Reliable,environmentally friendly method for the recycling of spent Ag/α-Al2O3 catalysts using(NH4)2Ce(NO3)6☆
2018-11-15XulongDongJianLiQingguiXiaoHuiZhangTaoQi
Xulong Dong ,Jian Li,Qinggui Xiao ,Hui Zhang ,*,Tao Qi,*
1 University of Chinese Academy of Sciences,Beijing 100049,China
2 National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology,Institute of Process,Chinese Academy of Sciences,Beijing 100190,China
3 Key Laboratory of Green Process and Engineering,Institute of Process,Chinese Academy of Sciences,Beijing 100190,China
Keywords:Cerium ammonium nitrate(CAN)Silver Spent catalyst Leaching Kinetics
ABSTRACT Traditionally,Ag-containing solid wastes are leached by nitric acid in order to recycle the noble metal.However,the huge amounts of emission of toxic nitrogen oxides demand the development of a new method for silver recycling.Recently,considering the Ce(IV)solution could be regenerated with electrolyzation method,our group invented a novel environmentally friendly process by using Ce(IV)as the oxidant to dissolve silver from the spent Ag/ɑ-Al2O3 catalysts without NO x emission.To find out the optimal parameters,in this work,the leaching reaction was thoroughly investigated with respectto the temperature,oxidantand HNO3 concentrations,stirring speed,and time.The optimized leaching reaction gave the leaching silver rate 99.8%in 1 h.The kinetic plots suggested a shrinking core model with the internal diffusion-controlled process and the activation energy of 38.83 kJ·mol-1.The order in which the experimental conditions in fluence the reaction was determined through orthogonalanalysis:temperature N oxidantconcentration N HNO3 concentration N stirring speed.
1.Introduction
Due to the increasing awareness of health and environmental protection,there has been a growing interest in the extraction of valuable components from recyclable substances using simple and green technology.As an important precious metal,silver is used in a wide range of applications,such as aviation technology,electronic materials,medicine,and catalysis[1].Moreover,the precious metal is a key element in many oxidation catalysts and constitutes a major part of these compounds[2].For example,in the silver-catalyzed oxidation of ethylene,the Ag/α-Al2O3catalystusually contains 8%-40%(w/w)ofsilver[1-3].The mixture resulting from this reaction contains a large amount of silver and various toxic substances.The recovery of the used silver after such a process is necessary because of both economic and environmental reasons.
Various agents,including cyanide,thiourea,thiosulfate,and halide[4-7],have been employed in the recycling of silver.In the recycling industry,however,almost only HNO3is used for the leaching of silver from contaminated or deactivated catalysts,which produces highly concentrated AgNO3solution.Salt or hydrochloric acid is added to the leaching solution to precipitate the silver ions,while the impurities remain in solution.The AgCl deposit can be then reduced to metallic sliver by glucose or N2H4[8,9].The catalysis of the leaching process by nitrogen species causes a strong reaction between Ag and concentrated HNO3[10].

