脊尾白虾不同发育期mtDNA拷贝数变化特征分析*
2018-12-19徐莞媛李志辉张庆起赖晓芳陈建华阎斌伦
张 培 徐莞媛 李志辉 高 焕 张庆起 赖晓芳 陈建华 阎斌伦
脊尾白虾不同发育期mtDNA拷贝数变化特征分析*
张 培1,2,3,4徐莞媛1,2,3,4李志辉1,2,3,4高 焕1,2,3,4张庆起5赖晓芳1,2,3,4陈建华1,2,3,4阎斌伦1,2,3,4①
(1. 江苏省海洋生物技术重点实验室 淮海工学院海洋生命与水产学院 连云港 222005; 2. 江苏省海洋生物产业技术协同创新中心 连云港 222001;3. 江苏省海洋资源开发研究院 连云港 222005; 4. 江苏省农业种质资源保护与利用平台 南京 210014;5. 连云港赣榆佳信水产开发有限公司 连云港 222100)
为了解脊尾白虾()在不同发育期线粒体基因组(mtDNA)拷贝数的变化特征,本研究共采集其8个时期(受精卵期、溞状幼体Ⅰ期、溞状幼体Ⅱ期、溞状幼体Ⅲ期、溞状幼体Ⅳ期、溞状幼体Ⅴ期、仔虾期和成虾期)的DNA样品,利用TaqMan实时荧光定量PCR技术,对上述各发育期单个细胞中的mtDNA拷贝数进行了测定。检测结果显示,脊尾白虾受精卵期、溞状幼体Ⅰ期、溞状幼体Ⅱ期、溞状幼体Ⅲ期、溞状幼体Ⅳ期、溞状幼体Ⅴ期、仔虾期和成虾期的mtDNA拷贝数的均值分别为2366、2648、2644、2873、3559、9948、6452和8872。进一步统计分析结果显示,mtDNA拷贝数与发育时间存在正相关性,相关系数为0.83。研究表明,随着脊尾白虾不断生长,其单个细胞中mtDNA拷贝数总体呈上升趋势。
脊尾白虾;TaqMan探针法;线粒体基因组;不同发育期;拷贝数
线粒体是细胞的能量工厂,通过氧化磷酸化为细胞活动提供能量(熊伟等, 2014)。线粒体功能的实现不仅依赖于线粒体基因组的完整性和核基因组的调控,还依赖其基因组的拷贝数(Hock, 2009)。不同细胞中的mtDNA拷贝数并不固定,从几个到几万个不等,这主要取决于细胞及机体所处的生理状况,如在人类()胚胎发育的不同阶段,肝脏和肌肉中的mtDNA拷贝数与胚龄呈显著正相关(Pejznochova, 2010),人类心肌细胞中的mtDNA拷贝数在不同年龄段也存在着较大的差异(Pohjoismäki, 2010)。mtDNA拷贝数在一定程度上反映了线粒体的数量,可作为衡量组织需氧及能量的一种指标(Kaaman, 2007; Justo, 2005)。在不同的生理状况下,mtDNA拷贝数的调控机制至今尚不清楚。目前,对于mtDNA拷贝数的研究主要集中在人类疾病发生方面,尤其是恶性肿瘤,如肝癌、结肠癌、乳腺癌、肺癌等(高原等, 2016; Linkowska, 2015; Lemnrau, 2015; Kim, 2014)。在此类疾病的发生过程中,mtDNA拷贝数会显著升高或者降低(Akbar, 2016; Mambo, 2005),如在肠癌等病变组织中,mtDNA拷贝数较正常组织显著升高(Qu, 2011),而在肺癌等病变组织中,mtDNA拷贝数较正常组织显著降低(Lin, 2008)。除此之外,在小鼠()、猪()、斑马鱼(、秀丽隐杆线虫(C)等物种中关于mtDNA拷贝数的研究也表明,机体在不同的生理状况下,其细胞中mtDNA拷贝数会出现显著的变化(Ikeda, 2015; 成文敏等, 2012; 周莉娟等, 2013; 叶侃, 2011),而在甲壳动物中,除了张培等(2016)对脊尾白虾mtDNA拷贝数响应汞胁迫的变化特征外,关于mtDNA拷贝数的研究则未见报道。
脊尾白虾()是我国的一种重要海洋经济虾类,具有繁殖周期短、生长速度快和环境适应性广等优点(Xu, 2010),3~4个月可繁殖1代,可以在短时间内观察到其不同生长阶段的特征,是开展甲壳动物基础遗传和遗传育种研究的良好模式生物。伴随着脊尾白虾的生长,其机体各项生理活动及指标也会变化。目前,脊尾白虾不同生长阶段的研究主要集中在消化和免疫防御、胚胎和幼体存活率、饵料系数等方面(Babulo, 2008; 索帅等, 2015; 李玉全, 2014; 冯艳艳等, 2017),而对于mtDNA拷贝数的研究甚少。mtDNA拷贝数的变化一定程度上反映了机体生命活动耗能的多少,也从侧面体现了生物体代谢与生长的快慢。本研究在建立一种快速、精确的脊尾白虾mtDNA拷贝数检测方法的基础上,研究了不同发育期脊尾白虾mtDNA拷贝数的变化特征,希望通过对脊尾白虾生长过程中mtDNA拷贝数的变化特征分析,为脊尾白虾在生长过程中的生长情况观测提供新的生物指标。
1 材料与方法
1.1 实验材料
实验用脊尾白虾取自江苏省连云港赣榆佳信水产开发有限公司的养殖池塘,是由池塘中的野生群体自繁而来。在实验室养殖环境条件下暂养24 h后,进行后续实验。实验期间正常投喂(早晚各1次),连续充气,水温保持在25℃左右,盐度为26,溶氧量≥5 mg/L,pH为8.0~8.5。
