF3as the main catalyst and alcohol,water or carboxylic acid as the initiator performed n-decene polymerization reaction in the typical PAO producing process with high yield and the narrow selectivity of product[9-13].Moreover,the reaction condition can be easily controlled.Then the BF3catalyst has drawn considerable attention in the field of catalyst [14-16].Darden et al.employed the boron trifluoride(BF3)and n-butanol(n-BuOH)as the co-initiator to perform the polymerization of C13or C14olefin.Then PAOs with medium viscosity index could be prepared eventually[17].Clarembeau reported the polymerization of n-decene and 1-dodecene was carried out by employing BF3in combination with alcohols [18].Similarly,Nandapurkar and Yang reported PAOs with high viscosity and superior low temperature performance could be prepared in a two-stage stirred tank applying BF3and diverse alcohols as catalyst for the polymerization of n-decene or n-dodecene [19].It was found that a ratio of diverse olefins could be cautiously controlled to decrease the pour point of the PAOs at an expectant level.Especially for BF3/n-BuOH catalytic system,the conversion of n-decene polymerization was nearly 100%and the product distributions of n-decene polymerization were mainly controlled at n-decene trimer and tetramer.Barrett L Cupples et al.[20]reported a two-stage continuous process for n-decene polymerization with BF3/n-BuOH complexes as catalyst.The process applied a stirred tank and a coiled tube reactor.By adjusting the ratio of conversion taking place in each stage,the product distribution and viscosity could be controlled by a two-stage process.There were other patents for olefin polymerization with BF3as catalyst.Akatasu et al.[21]reported that the mixture of n-octene,n-decene and n-dodecene could prepare the low viscosity PAOs efficiently employing BF3as main catalyst and alcohols or water as initiator in the presence of anhydrides.
However,neither have the catalytic active species of BF3/n-BuOH complexes been known in n-decene polymerization,nor the parameters of the preparation process for PAOs were reported systematically so far.With n-BuOH as the initiator,it was found that the polymerization of ndecene was carried out under the pressure of BF3varying within 0.1-0.5 MPa.The in-situ measurement was too dangerous to realize the characterization of BF3/n-BuOH complexes owing to the toxicity of BF3.The active species of BF3/n-BuOH complexes has not received much attention.Moreover,the condition parameter on the polymerization process has not been reported in detail.
To further explore the active species of BF3/n-BuOH complexes,we will report the preparation and characterization for BF3/n-BuOH complexes with different mass fractions of BF3under normal temperature and pressure.Meanwhile,theoretical study employing the density functional theory(DFT)has been performed to shed more light on the formation of the active species of BF3/n-BuOH complexes.Importantly,to explore the optimal parameters of the preparation process for PAOs,we are going to investigate the effects of polymerization conditions on the yield and selectivity of product systematically.Our work will be of great significance to the preparation of PAOs using BF3/n-BuOH complexes as catalyst.
2.Experimental
2.1.Materials and general instrumentation
The n-BuOH was purchased from Sinopharm Chemical Reagent Co.,Ltd.n-Decene was the polymerization stage and was purchased from Daqing Petrochemical Co.,Ltd.BF3was purchased from Henan Yangzi Chemical Co.,Ltd.Anhydrous ethanol was purchased from Tianjin Damao regent factory.
IR spectra were recorded on a Nicolet IR 750 infrared spectrometer with the KBr pellets method.1H NMR spectra and13C NMR spectra were recorded at Bruker-400 with tetramethylsilane(25°C)as an internal reference,and CFCl3was used as an internal reference for19F NMR spectra.D2O was put into a capillary glass tube(1 mm)with external standard method and was used as the BF3/n-BuOH complexes with different mass fractions of BF3lock field solvent.In addition,deuterated chloroform was used as the lock field solvent for n-BuOH.
GC analyses were carried out on VISFTA600 equipped with a 30 m and 0.22 mm inner diameter OV-101 capillary column.