Diluted nitric acid(≤1 mol·L-1)reacts with silver according to Eq.(2),leading to the emission of NO gas[1].However,when the concentration of nitric acid is more than 1 mol·L-1,NO2,a brown gas,is released instead.Nitrogen oxides are a common atmospheric pollutant,which is responsible for many harmful phenomena,such as acid rain,photochemical smog,ozone depletion,and the greenhouse effect[10].Various methods to absorb the toxic gas have been investigated.These include the use of the inorganic FeSO4solvent or the organic solvent Fe(II)EDTA to absorb the NOxgases[11,12].However,considering the challenging absorption of the gas into the solution,it is crucial that a novel,more efficient,and environmentally friendly method for leaching is developed.
Cerium ammonium nitrate(CAN)is a cheap and recyclable watersoluble oxidant,mainly used in organic synthesis,toxic organic decomposition,polymerization initiation and integrated circuit etching[13-18].For example,Ce4+-mediated electrochemical oxidation allows for the decomposition of EDTAand phenol[13].CANis environmentally friendly,highly reactive,and has low toxicity.Moreover,the standard potential of Ce4+/Ce3+(1.44 V)is much higher than that of HNO3/NO2(0.80 V)and Ag+/Ag(0.7996 V)[19].Thus,in a solution rich with Ce(IV),it is expected that the dissolution of silver occurs through a reaction of Ce4+and Ag,rather than HNO3and Ag,which prevents the emission of NOxgases.All of these advantages of CAN make it a good candidate for use in silver leaching.
In this work,CAN was used as a leaching agent in order to prevent the formation of nitrogen oxides.The acidic solution of CAN was mixed with Ag/α-Al2O3utilized catalysts.During the experiment,no bubbles were generated and no nitrogen oxide was detected in the air,indicating an environmentally friendly process.After the leaching process,the Ce(III)solution can be oxidized to Ce(IV)solution by electrolysis,so the leaching agent can be reused.The reaction temperature,concentration of CAN and HNO3,and stirring speed were varied in order to investigate their in fluence on the reaction.Additionally,the kinetic equation of the leaching reaction was established and a kinetic model was proposed.The optimized leaching conditions were determined through orthogonal analysis.
2.Materials and Methods
2.1.Materials
Spent Ag/α-Al2O3catalysts were obtained from Sino-Platinum Metals Resources(Yimen,China)Co.Ltd.The average mass fraction of silver(17.20%)was measured by inductively coupled plasma optical emission spectrometry(ICP-OES,Optima 5300DV,USA).The contents of the impurities were measured with X-ray fluorescence spectrometer(PANalytical,Netherlands).The data are shown in Table 1.

Table 1 Catalyst composition(mass fraction,%)
2.2.Experimental procedures
To prepare the lixiviant solution,a certain amount of CAN and nitric acid were dissolved in de-ionized water.The catalysts were used directly without grinding or any other pretreatment.The leaching experiments were carried out in a glass hermetic flask(250 ml).The catalyst was immerged in the lixiviant solution and since a mechanical stirrer could not run fluently,the stirring speed and temperature were controlled by using a rotary evaporator.Water evaporation was prevented by placing a rubber in the neck of the flask.
In all experiments,50 g of the spent catalyst was mixed with 75 ml CAN solution with the setting temperature.The reaction was continued for 2 h and the silver concentration was monitored over time by taking 0.5 ml of the solution every 20 min.After the reaction was completed,the mixture was filtered and the solid was washed five times in an ultrasonic cleaner with 50 ml of de-ionized water.The solid was dried at 100°C in a vacuum oven for 12 h and then ground to powder.The silver content of the residual solid and the initial catalyst samples was measured by leaching with concentrated HNO3.
2.3.Characterization
XRD analysis was conducted on an X'Pert Empyrean instrument(PANalytical,Netherlands)with Cu Kα radiation.The contents of silver and cerium in the samples were measured by using an inductively coupled plasma optical emission spectrometer(ICP-OES,Optimal 5300DV,Perkin-Elmer,USA).The content of impurities in the solid samples was determined with an X-ray fluorescence spectrometer(PANalytical,Netherlands).Morphologicalcharacterization and compositional analyses of catalysts were performed by scanning electron microscopy-energy-dispersive X-ray spectroscopy(SEM-EDS,JEOL JSM-6510A,Japan).
3.Results and Discussion
3.1.Leaching experiments
The XRD patterns of the initial spent catalyst and the residual solid are shown in Fig.1.The two phases in the initial catalyst were assigned to Ag(JCPDS 01-087-0597)and α-Al2O3(JCPDS 00-043-1484),respectively.After the leaching process(3),the silver peaks were not detected in the XRD pattern,implying that most of the silver had been dissolved into the solution by the lixiviant.The leaching reaction is as follows:


Fig.1.XRD patterns of the initial catalyst and the residual solid.
The ICP results showed thatthe leaching solution does notcontain Al ions,indicating that ɑ-Al2O3does not react with the lixiviant.
The SEM images of the initial catalyst and the residual solid are displayed in Fig.2.Due to the excellent conductivity of silver,the particles contained in the initial catalyst exhibited appeared as white dots under the gray background of Al2O3[Fig.2(a)],which was supported by the EDS images[Fig.2(b)].White dot disappeared after the sample was leached by CAN solution[Fig.2(c)].The residual solid was Al2O3,as the XRD pattern indicated.The EDS images of residual solid[Fig.2(d)]proved thatthe main ingredientwas Al2O3.The elemental mapping analyses of Ag and Al also revealed the disappearance of sliver,as shown in Fig.S1(supplements).
3.2.Effect of reaction temperature
The effect of the temperature on the leaching rate was investigated at 25 °C,40 °C,and 70 °C while stirring speed at 60 r·min-1.The lixiviant solution for this study consisted of 1.3 mol·L-1CAN and 0.1 mol·L-1HNO3.

