Investigation on and industrial application ofdegrading ofmethanol feed in methanol to propylene process☆
2018-11-15LixiangJiangChufuLiMingXuAihuaXingRuiFengJianjunWu
Lixiang Jiang ,Chufu Li,Ming Xu ,*,Aihua Xing ,Rui Feng ,Jianjun Wu
1 China University of Mining and Technology,Xuzhou 221116,China
2 China Energy Investment Corporation Limited,Beijing 100011,China
3 Shenhua Ningmei Coal Group Coal Chemical Industry Company,Yinchuan 750411,China
4 National Institute of Clean-and-Low-Carbon Energy,Beijing 102209,China
Keywords:MTP Methanol Distillation Simulation and optimization Energy saving
ABSTRACT At present,methanol to propylene(MTP)technology developed by Lurgi Company is adopted for commercial plants and re fined methanolwith the purity≥99.85 wt%is required as the feed of MTP unit in Lurgi's technology.Therefore,high energy cost for re fined methanol production is one of the bottlenecks to improve the economy of MTP technology.Reducing the grade of feed re fined methanol may be an effective method to save energy and reduce operation costs in MTP process.In this work,experiments and process simulation were carried out to investigate the in fluence and feasibility of degrading the methanol feed.Experiments were conducted to investigate the in fluence ofcrude methanolfeed on conversion and selectivity ofMTPreaction as wellas the performance of ZSM-5 catalyst.The experimental results showed that degrading the methanol feed had no obvious in fluence on the conversion and selectivity ofMTP reactions and the catalyst deactivation was caused by the carbon accumulation and metals deposition on the active sites.The process simulation results showed that the influence on the conversion and selectivity as well as the stream load of MTP process was negligible if 98mol%methanol was used as feed.Finally,industrial experiments were conducted by adjusting the operation parameters to degrade of feed methanol of the commercial 500 kt·a-1 MTP unit of Ningmei Group in China.The results of industrial application illustrated that annually 180 kt fuel coal and 150 kt desalted water as well as1770 MW·h-1 electricity would be saved when the water content increased from 0.01%to 0.4%.This work has identified the feasibility to improve MTP technology by degrading the methanol feed.
1.Introduction
With increasing demand of propylene as an important basic chemical,the conversion of methanol to light ole fins process as an alternative route to conventional cracking technology has attracted a huge attention during last decades[1-3].And coal based methanol-topropylene/ole fins(MTP/MTO)developed rapidly in China as coal is a major energy resource.With a high selectivity of propylene,ZSM-5 catalysts have attracted greatinterests and been widely used forpropylene production[4-7].To exploit the performance of ZSM-5 catalysts,a multiple-stage adiabatic fixed-bed reactor technology using was developed by Lurgi Company for MTP process[8].In this technology,a dimethyl ether(DME)reactor one pre-reactor where methanol converts to an equilibrium mixture of methanol,dimethyl ether and water and three parallel six-stage fixed-bed reactors among which two are performing the MTP reactions while the rest is regenerating the catalyst by burning off the coke and then stands by.The mixture from the pre-reactor is fed into each stage at a proper ratio and the undesired ole fins are recycled to the first stage of the reactor after rectification.This technology has been adopted by Ningmei Group with a propylene capacity of 500 kt·a-1unit in China and has successfully been brought on line in 2010[9,10].
At present,re fined methanol with the purity≥99.85 wt%is required as the feed of MTP unit in Lurgi's technology.As the production of re fined methanol is a high energy consuming stage,the cost of methanol feed has important in fluence on the economy of MTP process.Therefore,it is important and desired of attention on investigating how to reduce the cost of feed methanol production to improve the profit of MTP process.There are two feasible ways to reduce the cost of feed methanol:1)keep the feed methanol quality and reduce the energy consumption by process optimization[11];2)degrade the feed methanol quality for MTP and reduce the energy consumption during methanol production.As in feed methanol production stage,it accounts for about 20%cost of the feed methanol to distill crude methanol to re fined methanol.Hence,the second way seems to be potential and the accordingly possible technical routes have been reported[12,13].Butthere are few researches about the in fluence of degrading methanol feed and the reality of the technical routes.The research on the mechanism of methanol to hydrocarbon catalysis shows that a little amountof impurities in the methanol is bene ficial for the initiation of catalysis[14].And it is also reported that the impurity compounds such as ethanolin methanolcould form the active organic centers for the hydrocarbon pool-type mechanism,which is favorable to the MTP reaction[15,16].The experiments of MTP reactions with 94 wt%crude methanol feed illustrated that the impurities have little in fluence on the reaction and more propylene is produced[17].
In this work,we first analyzed the different components between crude methanol and re fined methanol to discuss the feasibility on how to use crude methanol as the feed for MTP reaction.Then experiments were conducted to investigate the in fluence of crude methanol feed on conversion and selectivity of MTP reaction.Based on the experimental results,the accordingly changes of operation conditions and in fluence on key equipment were computed with process simulation to verify the feasibility of degrading methanol feed for industrial application.Finally,we adjusted the operation conditions to apply degraded methanol feed for 500 kt·a-1MTP industrial unit.
2.Comparison of Crude and Re fined Methanol
The reaction mechanism is similar in technologies for methanol production including Lurgi,DAVY,TOPSOE and so on.Taking Lurgi technology for example,H2,CO and CO2are the feed materials to produce methanolwith Cu catalysts.And the main reactions are listed as follows:

The side reactions,producing by products such as hydrocarbons,other alcohols,aldehydes,esters,and acids,are listed as follows:

In Lurgi's MTP process,syngas firstly converts into crude methanol.Then crude methanol is distilled with a three-column rectification process to separate water and other impurities from it and to produce re fined methanol.Re fined methanol is fed into DME reactors and then intermediate mixture enters into MTP reactors to produce propylene and other products.The component contents of the crude and re fined methanol from 500 kt·a-1MTP plant of Ningmei Group in China are listed in Table 1.As shown in Table 1,in re fined methanol,the total molar content of impurities is lower than 0.01%.The main differencebetween crude and re fined methanol is that there is 11.25%water in crude methanol and 0.4%fusel in re fined methanol.

Table 1 Molar content of components in the crude and re fined methanol
Therefore,it could be concluded that most energy consumed in crude methanol distillation is to separate water from crude methanol.But from the view of the full MTP process,this operation has a potential to be optimized.Crude methanol is rectified to re fined methanol as the feed for MTP reaction.It is firstly fed into DME reactors and converted into mixture of methanol,dimethyl ether and water.And the feed materials that enter into MTP reactors include methanol,dimethyl ether,water,and recycle hydrocarbon mixtures.Water is separated in the methanol rectification stage,and it is fed into MTP reactors not only as intermediate but also as dilute to control the reaction temperature.Hence,water itself will not bring disadvantages for MTP reactions.Then we could try to save some energies from crude methanol rectification stage by increasing the content of water.As for the in fluence of minor fusel in crude methanol on the process,it is still in question.Therefore,we conducted experiments to investigate the in fluence of impurities in crude methanol including water and minor fusel on the conversion and selectivity of MTP reaction as well as the performance of ZSM-5 catalyst.
3.Experimental
3.1.Catalyst characterization
X-ray diffraction(XRD)tests were performed on a Bruker D8 ADVANCE X-ray diffractometer(40 kV,40 mA)in the 2θ range of 5o-90o.ICP-AES tests were conducted on a SPECTRO®SPECTO ARCOS spectroscopy.N2physisorption was measured at-193°C using a Micromeritics ASAP2460 instrument.NH3-TPD tests were performed on a Micromeritics AutoChem II 2920 instrument.The samples were preheated at 550 °C for 1 h in Ar flow,and then cooled down to 80 °C and absorbed NH3.After purging in Ar flow,the samples ramped in the temperature-programmed process,and the TCD signal was detected.TG/DTG analysis was performed on NETZSCH STA 449F3 simultaneous thermal analyzer with the heating rate of 10.0 K·min-1up to 900 K.
3.2.Catalytic reaction
The industrial MTP catalysts are used,which provided by Shenhua Ningmei Coal group and produced by Clariant.The catalytic reaction was carried out in 2 parallel DME reactors and 2 parallel fixed bed MTP reactors with diameter of 8 mm.A scheme of the experimental setup is shown in Fig.1.The components of feed methanol are listed in Table 1.The re fined methanol and crude methanol feed flow rates were controlled with metering pump and were fed to the vaporizers respectively.Then the streams entered into the according DME reactors to produce equilibriummixtures ofmethanol,dimethylether and water at T=280°C.Then the equilibrium mixtures entered into the MTP reactor as feed.Water was also introduced to each of MTP reactors with a constant flow rate.In MTP reactors,the feed were converted to propylene and other hydrocarbons at T=480°C and ambientpressure.The product streams from MTP reactors were then cooled and separated.The products were analyzed by Agilent7890 gas chromatographs equipped with a HP-PLOT-Q capillary column and Flame Ionization Detector(FID).