2.2.Synthesis of BF3/n-BuOH complexes with different mass fractions of BF3
Synthesis of BF3/n-BuOH complexes was performed in a 250 ml flask.The n-BuOH(74.1 g,1.0 mol)was added to the flask when the reaction temperature was controlled at −30°C and normal pressure.Before the complex reaction occurred,the mass of the n-BuOH was weighed by an analytical balance and recorded as m1.Then BF3was consecutively injected into the reactor and the flow rate of BF3was controlled at 15 mL/min−1.After the BF3was absorbed by the liquid for a period of time,the mass of the sample was again weighed by an analytical balance and recorded as m2,and wt(BF3)in the complex was calculated according to the difference between m2and m1.Repeating the above operation until the content of BF3reached the desired value.The 1%BF3n/n-BuOH complexes(liquid)were obtained.(The mass fraction of BF3and the surface stretching frequency and chemical shift were expressed as χ,ν and δ respectively.)
IR (KBr)(cm−1):3325,2960,2934,2875,1072,952;1H NMR(400 MHz,D2O,25°C):δ 1.32-1.36(t,3H,--CH3),1.76-1.83(m,2H,--CH2--),1.89-1.96 (t,2H,--CH2--),3.93-3.96 (m,2H,--CH2--),5.75(s,1H,--OH);13C NMR(101 MHz,D2O,25°C):δ 13.8,19.3,35.0,61.8.
Using the same synthetic approach,the flow rate of BF3was controlled at 30 ml·min−1for about 44 min.The 5%BF3/n-BuOH complexes were prepared.IR(KBr)(cm−1):3324,2960,2935,2875,107,952.1H NMR(400 MHz,D2O,25°C):δ 1.29-1.33(t,3H,--CH3),1.72-1.81(m,2H,--CH2--),1.89-1.93(t,2H,--CH2--),3.93-3.96(m,2H,--CH2--),6.38(s,1H,--OH).13C NMR(101 MHz,D2O,25°C):δ 13.7,19.2,34.8,61.9.
Using the same synthetic approach,the flow rate of BF3was controlled at 75 ml·min−1for about 33 min.The 10% BF3/n-BuOH complexes were prepared.Then the 10%BF3/n-BuOH complexes(3.700 g)were used to catalyze n-decene polymerization reaction.IR (KBr)(cm−1):3535,3320,2960,2936,2867,1253,1072,987;1H NMR(400 MHz,D2O,25°C):δ 1.27-1.30(t,3H,--CH3),1.69-1.79(m,2H,--CH2--),1.88-1.92 (t,2H,--CH2--),3.96(s,2H,--CH2--),7.14 (s,1H,--OH);13C NMR (101 MHz,D2O,25 °C):δ 13.6,19.1,31.7,34.5,62.1,64.4.
Using the same synthetic approach,the flow rate of BF3was controlled at 85 ml·min−1for about 115 min.The 30%BF3/n-BuOH complexes were prepared.Then the 30%BF3/n-BuOH complexes(1.233 g)were used to catalyze n-decene polymerization reaction.IR (KBr)(cm−1):3544,3249,2962,2937,2877,1252,1068,960;1H NMR(400 MHz,D2O,25°C):δ 1.17-1.21(t,3H,--CH3),1.60-1.70(m,2H,--CH2--),1.88-1.90 (t,2H,--CH2--),4.10(s,2H,--CH2--),9.10 (s,1H,--OH);13C NMR(101 MHz,D2O,25°C):δ 13.1,18.5,32.9,64.1.
Using the same synthetic approach,the flow rate of BF3was controlled at 90 ml·min−1for about 133 min.The 35%BF3/n-BuOH complexes were prepared.The 35%BF3/n-BuOH complexes were prepared.Then the 35%BF3/n-BuOH complexes(1.057 g)were used to catalyze n-decene polymerization reaction.IR(KBr)(cm−1):3533,3208,2964,2938,2876,1259,1068,1042,952;1H NMR(400 MHz,D2O,25°C):δ 1.15-1.18(t,3H,--CH3),1.58-1.67(m,2H,--CH2--),1.89-1.96(t,2H,--CH2--),4.22-4.26 (t,2H,--CH2--),8.74 (s,1H,--OH);13C NMR(101 MHz,D2O,25°C):δ 13.0,18.4,31.9,65.5.