Fig.2.SEM images of the(a)initial catalyst and(b)EDS of the initial catalyst;(c)residual solid and(d)EDS of the residual solid.

Fig.3.Effect of reaction temperature on the leaching rate of silver.
The results from these experiments revealed that the leaching rate increases proportionally to the rising reaction temperature(Fig.3).Increasing the temperature to 70°C gave excellent leaching rate(98.4%)after 80 min.Performing the reaction at 40°C,in contrast,generated a lower final leaching rate(73.0%).When decreasing the temperature even further(25°C),the reaction was slow and gave the lowestleaching rate(62.7%).These results imply that the reaction temperature has a significant in fluence on the leaching rate.This dependence could be due to an enhanced molecular motion caused from the increased temperature,which leads to a higher diffusion rate and results in faster reaction rates.According to literature procedures,the leaching of silver with 1 mol·L-1HNO3solution at 70 °C for 2 h,results in a leaching rate below 95%.High yields of around 99%can be achieved only after increasing the temperature even further(85°C),which indicated that the leaching effectwas not as good as that of CAN,and the reaction processproduced a large amountofNOgas[1].Compared to this previously reported research,the method presented in this study gives better results and is more environmentally friendly.
3.3.Effect of the CAN concentration
The CAN concentration effect on the leaching rate was investigated.Three CAN concentrations were examined(1.0,1.3,and 1.6 mol·L-1)while the concentration of HNO3in the lixiviant remained constant(0.1 mol·L-1).The stirring speed and reaction temperature were 60 r·min-1and 70 °C,respectively.