1.2 引物设计
根据(腺苷酸转移酶基因,GenBank No. KP892663.1,核基因组单拷贝基因)和6(三磷酸腺苷合成酶6,GenBank No. NC012566.1,线粒体基因组单拷贝基因)2个基因序列,设计实时定量PCR (qRT- PCR)扩增引物及TaqMan探针(已申请国家专利,申请号为201610906439.X)。引物和探针均由生工生物工程(上海)股份有限公司合成。
1.3 mtDNA和nDNA标准品的制备
提取脊尾白虾组织总DNA,并以此为模板,分别用和6引物进行扩增,10 µl PCR体系:7.0 µl ddH2O,1.0 µl 10×PCR缓冲液,0.6 µl MgCl2(25 mmol/L),0.2 µl dNTPs (10 µmol/L),0.2 µlDNA聚合酶(5 U/µl),上、下游引物各0.4 µl (10 µmol/L),0.2 µl mix-DNA (20 ng/µl)。扩增程序:95℃预变性3 min;95℃ 30 s、60℃ 30 s、72℃ 1 min、35个循环;最后,72℃延伸10 min。PCR产物用1.5%琼脂糖凝胶电泳检测。扩增产物经胶回收纯化后,按pEASY-T3 Zero Cloning Kit操作说明,把相应的片段连接到质粒载体中,再转化入Trans1-T1 phage Resistant感受态细胞,利用蓝白斑筛选获得阳性克隆菌株,挑取含有阳性克隆的单克隆菌落扩大培养,并送至生工生物工程(上海)股份有限公司测序鉴定。经鉴定为阳性质粒的,用NanoDrop 2000测定质粒浓度,利用双链DNA拷贝数计算器(http://cels.uri.edu/gsc/cndna.html)将质粒质量换算成拷贝数(拷贝/µl),作为mtDNA和nDNA 标准品。
表1、6基因实时定量PCR引物及TaqMan探针
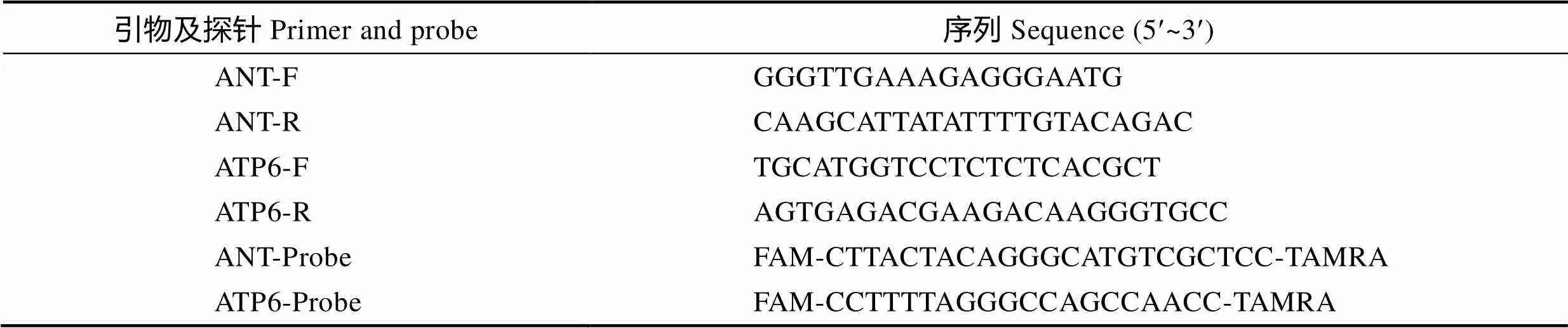
Tab.1 Quantitative real-time PCR primers and TaqMan probes of ANT and ATP6
1.4 mtDNA和nDNA标准曲线的建立
使用无DNA酶的ddH2O将质粒标准品10倍梯度稀释成107、106、105、104、103、102、101拷贝,各1000 μl,并以此制作标准曲线。
qRT-PCR采用10 μl扩增体系,反应体系:5 μl Premix Ex(qPCR Probe),0.2 μl上、下游引物 (10 µmol/L),0.2 μl 50×ROX Reference Dye,0.2 μl gDNA(20 ng/μl),0.4 μl TaqMan探针(0.6 μmol/L)。采用StepOnePlus™型荧光定量PCR仪(Applied Biosystems Inc.)进行扩增,扩增程序:95℃ 20 s;95℃ 1 s、60℃ 20 s,40个循环。
1.5 脊尾白虾不同发育期DNA的提取
脊尾白虾胚胎发育的过程一般分为2细胞期、 4细胞期、8细胞期、16细胞期、32细胞期、囊胚期、原肠期、无节幼体期、溞状幼体Ⅰ期、溞状幼体Ⅱ期、溞状幼体Ⅲ期、溞状幼体Ⅳ期、溞状幼体Ⅴ期、溞状幼体Ⅵ期和仔虾等时期(梁象秋等, 1988; 武文魁, 1984; 王绪峨, 1991)。本研究选取了受精卵期、溞状幼体Ⅰ期,溞状幼体Ⅱ期、溞状幼体Ⅲ期、溞状幼体Ⅳ期、溞状幼体Ⅴ期、仔虾期和成虾期8个时期的样品,每个时期各取3尾,经液氮研磨后用DNA快速抽提试剂盒[生工生物工程(上海)股份有限公司]提取总DNA,将DNA浓度稀释至20 ng/μl备用。
1.6 数据分析
采用SPSS 19.0对数据进行相关性分析。从脊尾白虾核型初步推测其为二倍体,因此,每个细胞中的线粒体基因组拷贝数的计算公式为:
mtDNA拷贝数=2×6拷贝数/拷贝数
2 结果
2.1 mtDNA和nDNA标准曲线的构建
以10倍梯度稀释重组质粒后,选取107~102的稀释标准品进行qRT-PCR扩增,分别获得基因与6基因的扩增曲线。6基因的标准曲线的回归方程=3.402+15.928;基因的标准曲线的回归方程=3.439+15.248,相关系数>0.99,见图1。
2.2 脊尾白虾不同发育期线粒体基因组拷贝数的变化
利用qRT-PCR,根据标准曲线获得各个样本平均每个细胞中线粒体基因组拷贝数,统计结果显示,各发育期的mtDNA拷贝数随着生长发育总体呈上升趋势(图2)。受精卵期、溞状幼体Ⅰ期、溞状幼体Ⅱ期、溞状幼体Ⅲ期、溞状幼体Ⅳ期、溞状幼体Ⅴ期、仔虾期和成虾期8个时期的mtDNA拷贝数均值分别为2366、2648、2644、2873、3559、9948、6452和8872,溞状幼体Ⅴ期和成虾期mtDNA拷贝数最多,分别是受精卵期的4.2倍和3.7倍。进一步统计分析发现,mtDNA拷贝数和发育时间存正相关性,相关系数为0.83,且溞状幼体Ⅴ期、仔虾和成虾期与其他发育期存在显著性差异(<0.05)。