Using the same synthetic approach,the flow rate of BF3was controlled at 95 ml·min−1for about 156 min.The 40%BF3·n-BuOH complexes were prepared.Then the 40%BF3·n-BuOH complexes(0.925 g)were used to catalyze n-decene polymerization reaction.IR (KBr,cm−1):3522,3252,2970,2934,2874,1256,1066,1040,953;1H NMR(400 MHz,D2O,25°C):δ 1.11-1.14(t,3H,--CH3),1.64-1.65(m,2H,--CH2--),1.88-1.95(t,2H,--CH2--),4.28-4.31(t,2H,--CH2--),8.31(s,1H,--OH);13C NMR(101 MHz,D2O,25°C):δ 12.9,18.2,31.4,66.9.Using the same synthetic approach,the flow rate of BF3was controlled at 120 ml·min−1for about 162 min.The 45%BF3·n-BuOH complexes were prepared.Then the 45%BF3/n-BuOH complexes(0.820 g)were used to catalyze n-decene polymerization reaction.IR(KBr)(cm−1):3524,3250,2964,2934,2876,1253,1062,940;1H NMR (400 MHz,D2O,25 °C):δ 1.10-1.14 (t,3H,--CH3),1.54-1.63 (m,2H,--CH2--),1.90-1.98 (t,2H,--CH2--),4.38-4.41 (t,2H,--CH2--),7.55 (s,1H,--OH);13C NMR(101 MHz,D2O,25°C):δ 12.5,17.8,30.45,70.0.
In the end,IR,1H NMR and13C NMR of n-BuOH were also employed to compare with BF3/n-BuOH complexes with different mass fractions of BF3.The detailed data were listed as follows.IR(KBr,cm−1):3335,2959,2913,2875,1072;1H NMR(400 MHz,CDCl3,25°C):δ 0.93-0.96(t,3H,--CH3),1.37-1.43(s,2H,--CH2--),1.51-1.58(t,2H,--CH2--),3.60-3.63(m,2H,--CH2--)2.51(s,H,--OH);13C NMR(101 MHz,CDCl3,25°C):δ 13.7,18.8,34.8,62.4.
2.3.General procedure for n-decene polymerization
n-Decene polymerization reaction was carried out in a 250 ml stainless steel reactor equipped with a magnetic stirrer.The reactor was cleaned with nitrogen gas three times.n-Decene(100 ml)was injected into the reactor with a syringe.Then the desired amount of BF3/n-BuOH complexes was injected into the reactor.The reaction mixture was allowed to be stirred for 1 h at room temperature and at a certain text pressure.After the reaction finished,the upper organic phase was taken out to investigate the conversion rate of n-decene by gas chromatography(GC).
Using the same procedure to carry out the oversaturated BF3for ndecene polymerization,the desired account of 45%BF3/n-BuOH complexes was injected into the reactor.Then the BF3was introduced into the reactor to maintain the desired pressure.The expected temperature was controlled by the water bath.After the desired reaction time,the upper organic phase was taken out to investigate the conversion rate of n-decene by gas chromatography(GC).
2.4.Theoretical calculation method
All calculations were performed by density functional theory(DFT)and carried out with Gaussian 09 program package.It has been reported that two types of complexes:BF3·ROH and BF3·2ROH[22]could be obtained by BF3and alcohols.Thus we established the model of BF3·(n-BuOH)2and BF3·n-BuOH.On that basis,the structures of BF3and n-BuOH monomers were optimized by B3LYP/6-311++g(3df,2pd).BF3·n-BuOH molecule pair and BF3·(n-BuOH)2molecule triad were optimized by B3LYP-D3 method at 6-311++g(3df,2pd)level.The charge population of each atom was calculated applying natural bond orbital(NBO)methods[23].The corresponding1H NMR and13C NMR spectra were also calculated and plotted by the data of the atomic chemical shifts.
3.Result and Discussion
3.1.Characterization of BF3/n-BuOH complexes
In order to explore the catalytic active species(whether BF3·n-BuOH or BF3·(n-BuOH)2)and their forming conditions,we synthesized BF3/n-BuOH complexes with different mass fractions of BF3.It has been reported that the structure of BF3·ROH was different from that of BF3·2ROH.BF3·ROH showed that the B atom linked to the O atom on the alcohols by dipole-dipole interaction.BF3·2ROH showed that the other alcohol molecule connects to the oxygen atom on BF3·ROH by hydrogen bonds [24].On the basis of the structure of BF3·ROH and BF3·2ROH,we established the model of BF3·(n-BuOH)2and BF3·n-BuOH in calculation,and the detailed chemical structural formula of n-BuOH,BF3·n-BuOH and BF3·(n-BuOH)2was given in Fig.1.Besides,we compared the δ values of BF3/n-BuOH complexes with the experiment.