Fig.4.Effect of CAN concentration on the leaching rate of silver.
As Fig.4 shows,the leaching rate increases proportionally to the rising concentration of CAN.When performing the reaction atCAN concentration of 1 mol·L-1for 2 h,the leaching rate was 91.4%.Increasing the concentration of CAN to 1.3 mol·L-1led to a significant enhancement,giving a leaching rate of 98.7%.Similarly,when the concentration was 1.6 mol·L-1,the leaching rate increased to 99.9%.The reactions with the higher CAN concentrations(1.3 and 1.6 mol·L-1)also exhibited increased reaction rates,giving the product almost in full after about 80 min.In contrast,further increase in the CAN concentration could affect the solubility of the silver nitrate,thereby reducing the leaching rate.Consequently,1.3 mol·L-1seems to be the most optimal concentration in terms of increased reaction rates and milder reaction conditions.
3.4.Effect of the HNO3 concentration
The concentration of HNO3was varied(0.1 mol·L-1,0.3 mol·L-1,and 1.0 mol·L-1)in order to investigate the concentration dependence of the reaction(Fig.5).The remaining conditions such as stirring speed(60 r·min-1),CANconcentration(1.3 mol·L-1)and temperature(70 °C)were kept constant.
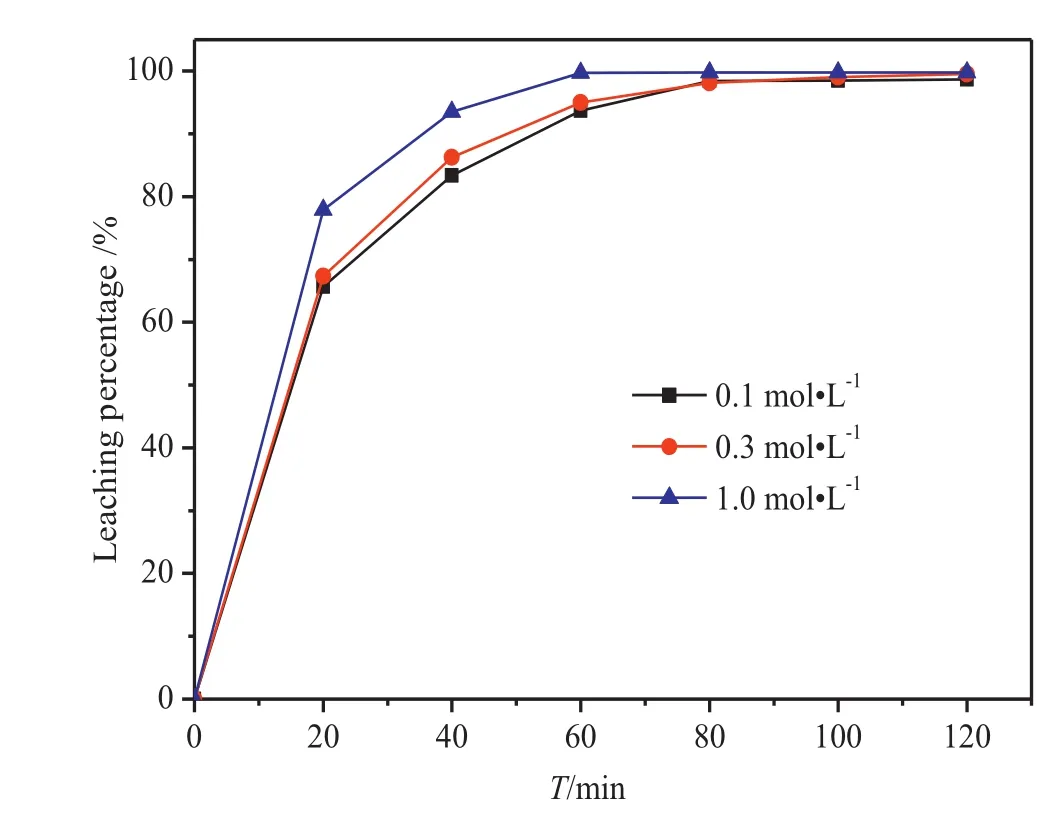
Fig.5.Effect of HNO3 concentration on the leaching percentage of silver.
Fig.5,it can be inferred that the nitric acid concentration has a minimal effect on the leaching rate of silver,which reaches about 99%after 80 min despite the change in concentration.The rate of the leaching process,in contrast,is affected by the change in concentration.Although this is not apparent when the HNO3concentration is increased from 0.1 to 0.3 mol·L-1,at a HNO3concentration of 1 mol·L-1the reaction rate increases significantly(78%after 20 min).This change could be due to the high H+concentration,which makes Ce4+difficult to hydrolyze and leads to enhanced oxidation ability[20].In addition,when the acidity of the solution increases,the Ce4+self-assembled binuclear complex[Ce4+—O—Ce4+]6+undergoes the following reduction[21]:

The increase in the concentration of hydrogen ions causes acceleration of the forward reaction due to equilibrium reasons.
3.5.Effect of the stirring speed
The in fluence of different stirring speeds(0 r·min-1,30 r·min-1,and 60 r·min-1)on the leaching rate was investigated(Fig.6).All reactions were performed at 70 °C and a lixiviant solution of 1.3 mol·L-1CAN and 0.1 mol·L-1HNO3was utilized.

Fig.6.Effect of stirring speed on the leaching rate of silver.
The experimental results showed that the leaching rate increases with increasing stirring speed.When the stirring speed was 0 r·min-1,the leaching rate was only 76.0%.Increasing the stirring speed to 30 r·min-1led to a significant increase in the leaching rate 96.3%.Similar but smaller increase in leaching rate(98.7%)was obtained when the reaction was stirred at 60 r·min-1.The fact that the increase in stirring speed improves the leaching rate,suggests that the leaching reaction is diffusion-controlled.This assumption was verified by dynamic calculations and is presented later.
According to literature procedures in which HNO3was employed as a leaching reagent,a reaction of the powdered used catalyst at 85°C,stirred at 200 r·min-1generates 95%leaching rate[1].Compared to these results,the method presented in this study gives significantly higher leaching rate despite the fact thatthe catalyst was not pulverized.
3.6.Orthogonal experiments
The orthogonal studies were carried out in L9(34),with the use of four factors and three variables,(Table 2).The relative leaching rate was calculated based on the silver content of the residual solid,and the absolute leaching rate was determined according to the standard volume of the leachate(75 ml).The final orthogonal analysis was based on the relative leaching rate.