3 讨论
目前,检测mtDNA拷贝数的方法主要有SYBR GreenⅠ染料法(何静等, 2014)和TaqMan探针法(Chiu, 2003) 2种。由于SYBR GreenⅠ染料法的特异性不强,多数mtDNA拷贝数测定还是采用TaqMan探针法。采用TaqMan探针法建立的标准曲线具有良好的重复性,且引物、探针序列和引物探针相互作用关系的双重保证克服了普通PCR假阳性的缺陷(Pohjoismäki, 2010)。本研究通过构建脊尾白虾线粒体及核基因组单拷贝基因的质粒作为标准品,建立了一种快速、高效、精确的检测脊尾白虾mtDNA拷贝数的方法,并在此基础上,对脊尾白虾不同发育期的mtDNA拷贝数进行了精确定量。
早期胚胎中的ATP水平代表着机体胚胎的发育水平(Dumollard, 2008),线粒体产ATP能力又与细胞内mtDNA拷贝数有密切联系(Dumollard, 2007; Tsai, 2015),至今有许多学者将mtDNA拷贝数作为评估卵母细胞发育水平的一个指标(Van Blerkom, 2011; Santos, 2006; Zeng, 2007),即mtDNA拷贝数越高,卵母细胞成熟度越高,受精能力越强,胚胎发育潜能越高。所以,mtDNA拷贝数对机体的胚胎发育有着深远影响。本研究结果显示,随着脊尾白虾的生长,细胞内的mtDNA拷贝数在不断增加,总体呈上升趋势,这与谢燕梅(2014)在对猪不同生长阶段不同组织mtDNA拷贝数变化研究中得到的结论相似,即伴随着脊尾白虾的生长,其活动量增加,机体能量需求增大,mtDNA拷贝数也会随之增加。mtDNA拷贝数在一定程度上反映了脊尾白虾不同生长阶段的能量需求情况,为评估脊尾白虾生长发育状况提供了一个生物指标。
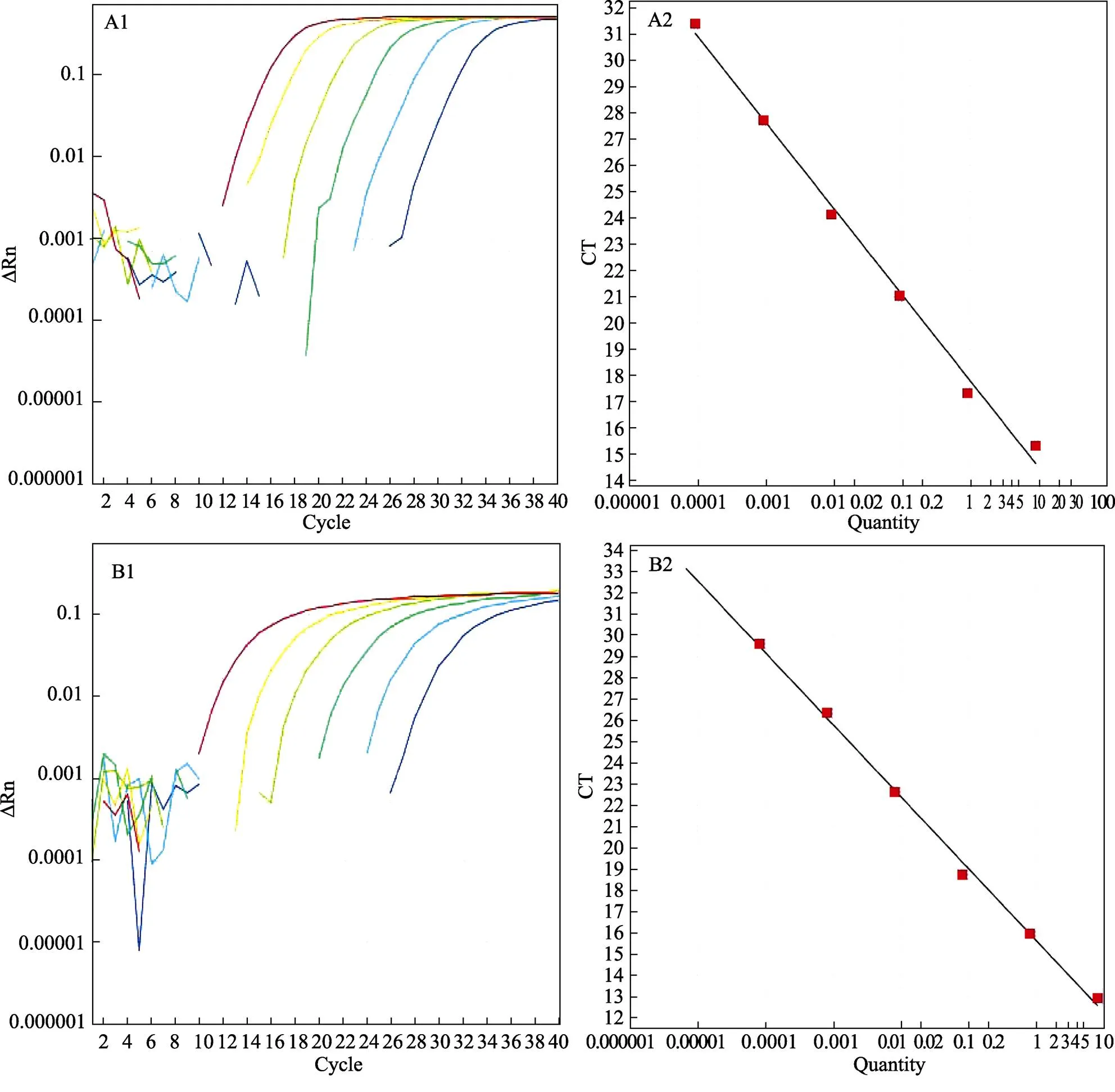
图1 ATP6(A)与ANT(B)荧光定量PCR扩增曲线及标准曲线
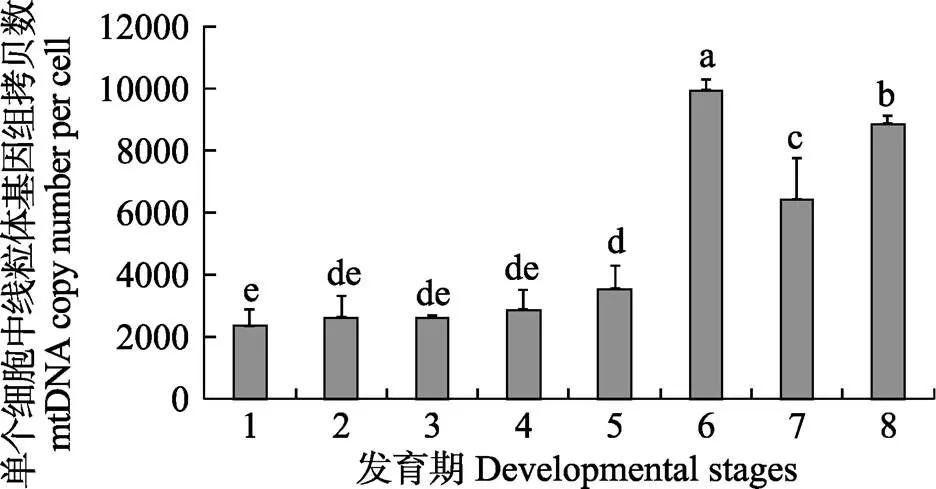
图2 不同发育期线粒体基因组拷贝数变化
1:受精卵期;2:溞状幼体Ⅰ期;3:溞状幼体Ⅱ期; 4:溞状幼体Ⅲ期;5:溞状幼体Ⅳ期;6:溞状幼体Ⅴ期; 7:仔虾期;8:成虾期。不同字母表示差异显著(<0.05)
1: Zygote; 2: Zoea Ⅰ; 3: Zoea Ⅱ; 4: Zoea Ⅲ; 5: Zoea Ⅳ; 6: Zoea Ⅴ; 7: Post larvae; 8: Adult shrimps. Different letters indicated significant differences (<0.05)
甲壳动物具有独特的蜕皮生长方式,在蜕皮生长期间会出现幼体前期的慢生长和幼体后期的快生长,并伴随着机体变态,二者相对独立又互相联系(Gore, 1985; Anger, 1987)。在十足类幼体发育阶段,变态期间的快速生长期一般仅限制在幼体生长的倒数第一期和第二期,此时机体形态变化大、消耗能量多,是早期幼体慢生长及能量积累的结果。进入成体之后,机体蜕皮生长间隔变长,能量累积也随之变缓(McConaugha, 1982、1985)。机体内能量需求越大,mtDNA拷贝数越高,这可能是本研究中溞状幼体Ⅴ期mtDNA拷贝数高于其他时期的原因之一。另外,还有可能是机体内mtDNA拷贝数调控机制的作用,目前报道的mtDNA拷贝数受线粒体转录因子A (Lu, 2013)、同源重组修复蛋白Rad51(Sage, 2011)、DNA聚合酶γ(Kelly, 2012)等因素影响,具体如何调控有待进一步研究。
Akbar M, Essa MM, Daradkeh G,. Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Research, 2016, 1637: 34‒55
Anger K. The D0threshold: A critical point in the larval development of decapod crustaceans. Journal of Experimental Marine Biology and Ecology, 1987, 108(1): 15‒30
Babulo B, Muys B, Nega F,. Household livelihood strategies and forest dependence in the highlands of Tigray, Northern Ethiopia. Agricultural Systems, 2008, 98(2): 147‒155