Fig.1.Molecular formula:(a)n-BuOH,(b)BF3·n-BuOH and(c)BF3·(n-BuOH)2.
3.2.1H NMR and 13C NMR spectrum
To illuminate χ inducing the variation of structures of BF3/n-BuOH complexes in detail,this paper combined the experiment with the theory method to characterize the structures of BF3/n-BuOH complexes by1H NMR and13C NMR spectra.The detailed results were given in Figs.2 and 3.
As shown in Fig.2(a),the peaks of the BF3/n-BuOH complexes(1%-45%)at 1.76-1.83,1.89-1.96 and 3.93-3.96 were attributed to the characteristic proton on α-C,β-C and γ-C respectively.The peaks of the BF3complexes(1%-45%)at 1.32-1.36 were the characteristic proton on ω-C on n-BuOH ligand.Meanwhile,1H NMR spectra of n-BuOH were also compared with BF3/n-BuOH complexes(1%-45%).Due to the BF3molecule having a stronger ability to withdraw electrons,it was found that the δ of H on α-C increased with χ.It showed the shielding effect weakened.It can be inferred that the density of electrons around H on the α-C decreased.Compared with the H on n-BuOH,the H on β-C,γ-C and ω-C on BF3/n-BuOH complexes(1%-45%)also had a trend of moving to the low field.It showed that BF3also had the induced effect on the different the H on BF3/n-BuOH complexes.Meanwhile,it could be seen that the peaks of the H on β-C,γ-C and ω-C decreased with the increase of χ,indicating the decrease of induced effect of the H on β-C,γ-C and ω-C.
As can be seen from Fig.2(a),the δ value of hydroxyl H firstly increased and then decreased.This trend was consistent with previous results for BF3/n-CH3OH complexes[25].As for the increase of the δ value of hydroxyl H,the possible reason was that the different hydroxyl H exchanged rapidly with χ in the presence of excess n-BuOH.As for the decrease of δ value of hydroxyl H,the possible reason was that the exchange rate of hydroxyl H was slow gradually when all n-BuOH in connection with BF3is in a 1:1 ratio.Additionally,we also investigated the effect of χ on the different carbon atoms.The detailed results were shown in Fig.3(a).
As can be seen from Fig.3(a),the peaks at around 62,32,19 and 14 were assigned to the characteristic peaks on α-C,β-C,γ-C and ω-C atoms respectively.The major variation of δ focused on the α-C and β-C atoms of BF3/n-BuOH complexes.When χ increased to 10%,there were two new characteristic peaks appearing at around 65(α-C)and 35(β-C)respectively.With χ increasing,the characteristic peak of α-C on BF3/n-BuOH complexes moved gradually to the lower field.Instead,as for the β-C peak,the δ value decreased.
Importantly,to confirm the detailed the structures of BF3/n-BuOH complexes,the1H NMR and13C NMR spectra of n-BuOH,BF3·(n-BuOH)2and BF3·n-BuOH were also depicted in Figs.2(b)and 3(b),according to the δ values obtained by calculation.From Fig.2(b),the δ value of H15also increased from n-BuOH to BF3·(n-BuOH)2to BF3·n-BuOH.The variation showed an excellent agreement with experiment results.Meanwhile,as shown in Fig.3(b),the variation of δ value for C8and C11from n-BuOH to BF3·(n-BuOH)2to BF3·n-BuOH were consistent with the trend of the α-C and β-C atom on BF3/n-BuOH complexes with χ increasing in experiment.Based on the above research,it proved that the micro-structure of BF3/n-BuOH complexes shifted from BF3·(n-BuOH)2to BF3·n-BuOH with χ increasing.Meanwhile,the theoretical model we established was reasonable in our work.