Fig.1.Schematic flowchart of experimental setup.
The catalysts were hydrothermally treated under 530°C for 17 h.And then,2.0 g of hydrothermally treated catalyst was used and the methanol WHSV=1 or 2 h-1.And 2.0 g of quartz sand also was used,whose role was mainly to ensure the thickness of the bed and decrease the in fluence of reaction heat on reaction temperature.The reaction conditions are listed in Table 2.

Table 2 Parameters of experimental
4.Results and Discussion
4.1.Catalytic performance
The performance result comparisons between crude methanol feed and re fined methanol feed are shown in Figs.2-4.As shown in Fig.2,the reaction period of each experiment was divided into two stages and that the earlier stage was carried out under WHSV=1.0 h-1while the later stage was carried out under WHSV=2.0 h-1.For crude methanol feed case,the earlier stage lasted for 187 h and the average conversion was 99.96%while the average molar selectivity of propylene was 40.12%;the later stage lasted for 350 h and the average conversion was 99.44%while the average molarselectivity of propylene was 44.03%.For re fined methanol feed case,the earlier stage lasted for 150 h and the average conversion was 99.98%while the average molar selectivity of propylene was 39.94%;and the later stage lasted for 300 h and the average conversion was 99.49%while the average molar selectivity of propylene was 45.5%.And the molar selectivity of ethylene of crude and re fined methanol feed was also very close.Hence,the results illustrate that crude methanol feed has no disadvantage in performance compared with re fined methanol feed.
The molar selectivities of CH4of crude and re fined methanol along with time were shown in Fig.3.The result illustrated that the molar selectivity of CH4of crude methanol feed was a little lower than that of re fined methanol feed at the earlier time while they were close at the middle time;at later time,it was higher than that of re fined methanol feed case.This difference may be caused by the fusel oil in the crude methanol.
The molar selectivity of C4,C5+C6and C3H8of crude and re fined methanol fed was shown in Fig.4.The result illustrated that the tendency of molar selectivity of C4,C5+C6and C3H8of crude methanol feed along time was similar with that of re fined methanol feed case.At the earlier reaction stage under WHSV=1.0 h-1,the molar selectivity of C4with crude methanol fed fluctuated around 19%and then increased to 21%while that of re fined methanol fed changed slightly and increased to 21%;the molar selectivity of C5+C6with crude methanol fed fluctuated around 9%while that of re fined methanol fed fluctuated around 11%.As the reaction time evolved and WHSV increased to 2.0 h-1,the molar selectivity of C4with crude methanol fed firstly fluctuated around 22%and then decreased after another 200 h while that of re fined methanol fed decreased after another 100 h;the molar selectivity of C5+C6had an opposite tendency and they increased in both case.At all the reaction times,the selectivity of C3H8of crude and re fined methanol fed was slightly decreased.

Fig.2.Performance comparison of crude methanol feed with re fined methanol feed.Note that the dark line and dash line mark the shift of methanol WHSV from 1.0 h-1 to 2.0 h-1 for crude methanol feed and re fined methanol feed,respectively.
4.2.Catalyst characterization
Fig.5 shows the XRD patterns of the MTP catalysts before and after crude methanol feed for 525 h and re fined methanol feed for 450 h.It is found that the diffraction patterns of reacted catalysts were similar to that of fresh catalyst.This suggests that the crystal structure of HZSM-5 was remained and hardly changed for the reacted catalysts.The intensities of XRD peaks of the catalysts after reaction were slightly lower,92.3%of that of the fresh catalyst for the re fined methanol feed case and 96.3%for the crude methanol feed case respectively,possibly due to the deposition on catalyst after reaction.
As shown in Table 3,the ICP results indicate that the metal contents in the catalysts,such as Na,Mg and Fe,were insignificantly increased after reaction,suggesting that much more metals deposited on the catalyst surface.The existed metal impurities are usually introduced by the process steam during reaction.Comparing with the case of fed with re fined methanol,Ca is more significantly concentrated in case of fed with crude methanol.