Cheng WM, Wei HJ, Pan WR,. Genetic variation and phylogenetic analysis ofgenes of 63 isolate strains of IBV. China Animal Husbandry and Veterinary Medicine, 2012, 39(1): 127‒131 [成文敏, 魏红江, 潘伟荣, 等. 猪孤雌激活早期胚胎线粒体分布及mtDNA拷贝数变化的研究. 中国畜牧兽医, 2012, 39(1): 127‒131]
Chiu RWK,Chan LYS,Lam NYL,. Quantitative analysis of circulating mitochondrial DNA in plasma. Clinical Chemistry, 2003, 49(5): 719‒726
Dumollard R, Campbell K,Halet G,. Regulation of cytosolic and mitochondrial ATP levels in mouse eggs and zygotes.Developmental Biology, 2008, 316(2): 431‒440
Dumollard R, Duchen M, Carroll J. The role of mitochondrial function in the oocyte and embryo. Current Topics in Developmental Biology, 2007, 77: 21‒49
Feng YY, Li J, Li JT,. Effects of florfenicol on the immune and antioxidant activities of. Progress in Fishery Sciences, 2017, 38(5): 140‒147 [冯艳艳, 李健, 李吉涛, 等. 氟苯尼考对脊尾白虾()免疫和抗氧化功能的影响. 渔业科学进展, 2017, 38(5): 140‒147]
Gao Y, Nie HJ, Yang D,. Changes of the mitochondrial DNA copy number and the antioxidant system in the PBMC of hepatocellular carcinoma. Chinese Journal of Applied Physiology, 2016, 32(1): 1‒5 [高原, 聂鸿靖, 杨栋, 等. 肝癌患者单个核细胞线粒体DNA拷贝数与抗氧化能力的变化. 中国应用生理学杂志, 2016, 32(1): 1‒5]
Gore RH. Molting and growth in decapod larvae. Crustacean Issue 2-Larval Growth, 1985, 52–65
He J, Xia H, Zhang CM,. The real-time PCR assay for mitochondrial DNA copy number and its applications in kidney disease diagnosis. Chinese Journal of Nephrology, Dialysis and Transplantation, 2014, 23(4): 342‒348 [何静, 夏虹, 张昌明, 等. 线粒体DNA拷贝数检测方法的建立及在肾脏疾病诊断中的应用. 肾脏病与透析肾移植杂志, 2014, 23(4): 342‒348]
Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annual Review of Physiology, 2009, 71: 177‒203
Ikeda M,Ide T, Fujino T,.Overexpression of TFAM or twinkle increases mtDNA copy number and facilitates cardioprotection associated with limited mitochondrial oxidative stress. PLoS One, 2015, 10(3): e0119687
Justo R, Oliver J, Gianotti M. Brown adipose tissue mitochondrial subpopulations show different morphological and thermogenic characteristics. Mitochondrion, 2005, 5(1): 45‒53
Kaaman M, Sparks LM, van Harmelen V,. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia, 2007, 50(12): 2526‒2533