3.3.IR spectra
IR spectra of n-BuOH and BF3/n-BuOH complexes were given in Fig.4.The stretch vibration frequencies at around ν1=3320 cm−1belonged to O--H on n-BuOH,which gradually disappeared with the increase of χ.Moreover,the stretch vibration frequencies appeared at around the ν2=3540 cm−1and ν3=3250 cm−1respectively when χ was above 10%.The stretch vibration frequencies at around 3250 cm−1and 3540 cm−1were attributed to--OH group in the chains and the terminal--OH groups.The similar absorption peaks also existed in BF3·(n-CH3OH)2[26].Based on the above result,it showed BF3·(n-BuOH)2existed in the solution when χ was above 10%.Additionally,as can be seen from Fig.4,there were no the stretch vibration frequencies for BF3·n-BuOH.The possible reason was that the concentration of BF3·n-BuOH was too low to be detected.
3.4.Theoretical calculation
To elucidate the mechanism for the variation of structures of BF3/n-BuOH complexes in detail,it was necessary for the theoretical calculation to study on electronic and geometric structures of BF3,n-BuOH,BF3·(n-BuOH)2and BF3·n-BuOH.The geometric parameters of BF3,n-BuOH,BF3·(n-BuOH)2and BF3·n-BuOH were listed in Tables S1 and S2.The molecular model of BF3,n-BuOH,BF3·(n-BuOH)2and BF3·n-BuOH was established as shown in Fig.5.

Fig.2.1H NMR spectra of(a)BF3/n-BuOH complexes(in experiment)and(b)n-BuOH,BF3·(n-BuOH)2and BF3·n-BuOH(in theory).

Fig.3.13C NMR spectra of(a)BF3/n-BuOH complexes(in experiment)and(b)n-BuOH,BF3·(n-BuOH)2,and BF3·n-BuOH(in theory).

Fig.4.IR spectra of BF3/n-BuOH complexes with the different mass fractions of BF3.
3.5.Natural bond orbital(NBO)charge population analysis
To study the charge variation in the process of reaction,we compared and analyzed the charge population of the corresponding atoms in different complexes.All the charge population was listed in Table S3.The main data of Table S3 were selected in Table 1.
From Table 1,it was obvious that there were some differences in the corresponding atom from BF3to n-BuOH to BF3·(n-BuOH)2to BF3·n-BuOH.Comparing the corresponding atom on n-BuOH and those on BF3·(n-BuOH)2,it was worth noting that the H15on BF3·(n-BuOH)2had less negative charge about 0.071 than that on n-BuOH,while the O14on BF3·(n-BuOH)2had more positive charge about 0.027 than that on n-BuOH.At the same time,the H13and H12have lost electrons about 0.03 from n-BuOH to BF3·(n-BuOH)2.Besides,from BF3to BF3·(n-BuOH)2,the B16on BF3·(n-BuOH)2had less positive charge about 0.074 than that on BF3,while the F18had more negative charge about 0.082 than that on BF3.

Fig.5.The structures of(a)BF3,(b)n-BuOH,(c)BF3·(n-BuOH)2and(d)BF3·n-BuOH with the labeled atoms.
Based on the variation in charge population of corresponding atoms,we found that n-BuOH supplied BF3with plenty of electrons from O atom and H atoms when BF3reacted with n-BuOH to form BF3·(n-BuOH)2by the complex reaction,leading to the arrangement of charge population of partial atoms.Owing to BF3on BF3·(n-BuOH)2as the acceptor for electrons,it can be seen from Table 1 that the positive charge of B16decreased and the negative charge of F18increased.Moreover,the F atoms with the strong electronegativity on BF3·(n-BuOH)2,the result was that the F18atom on BF3·(n-BuOH)2possessed more electrons than that on B16.