Fig.3.Comparison of molar selectivity of CH4 between crude and re fined methanol feed.

Fig.4.Comparison of molar selectivity of C4,C5+C6,and C3H8 between crude and re fined methanol feed.

Fig.5.XRD patterns of catalysts before and after reaction.
The NH3-TPD test is used to measure the catalyst acidity and the results are shown in Table 4.Compared to the fresh catalyst,the total acid amounts of the catalysts after reaction decreased clearly.This might be attributed to the poisoning of acidic sites of zeolite catalysts by metal impurities as shown in Table 3.Meanwhile,the coverage of coking species on the acid sites of catalysts is probably a reason of the decrease in catalyst acidities.In addition,the relative percentages of strong acid and weak acid of catalysts are both similar with cases of fed with crude and re fined methanol,indicating that the change of acidic properties is not in fluenced by methanol properties.
The N2physisorption results of the fresh and reacted catalysts are demonstrated in Table 5.Compared to the fresh catalyst,the surface areas,micro pore areas and pore volumes of the catalysts after reaction were significantly decreased.This indicates that,the internal pores of catalysts are blocked by carbon deposited on catalyst surfaces.Besides the carbon deposition,the decrease in the micropore areas and volumesis also possibly caused metal elements deposition.During reaction,metal elements could enter into micropores and replace H proton of ZSM-5,resulting in the decrease in the catalyst acidity and coverage of accumulated metal elements,as seen in Table 4.The results shown in Table 5 also demonstrated that the BET differences in the case of fed with crude and re fined methanol are actually very small.

Table 3 Result of contents of elements with ICP test
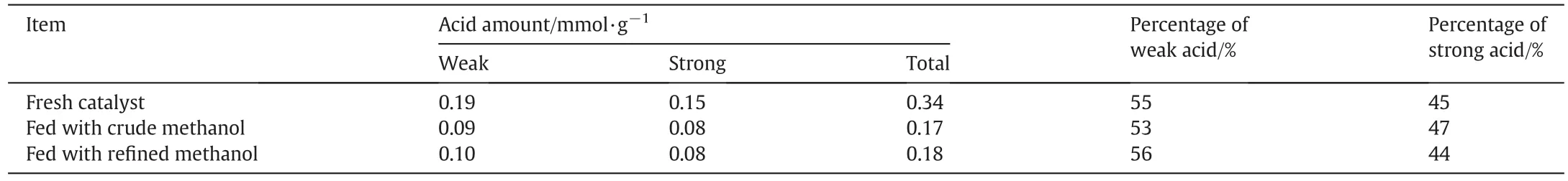
Table 4 Acidity results with NH3-TPD test of catalyst before and after reaction

Table 5 N2 physisorption results of the fresh and reacted catalysts

Fig.6.TG results of reacted catalysts:(a)fed with crude methanol;(b)fed with re fined methanol.

Fig.7.DTG results of reacted catalysts.
The TG results of reacted catalysts shown in Fig.6 illustrate that there exist two distinct weight loss areas.The weight loss areas under 400 K may be caused by the water and hydrocarbons in the catalysts as the losses are 1.36%and 1.45%respectively for the cases of fed with crude methanol and re fined methanol.The weight loss areas from about 600 K present the content of carbon,and there is 7.3 wt%carbon accumulated on the reacted catalyst with crude methanol feeding while it is 6.54 wt%in the case of re fined methanol feeding.As shown in Fig.7,the DTG results of reacted catalysts indicate that there exists one distinct weight loss peak around 600 K for both cases,which means that the carbon accumulated on the reacted catalysts is similar.

Fig.8.Flowsheet diagram for fixed bed MTP process.