Kelly RDW, Mahmud A, McKenzie M,. Mitochondrial DNA copy number is regulated in a tissue specific manner by DNA methylation of the nuclear-encoded DNA polymerase gamma A. Nucleic Acids Research, 2012, 40(20): 10124‒10138
Kim C, Bassig BA, Seow WJ,. Pooled analysis of mitochondrial DNA copy number and lung cancer risk in three prospective studies. Cancer Epidemiology Biomarkers and Prevention, 2014, 23(12): 2977‒2980
Lemnrau A, Brook MN, Fletcher O,. Mitochondrial DNA copy number in peripheral blood cells and risk of developing breast cancer. Cancer Research, 2015, 75(14): 2844‒2850
Li YQ. Effects of salinity on growth, feeding and conversion rate of. Jiangsu Agricultural Sciences, 2014, 42(9): 191‒193 [李玉全. 盐度对脊尾白虾生长、摄食及饵料转化率的影响. 江苏农业科学, 2014, 42(9): 191‒193]
Liang XQ, Li YJ, Zhou ZM. The larval development of(holthuis). Journal of Fisheries of China, 1988, 12(2): 157‒168 [梁象秋, 李亚娟, 周昭曼. 脊尾白虾的幼体发育. 水产学报, 1988, 12(2): 157‒168]
Lin CS, Wang LS, Tsai CM,. Low copy number and low oxidative damage of mitochondrial DNA are associated with tumor progression in lung cancer tissues after neoadjuvant chemotherapy. Interactive Cardiovascular and Thoracic Surgery, 2008, 7(6): 954‒958
Linkowska K, Jawień A, Marszałek A,Mitochondrial DNA polymerase γ mutations and their implications in mtDNA alterations in colorectal cancer. Annals of Human Genetics, 2015, 79(5): 320‒328
Lu B, Lee J, Nie XB,. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+Lon protease. Molecular Cell, 2013, 49(1): 121‒132
Mambo E, Chatterjee A, Xing M,. Tumor-specific changes in mtDNA content in human cancer. International Journal of Cancer, 2005, 116(6): 920‒924
McConaugha JR. Nutrition and larval growth. In: Wenner AM, Balkema AA(ed.), Larval growth. Rotterdam, 1985, 127‒154
McConaugha JR. Regulation of crustacean morphogenesis in larvae of the mud crab. Journal of Experimental Zoology Part A Ecological Genetics and Physiology, 1982, 223(2): 155‒163