Table 1 The NBO charge population of the selected corresponding atoms on the different molecules①,②
Comparing the n-BuOH on BF3·n-BuOH with the corresponding n-BuOH on BF3·(n-BuOH)2,most of the charge populations on the corresponding atoms were the same except for O14and H15.However,comparing the n-BuOH on BF3·n-BuOH with the other one on BF3·(n-BuOH)2,it was noticeable that the charge population on the other n-BuOH molecule changed greatly when the structures shifted from BF3·(n-BuOH)2to BF3·n-BuOH.For instance,not only did the O14and C11atoms on BF3·n-BuOH have less negative charge than O33and C30on BF3·(n-BuOH)2,but also the H15,H13,H12,H10and H9atoms on BF3·n-BuOH had more positive charge than that for H34,H32,H31,H29and H28atoms.By contrast,the C8on BF3·n-BuOH had more negative charge than C27on BF3·(n-BuOH)2.The reason was that the O33did not provide the excessive BF3with enough electrons to generate new BF3·n-BuOH.Therefore,the adjacent atoms which contained H34,O33,H32,H31,C30,H29and H28atoms supplied the excessive BF3with some electrons by the descent of the charge itself.
The net charge on n-BuOH molecule on different complexes was also calculated by the theoretical calculation,as shown in Table 2.From Table 2,it was obvious that the net charge of BF3decreased from BF3·(n-BuOH)2to BF3·n-BuOH.Meanwhile,the net charge on both the corresponding n-BuOH and the other n-BuOH increased from BF3·(n-BuOH)2to BF3·n-BuOH.The reason was that the associated n-BuOH molecule both provided BF3with more electrons than the single n-BuOH on BF3·n-BuOH.Then we saw that n-BuOH on BF3·n-BuOH possessed more charge than that on BF3·(n-BuOH)2.
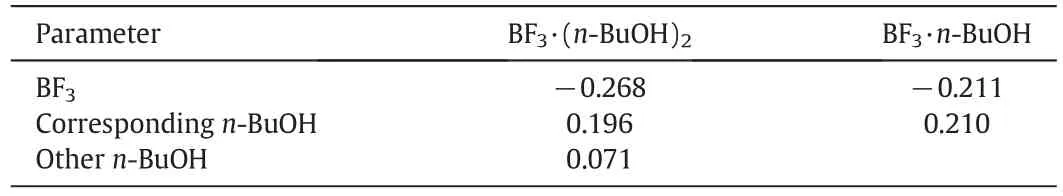
Table 2 Net charge of BF3,corresponding n-BuOH on BF3·n-BuOH and BF3·(n-BuOH)2,the other n-BuOH on BF3·(n-BuOH)2
Based on the variation of charge population for the above atoms,we inferred the detailed reaction mechanism,as shown in Scheme 1.From Scheme 1,BF3reacted with n-BuOH to form BF3·(n-BuOH)2by the complex reaction,leading to the electrons of partial atom arrangement.The number of BF3·(n-BuOH)2molecule increased with χ.When the ratio of BF3/n-BuOH is going to approach 1:1,the O on the other n-BuOH connected with the B on the excess of BF3.The charge population of partial atom was rearranged.As a result,the hydrogen bond between the O on the corresponding n-BuOH and H of hydroxyl on the other n-BuOH would be destroyed by the excess of BF3molecule,forming BF3·n-BuOH.electrostatic potential of O on n-BuOH was negative and the electrostatic potential of B on BF3was positive.By the variation of electrostatic potential above,we inferred that the B and O were the active sites for the complex reaction.Additionally,comparing the corresponding n-BuOH on BF3·n-BuOH with that on BF3·(n-BuOH)2,the H of hydroxyl on BF3·n-BuOH possessed the positive electrostatic potentials,while the electrostatic potentials of both the corresponding H of hydroxyl on BF3·(n-BuOH)2and the O on other n-BuOH approached neutral.This showed that,for BF3·(n-BuOH)2,there was a hydrogen bond lying between the H of hydroxyl and the O on the other n-BuOH.Moreover,with regard to the geometric structure,it could be seen that the H of hydroxyl on BF3·(n-BuOH)2had larger steric hindrance than that on BF3·n-BuOH.

Scheme 1.Structural transformation from BF3·(n-BuOH)2to BF3·n-BuOH.