Table 6 Composition of the outlet gas(S5)from the DME reactor(mol%)
With the results of catalytic performance experimental,it could be concluded that the little amount of impurities in the crude methanol have little in fluence on the product distribution and catalyst deactivation.The results of catalyst characterization illustrate that feeding with crude methanol would not result in changes of catalyst structures and the deactivation pattern is similar with that of feeding with re fined methanol.The results could also be explained with other researchers'work on methanoland otheralcoholsto hydrocarbon reaction,Johansson et al.[18]proposed that the reaction of ethanol to hydrocarbon proceeds with a “hydrocarbon pool”mechanism similar to that of methanol to hydrocarbon reaction.According to the “hydrocarbon pool”mechanism proposed by Kolboe et al.[19],the alkylated ole fins and carbenium ions in the zeolite pores can further react with the feedstock,(i.e.,methanol or ethanol)and other reaction intermediates to produce benzenes and C2-C4ole fins.Therefore,alcohols in crude methanol could be seemed as intermediates during the MTP reaction and they would have little in fluence on the final production as their contents are small.Also,other researchers compared conversions of methanol,ethanol,and i-propanol under methanol-to-gasoline(MTH)-like conditions over zeolite H-ZSM-5[20].The results showed that the catalyst lifetimes and conversion capacities were comparable for methanol and ethanol,while the catalyst lifetime was increased dramatically when i-propanol was used as the reactant.Thus,it could also identify that the alcohol impurities in crude methanol have no obvious negative in fluences on the catalyst lifetime.But from the experimental results,it should be noted that more Ca will deposit on the catalyst and the removal of Ca from the feeding should be paid attention.
5.Model and Simulation of MTP Process
5.1.Process description
MTP process is mainly divided into two steps:1.converting methanol into DME(Dimethyl Ether);2.converting the equilibrium mixture and processing methanol into propylene and other products[21].Fig.8 shows a flowsheet diagram of the fixed bed MTP process.Methanol is preheated by the DME reactor tail gas in a heat exchanger,and then heated for vaporization.After the temperature reaches about 270°C,the methanol is fed to the DME reactor,where most of the raw material is converted into DME.Part of the DME reactor tail gas is successively cooled by the recycle hydrocarbon gas,cold methanol and circulating cooling water,and its temperature decreases to around 150°C.Subsequently,the gas-liquid mixture is separated in a gas-liquid separator.The gas is heated again while the liquids cooled before they are fed to a fixed bed MTP reactor.The rest tail gas of DME reactor mixed with recycle hydrocarbon gas and steam is heated to about 460°C in the furnace,and also sent to the fixed bed MTP reactor where nearly all methanol and DME are converted into propylene and other hydrocarbon.
5.2.Process modeling
A process model integrated with the DME and MTP reactor model is developed for the fixed bed MTP process.In the model,the SR-POLAR physical property is employed[22,23].The DME reactor is modeled as an equilibrium reactor,while the fixed bed MTP reactor is modeled as a yield reactor model.
Methanol to DME is a rapid reaction close to thermodynamic equilibrium,thus it could be modeled as an equilibrium reactor with a 35°C equilibrium temperature interval.Table 6 demonstrates the simulation results of the DME reactor with re fined methanol.It is discovered that the simulated composition of outlet gas is very close to the design values,with the errors less than 0.2%.
5.3.Simulation and analysis of MTP process with crude methanol
5.3.1.Equilibrium conversion and composition in the DME reactor
Fig.9 reveals that the equilibrium conversion and composition change as the water content in the crude methanol varies in the DME reactor.When water content increases,the equilibrium conversion and content of methanol decrease linearly.For example,when water content increases from 0%to 20mol%,the equilibrium conversion of methanol decreases from 81.1%to 77.8%,and the equilibrium content of DME decreases from 40.6%to 31.1%,as the equilibrium content of methanol decreases from 18.9%to 17.8%.
5.3.2.Simulation results of MTP process with crude methanol
Two cases are investigated to evaluate the in fluence of crude methanol on the fixed bed MTP process.Case 1 uses the crude methanol(98mol%)as the feed methanol,case 2 uses the crude methanol(88mol%)as the feed methanol,and base case uses the re fined methanol(N 99.99mol%)as the feed methanol.
Table 7 presents the simulation results of the MTP reactor in a 500 kt·a-1scale MTP plant with crude methanol under keeping the same propylene output.Compared with the MTP process with re fined methanol,the inlet and outlet molar flowrate variations are less than 1%,illustrating little in fluence on the operation as maintaining the same propylene output.In the fixed bed MTP reactor,the conversion of methanol is about 96%,and the conversion of DME is about 99.4%.