Pejznochova M, Tesarova M, Hansikova H,. Mitochondrial DNA content and expression of genes involved in mtDNA transcription, regulation and maintenance during human fetal development. Mitochondrion, 2010, 10(4): 321‒329
Pohjoismäki JI, Goffart S, Taylor RW,. Developmental and pathological changes in the human cardiac muscle mitochondrial DNA organization, replication and copy number. PLoS One, 2010, 5(5): e10426
Qu FL, Liu XN, Zhou F,. Association between mitochondrial DNA content in leukocytes and colorectal cancer risk. Cancer, 2011, 117(14): 3148‒3155
Sage JM, Gildemeister OS, Knight KL. Discovery of a novel function for human Rad51: Maintenance of the mitochondrial genome. Mitochondrion, 2011, 11(4): 676
Santos TA, Shourbagy EIS, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertility and Sterility, 2006, 85(3): 584‒591
Suo S, Li J, Li JT,. Effects of dietary protein and lipid levels on growth, non-specific immunity and disease resistance of. Chinese Fishery Quality and Standards, 2015, 5(4): 40‒47 [索帅, 李健, 李吉涛, 等. 蛋白质和脂肪水平对脊尾白虾生长、非特异性免疫力及抗病力的影响. 中国渔业质量与标准, 2015, 5(4): 40‒47]
Tsai S, Chen JC, Spikings E,. Degradation of mitochondrial DNA in cryoprotectant-treated hard coral (spp.) oocytes. Mitochondrial DNA, 2015, 26(3): 420‒425
Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion, 2011, 11(5): 797‒813
Wang XE. The larval development of. Marine Sciences, 1991, 15(1): 12‒14 [王绪峨. 脊尾白虾的幼体发育. 海洋科学, 1991, 15(1): 12‒14]
Wu WK. The study on the larval development of. Acta Oceanologica Sinica, 1984, 5(sup.): 953‒ 962 [武文魁. 脊尾白虾幼体发育的研究. 海洋学报, 1984, 5(sup.): 953‒962]
Xie YM. Quantitative changes of mitochondrial DNA copy number in various tissues of pigs during growth. Master´s Thesis of Sichuan Agricultural University, 2014, 1‒56 [谢燕梅. 猪不同生长阶段不同组织线粒体DNA(mtDNA)拷贝数变化. 四川农业大学硕士研究生学位论文, 2014, 1‒56]
Xiong W, Zhang HY, Yang HJ,. Research advances in transcription and regulation mechanism of mammalian mitochondrial genome. Journal of Dali University, 2014, 13(8): 26‒30 [熊伟, 张海洋, 杨红娟, 等. 哺乳动物细胞线粒体基因组的转录及调控机制研究进展. 大理学院学报, 2014, 13(8): 26‒30]
Xu W, Xie J, Shi H,.infections in cultured ridgetail white prawns,, in eastern China. Aquaculture, 2010, 300(1‒4): 25‒31