3.6.Electrostatic potentials
To investigate the active site on the catalyst for catalyzing n-decene polymerization,the electrostatic potentials[27]of BF3,n-BuOH,BF3·(n-BuOH)2and BF3·n-BuOH were analyzed,as shown in Fig.6.We found that the BF3on BF3·n-BuOH possessed more negative electrostatic potentials than the pure BF3.Moreover,comparing the corresponding atom on n-BuOH,most atoms on BF3·n-BuOH had more positive electrostatic potentials than those on n-BuOH.As for BF3·n-BuOH,it was rather remarkable that the electrostatic potentials of O approached neutral and the B possessed the negative electrostatic potentials.Nevertheless,the Thus,it could be inferred that the H of hydroxyl on BF3·n-BuOH had the stronger activity than that on BF3·(n-BuOH)2for electrophilic reaction.Our findings should be significant for studying the activation process of Lewis acid for catalyzing n-decene polymerization reaction.
3.7.Polymerization of n-decene
The catalytic performance of BF3/n-BuOH complexes(10%-45%)as catalyst was evaluated in n-decene polymerization reaction.And then the effects of polymerization conditions(reaction temperature,reaction pressure,reaction time,and m(n-BuOH)/m(n-decene)on product distributions were also investigated when BF3was continuously introduced into the reaction system.In the subsequent section,the detailed product distributions and experiment findings were also analyzed.

Fig.6.Electrostatic potentials molecule surfaces:(a)BF3,(b)n-BuOH,(c)BF3·(n-BuOH)2and(d)BF3·n-BuOH.
3.8.n-Decene polymerization catalyzed by BF3/n-BuOH complexes with different mass fractions of BF3
Plenty of study results showed that the mass fraction of BF3had a great effect on n-decene conversion.In order to investigate the detailed relationship between mass fraction of BF3and n-decene conversion,ndecene polymerization reaction was carried out using BF3/n-BuOH complexes with the different mass fractions of BF3as catalyst.

Fig.7.Effect of mass fraction of BF3on n-decene conversion.

Table 3 Product distributions using BF3/n-BuOH complexes as catalyst①,②,③
As can be seen from Fig.7,when χ(mass fraction of BF3)was between 10%and 30%,the conversion of n-decene polymerization was changed from 0.72% to 0.68%.The BF3/n-BuOH complexes showed nearly no catalytic activities for n-decene polymerization reaction.Nevertheless,the n-decene conversion polymerization reaction was significantly increased when χ was above 40%.At the same time,as can be seen in Table 3,the product distribution in n-decene polymerization reaction focused on monomer and dimer.Meanwhile,the trimer and tetramer had slightly increased when χ was above 40%.Based on the above results,we inferred that BF3·n-BuOH as active species which could release cations to n-decene monomer.So when the ndecene polymerization was triggered,a large number of n-decene monomers were consumed.
3.9.Effect of reaction pressure on catalytic performance
As shown in Table 4,variation of the reaction pressure in the range of 0.1-0.5 MPa revealed a distinct impact on the product distributions and n-decene conversion.Compared with the 45%BF3/n-BuOH complexes,the oversaturated BF3increased n-decene conversion and the catalytic activity of BF3/n-BuOH complexes.The possible reason was that the excessive BF3enhanced the dynamics for polymerization.Therefore,the catalytic performance increased with the pressure.Obviously,the ndecene conversion was 99% when the reaction pressure was at 0.2 MPa.It showed that a large number of active species of BF3/n-BuOH complexes could release cations to n-decene with the increase of reaction pressure.The result was that the chain propagation was initiated adequately.Nevertheless,the n-decene conversion was relatively low when variation of the reaction pressure was in the range of 0.3-0.5 MPa,indicating excessive BF3pressure probably obstructed the acceptability of the active sites to n-decene.Thus,the n-decene conversion was slightly decreased.Meanwhile,we have found that the product distributions were mainly controlled at trimer and tetramer,and n-decene conversion was almost 99%when the pressure of BF3was at 0.2 MPa.Taking the economic factors,the optimum reaction pressure was selected at 0.2 MPa.
3.10.Effect of reaction temperature on catalytic performance
As shown in Table 5,the effect of polymerization temperature was also studied in detail by arranging polymerization temperature from 10°C to 50°C.Under the low reaction temperature(from 10°C to 20°C),pentamer and hexamer had higher product distributions than that under other temperatures.The possible reason was that the low temperature could reduce termination reaction caused by chain transfer reaction and extended the life of active species.The low temperature would be beneficial to increase the content of pentamer and hexamer.Otherwise,the chain transfer reaction was accelerated when the temperature was high.The polymerization was easy to terminate.Thus,the contents of pentamer and hexamer were low,while the monomer and dimer were high relatively.Additionally,we also made an analysis for the product of distribution.The results were that the selectivity of trimer and tetramer was the highest when temperature was 30°C.Therefore,30°C was selected as the optimal temperature.