Table 2 Orthogonal optimization experiments of the leaching reaction
An almostleaching rate(99.9%)was obtained when the reaction was carried out at 70 °C in a leaching solution of 1.3 mol·L-1CAN and 0.1 mol·L-1HNO3under stirring at 60 r·min-1.From the orthogonal analysis it can be inferred that the main factors affecting the leaching of silver by CAN are:temperature N CAN concentration N HNO3concentration N stirring speed.
3.7.Analysis of the main factors affecting the leaching rate
The results from the different reaction studies were summarized in Table 3.The dependence of the reaction on temperature,CAN and nitric acid concentrations,and stirring speed,is also presented graphically in Fig.7.
As can be observed from Fig.7,the leaching rate increased almost linearly with the increasing temperature,CAN concentration,and stirring speed.The larger slopes ofthe rate dependence on the temperature and CANconcentration indicate thattheir in fluence on the leaching rate is significantly greater than that the stirring speed.The slope of the stirring-speed-dependent leaching curve is rather small,indicating that it does not have a strong impact on the leaching rate.
3.8.Optimal conditions experiment
After obtaining the optimal reaction conditions via the orthogonal analysis(Table 2),the reaction was monitored over time in 20 min intervals(Fig.8).The experiment was performed at 70°C,with a lixiviant solution of 1.6 mol·L-1CAN and 0.1 mol·L-1HNO3and under stirring(60 r·min-1).The reaction gave high leaching rate already after 20 min(82.7%)and achieved almost quantitative yields after 60 min(99.8%).
3.9.Kinetic analysis
The most significant step in the leaching process is the solid-liquid reaction of the CAN solution and the solid Ag particles on an α-Al2O3carrier.The process of leaching silver can be analyzed using the shrinking core model under ideal conditions.Assuming that the spent Ag/α-Al2O3catalysts are spherical,the leaching rate of silver could be controlled either by a chemical reaction process or an internal diffusion process.The following kinetic equations can be applied to these ratecontrolling processes[22].
(1)Internal diffusion-controlled process:

(2)Chemical reaction-controlled process:

x:the silver leaching rate(%) k1,k2:apparent rate constants
The plots of 1-3(1-x)2/3+2(1-x)versus t at different temperatures are shown in Fig.9.The experiments were performed under stirring(60 r·min-1)and the CAN and HNO3concentration in the leaching solution were controlled to 1.3 and 0.1 mol·L-1nitric,respectively.Table 4 presents the linear correlation coefficients for the two control models atdifferenttemperatures(25 °C,40 °C,55 °C and 70 °C).The linear correlation coefficient observed for the internal diffusion-controlled process was larger than 0.99,which indicates a well linear relationship with time.Thus,the leaching is an internal diffusion-controlled process,rather than a chemically controlled process.
Substituting the diffusion model equation into the Arrhenius equation gave the following[22]:

Ea:apparent activation energy(kJ·mol-1) A:Arrhenius constant(s-1)R:molargas constant(8.314 J·mol-1·K-1) T:reaction temperature(K)

Table 3 Summary of the results from the different reaction studies

Fig.7.Graphical representation of the reaction dependence on temperature,CAN and nitric acid concentrations,and stirring speed.
In order to calculate the apparent activation energy of the leaching reaction,the E/R value was obtained by plotting ln k versus 1/T and taking the slope of the formed line(Fig.10).The activation energy and the Arrhenius constant were then calculated to be 38.83 kJ·mol-1and 9701 s-1,respectively.
To sum up,the leaching process of spent Ag/α-Al2O3catalysts is a diffusion-controlled reaction.Under optimized conditions,60 r·min-1stirring,1.3 mol·L-1CAN and 0.1 mol·L-1nitric acid,the kinetic equation of the reaction can be expressed as:

Fig.8.Optimal experimental parameters leaching test.
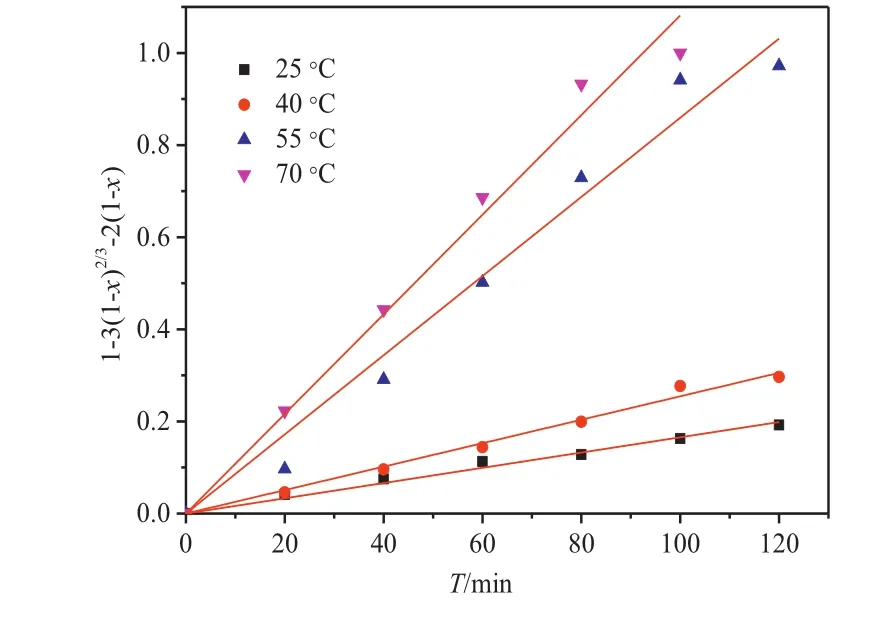
Fig.9.Plots of 1-3(1-x)2/3+2(1-x)vs.t at different temperatures.

Table 4 Linear correlation coefficients(R2)of the two rate-controlling processes

Fig.10.Plot of ln k vs.T-1.

4.Conclusions
In this paper,we present a new and environmentally friendly method of leaching silver from spent Ag/α-Al2O3catalysts.The application of CAN as lixiviant resulted in fast reaction rates and excellent leaching rate(99.8%in 1 h)without any NOxemission.Bene fiting from the results,the novel process has been successfully utilized in a plant.
(1)The leaching rate of silver increased with proportionally to the rises in the reaction temperature,CAN and HNO3concentrations,and stirring speed.The factors that in fluenced the reaction most were determined to be the reaction temperature and CAN concentration.The optimized reaction was performed at 70°C in a lixiviant solution of 1.6 mol·L-1CAN and 1.0 mol·L-1nitric acid under stirring at 60 r·min-1.The reaction gave high leaching rate already after 20 min(82.7%)and achieved almost quantitative yields(99.8%)just in 1 h.The final leaching rate was 99.9%in 2 h.
(2)Ashrinking core modelwasapplied to describe the leaching rate.The study showed that the leaching reaction can be described by an internal diffusion-controlled model,and the activation energy was calculated to be 38.83 kJ·mol-1.The leaching kinetics of the leaching process can be expressed by the following equation:1-3(1-x)2/3+2(1-x)=9701e138830/RTt
Supplementary Material
Supplementary data to this article can be found online athttps://doi.org/10.1016/j.cjche.2018.04.018.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Online measurement of solids motion in fluidized bed reactor with different distributor for Fischer-Tropsch synthesis☆
- Compressibility induced bubble size variation in bubble column reactors:Simulations by the CFD-PBE
- Meniscus behaviors and capillary pressures in capillary channels having various cross-sectional geometries☆
- Comparison of continuous homogenous azeotropic and pressure-swing distillation for a minimum azeotropic system ethyl acetate/nhexane separation☆
- Pressure relief-dipping-microwave assisted polymerization of melamine-L-aspartic acid resin at activated carbon for purification of L-threonine fermented crude product☆
- Conceptual design of an extractive distillation process for the separation of azeotropic mixture of n-butanol-isobutanol-water☆