Ye K. Study on the radiation damage of growth and the protection in. Doctoral Dissertation of Suzhou University, 2011 [叶侃. 辐射对秀丽线虫的生长损伤及其防护研究. 苏州大学博士研究生学位论文, 2011]
Zeng HT, Ren Z, Yeung WS,. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope inmatured human oocytes. Human Reproduction, 2007, 22(6): 1681‒1686
Zhang P, Li ZH, Zhao L,. Profiles of mtDNA copy number in response to mercury stress in. Journal of Huaihai Institute of Techology (Natural Science Edition), 2016, 25(4): 78‒81 [张培, 李志辉, 赵莲, 等. 汞胁迫下脊尾白虾线粒体基因组拷贝数变化特征分析. 淮海工学院学报(自然科学版), 2016, 25(4): 78‒81]
Zhou LJ, Shi CM, Song GX,. Effect of FABP3 overexpression on heart development and mitochondrial function in zebrafish embryo. Jiangsu Medical Journal, 2013, 39(6): 621‒624 [周莉娟, 史春梅, 宋桂仙, 等. FABP3过表达对斑马鱼胚胎心脏发育及线粒体DNA拷贝数的影响. 江苏医药, 2013, 39(6): 621‒624]
Profiles of mtDNA Copy Number at Different Developmental Stages of
ZHANG Pei1,2,3,4, XU Wanyuan1,2,3,4, LI Zhihui1,2,3,4, GAO Huan1,2,3,4, ZHANG Qingqi5, LAI Xiaofang1,2,3,4, CHEN Jianhua1,2,3,4, YAN Binlun1,2,3,4①
(1. Jiangsu Key Laboratory of Marine Biotechnology, College of Marine Life and Fisheries, Huaihai Institute of Technology, Lianyungang 222005; 2. Co-Innovation Center of Jiangsu Marine Bio-Industry Technology, Lianyungang 222001; 3. Marine Resource Development Institute of Jiangsu, Lianyungang 222005; 4. Jiangsu Provincial Platform for Conservation and Utilization of Agricultural Germplasm, Nanjing 210014; 5. Ganyu-Jiaxin Aquaculture Limited Company of Lianyungang, Lianyungang 222100)
Mitochondria are important organelles in cells. Mitochondria can produce ATP and provide energy for almost all biological activities. Mitochondria have a relatively independent genetic system, and nuclear genes also encode certain enzymes responsible for mitochondrial DNA (mtDNA) replication and transcription. Therefore, the number and function of mitochondria is essential for embryonic development. Mitochondrial function depends on the integrity of the mitochondrial genome or the regulation of nuclear genome, as well as the