Table 4 Effect of reaction pressure on the product distributions①,②,③

Table 5 Effect of reaction temperature on the product distributions①,②,③

Table 6 Effect of reaction time on the product distributions①,②,③
3.11.Effect of reaction time on catalytic performance
The effect of reaction time on product distributions was also investigated and the results were given in Table 6.As can be seen from Table 6,we found the n-decene conversion increased with the reaction time.Notably,the product distributions of pentamer and hexamer were gradually increased with elongation of reaction time.Contrary to this,the product distributions of monomer and dimer were decreased.It proved elongation of reaction time increased the effective collision chances between active species and oligomers of n-decene.It was benefited to the transformation from shorter chains to long chains.Meanwhile,the product distributions of monomer and dimer were almost stable at 4%when time was above 2 h.Therefore,the optimal reaction time was determined at 2 h.
3.12.Effect of m(n-BuOH)/m(n-decene)on catalytic performance
Variation of the mass ratio between n-BuOH and n-decene in the range of 0.1%to 0.9%indicated a great impact on n-decene conversion and product distributions(Table 7).The product distributions of monomer and dimer were almost stable at 4%when the mass ratio between n-BuOH and n-decene was above 0.5%.Furthermore,the yields of trimer and tetramer were controlled at 80%.It basically coincided with the requirement of lubricant.Therefore,the rational m(n-BuOH)/m(ndecene)was selected at 0.5%.
4.Conclusions
A series of BF3/n-BuOH complexes were synthesized and the structures of BF3/n-BuOH complexes were characterized in both experiment and theory.The nuclear magnetic spectra of BF3·(n-BuOH)2and BF3·n-BuOH obtained by calculation showed an excellent agreement with experiment results.The results proved that BF3·(n-BuOH)2was synthesized in solution when χ was between 10% and 30%,while BF3·n-BuOH was synthesized when χ was above 35%.Crucially,for geometric and electronic structures of BF3·(n-BuOH)2,we found that the O…H hydrogen bond could be destroyed by excessive BF3.The result was that two BF3·n-BuOH complexes generated.By analyzing the electrostatic potentials of the BF3,n-BuOH,BF3·(n-BuOH)2and BF3·n-BuOH,we inferred the H of hydroxyl on BF3·n-BuOH had stronger activity than that on BF3·(n-BuOH)2for catalyzing n-decene polymerization.In thefuture research work,we will further study the reaction mechanism of n-decene polymerization catalyzed by BF3/n-BuOH complexes.

Table 7 Effect of m(n-BuOH)/m(n-decene)on the product distributions①,②,③
The catalytic performances of BF3/n-BuOH complexes and the polymerization conditions were investigated.The highest n-decene conversion could reach 99% and the most excellent selectivity of n-decene trimer and tetramer could be reached up to 80%.The optimal reaction condition of polymerization was that the pressure was 0.2 MPa,temperature was 30°C,time was 1 h and the m(n-BuOH)/m(n-decene)was 0.5%.Our work was a significant study for manufacturing PAO process which used BF3/n-BuOH complexes as catalyst for n-decene polymerization.
Acknowledgments
We are grateful to the group of Xiaoyang Liu(State Key Laboratory of Inorganic Synthesis and Preparative Chemistry,College of Chemistry,Jilin University)for characterization suggestions and microscopic test center of the Northeast Petroleum University.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2019.05.006.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scaling of the bubble/slug length of Taylor flow in a meandering microchannel☆
- Analyzing of mixing performance determination factors for the structure of radial multiple jets-in-crossflow☆
- Particle-resolved simulation of packed beds by non-body conforming locally refined orthogonal hexahedral mesh☆
- Visual study on the characteristics of liquid and droplet in a novel rotor-stator reactor☆
- Molecular dynamics simulation of supercritical CO2microemulsion with ionic liquid domains:Structures and properties☆
- Modeling bubble column reactor with the volume of fluid approach:Comparison of surface tension models☆