Fig.9.Equilibrium conversion and composition in the DME reactor.(35°C equilibrium temperature interval).

Table 7 Simulation results for the inlet and outlet streams of the fixed-bed MTP reactor
Table 8 displays the simulation outcome about the heat load of the heat exchangers,heaters and coolers in the 500 kt·a-1MTP plant with crude methanol.The heat loads of cooler 1 and cooler 2 will increase significantly as the water content of crude methanol increases.In case 2,the heat loads of cooler 1 and cooler 2 almost are two times of the MTP process with re fined methanol,because additional water in crude methanolfeed has been chilled,which should be concerned more.

Table 8 Simulation results for the heat load for the heat exchangers,heaters and coolers
6.Industrial Application
Based on the experimentaland process simulation results,degrading methanol feed was applied to the 500 kt·a-1commercial MTP equipment of Ningmei Group.Firstly,the operation conditions of the three columns rectification process are adjusted to increase the watercontent in feed methanol.In the case of major adjustment of operation parameters and for the consideration of steady production,the molar water content increased from<0.01%to 0.4%.The analysis results of sampling at different times after adjustment were shown in Table 9.After operation condition adjustment,the water content of methanol from predistillation tower decreases from 9%to 7%,which meant that about 20 t·h-1water was saved.And the reflux ratio of the pressure tower decreased from2.2 to 1.5 while thatofthe atmospheric towerdecreased from 1.5 to 0.7.As shown in Fig.10,the production of methanol was very steady and steamsaved was 2606 t·d-1.The steam consumption decreased from 1.15 t·(t methanol)-1to 0.68 t·(t methanol)-1,which meant that 39.1%of energy consumption was saved in methanol rectification process.

Table 9 Water content in methanol after adjustment

Fig.10.The production of methanol after operation conditions adjustment.
The selectivity of main product from MTP reactors with re fined methanol feed and degraded methanol feed was compared and shown in Fig.11.The result illustrated that the production is steady and no significant change of main product selectivity.After methanol feed degrading,the average selectivity of propylene slightly increased from 29.96%to 30.59%,while that of ethylene slightly increased from 12.41%to 12.61%.The average selectivity of C4decreased a little from 26.50%to 25.74%,while that of C5increased from 15.05%to 16.34%.
The operation results after methanol feed degrading shows that the operation and production are steady.The resources saved are listed as follows:(1)annually 180 kt fuel coal would be saved as 2606 t·d-1steam consumption was reduced;(2)annually 150 kt desalted water would be saved as the water content in methanol from pre-distillation tower decreased;(3)annually 1770 MW·h-1electricity would be saved as 6 coolers for atmospheric tower were shutdown.
7.Conclusions
In this work,the feasibility of degrading methanol feed in MTP processhasbeen investigated by analysis,experimental,process simulation and industrial application.It is found that the conversion and selectivity ofcrude methanolfeed are similarwith thatofre fined methanolfeed by lab experiments and the catalyst is deactivated by carbon accumulation and metal element deposition on the active sites.The process simulation results show that the in fluence on the conversion and selectivity as well as the stream load of MTP process is negligible if 98%mol methanol is used as feed.The results of industrial application illustrated that annually 180 kt fuel coal and 150 kt desalted water as well as1770 MW·h electricity would be saved when the water content increased from 0.01%to 0.4%.This work has shown the effective saving of energy and resource and identified the feasibility to improve the economy of MTP technology by degrading the methanol feed.

Fig.11.comparison of selectivity between re fined methanol feed and degraded methanol feed.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Online measurement of solids motion in fluidized bed reactor with different distributor for Fischer-Tropsch synthesis☆
- Compressibility induced bubble size variation in bubble column reactors:Simulations by the CFD-PBE
- Meniscus behaviors and capillary pressures in capillary channels having various cross-sectional geometries☆
- Comparison of continuous homogenous azeotropic and pressure-swing distillation for a minimum azeotropic system ethyl acetate/nhexane separation☆
- Pressure relief-dipping-microwave assisted polymerization of melamine-L-aspartic acid resin at activated carbon for purification of L-threonine fermented crude product☆
- Conceptual design of an extractive distillation process for the separation of azeotropic mixture of n-butanol-isobutanol-water☆