mtDNA copy number. Many studies have investigated mtDNA copy numbers in animals, including studies on oocyte maturation, mammalian embryonic development, and human disease, but none have reported the mtDNA copy number in. To study the profiles of mtDNA copy number at different developmental stages of,and the energy expenditure of tissues at the different stages of development, total DNA from zygote, zoea Ⅰ, zoea Ⅱ, zoea Ⅲ, zoea Ⅳ, zoea Ⅴ, post larvae, and adult shrimps were used to analyze the mtDNA copy number. One TaqMan probe-based real-time PCR was established to assess the mtDNA copy number. Our results showed that the average copy number of mtDNA per cell was 2366, 2648, 2644, 2873, 3559, 9948, 6452, and 8872 in the zygote, zoea Ⅰ, zoea Ⅱ, zoea Ⅲ, zoea Ⅳ, zoea Ⅴ, post larvae, and adults, respectively. These results show that mtDNA copy number is positively correlated with age, andthe correlation coefficient () was 0.83, i.e. the copy numbers of mtDNA tend to increase with progressing developmental stages of.
; TaqMan real-time PCR; Mitochondrial genome; Different developmental stages; Copy number
YAN Binlun, E-mail: yanbl@hhit.edu.cn
张培, 徐莞媛, 李志辉, 高焕, 张庆起, 赖晓芳, 陈建华, 阎斌伦. 脊尾白虾不同发育期mtDNA拷贝数变化特征分析. 渔业科学进展, 2018, 39(6): 173–179
Zhang P, Xu WY, Li ZH, Gao H, Zhang QQ, Lai XF, Chen JH, Yan BL. Profiles of mtDNA copy number at different developmental stages of. Progress in Fishery Sciences, 2018, 39(6): 173–179
* 江苏高校优势学科建设工程资助项目、江苏省高等学校自然科学研究重大项目(17KJA240001)、江苏省“六大人才高峰”创新人才团队资助项目(2016-HYGC-CXTD-004)和江苏省研究生实践创新计划(KYLX16_1417)共同资助[This work was supported by Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD), Natural Science Foundation of the Jiangsu Higher Education Institutions of China(17KJA240001), “Six Talent Summit” Innovative Talents Team of Jiangsu Province (2016-HYGC-CXTD-004), and Practice Innovation Program for Graduate Students in Jiangsu Province (KYLX16_1417)]. 张 培,E-mail: 2272525096@qq.com
阎斌伦,教授,E-mail: yanbl@hhit.edu.cn
2017-09-21,
2017-11-13
10.19663/j.issn2095-9869.20170921001
S917.4
A
2095-9869(2018)06-0173-07
(编辑 马璀艳)
