Optimization of conditions for preparation of ZSM-5@silicalite-1 core-shell catalysts via hydrothermal synthesis☆
2018-11-15ChuangLiuYihuaLongZhengbaoWang
Chuang Liu,Yihua Long,Zhengbao Wang*
Zhejiang Provincial Key Laboratory of Advanced Chemical Engineering Manufacture Technology,College of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310027,China
Keywords:ZSM-5 Silicalite-1 Core-shell catalyst Alkylation Para-selectivity
ABSTRACT Although the preparation of ZSM-5@silicalite-1(ZS)core-shellcatalysts has been reported in the literature,their selectivity to para-xylene(PX)in the toluene alkylation with methanolis difficultto control.Here we presentthe effects of water and ZSM-5 adding amounts in the synthesis solution,the hydrothermal synthesis time,and the Si/Al ratio of core ZSM-5 on the catalytic performance of ZS core-shell catalysts.The ZS core-shell catalysts were characterized by X-ray diffraction(XRD),N2 adsorption,and NH3 temperature-programmed desorption(NH3-TPD)techniques.The highest PX selectivity of 95.5%was obtained for the ZS(Si/Al=140)catalyst prepared in the synthesis solution with a molar ratio of 0.2TPAOH:1TEOS:250H2O at 175 °C and 10 r·min-1 for only 2 h and the corresponding toluene conversion is as high as 22.8%for the alkylation of toluene with methanol.
1.Introduction
Para-xylene(PX)is a very important hydrocarbon compound and basic chemical material,e.g.,it is the raw material for the production of polyethylene terephthalate(PET).PET is widely used in production of fibers,packaging materials and PET bottles.The demand for PX is growing annually[1].In traditional industry,xylene isomers and other aromatics(e.g.,benzene and toluene)are mainly produced from the catalytic reforming of naphtha and pure PX is separated from its isomer mixture.However,the separation of PX from its isomers,especially from meta-xylene(MX)would cost very much because of their similar physicochemical properties[2].PX can also be produced by the transalkylation ofmethylbenzenes,the dealkylation,the xylene isomerization,the disproportionation of toluene and the toluene alkylation with methanol(i.e.,methylation of toluene).Toluene has a lower demand than benzene and xylenes.Therefore,converting surplus toluene into PXis strongly desired by the methylation and disproportionation of toluene[3-10].The toluene alkylation with methanol to PX is capable if the methanolprice is low and the selectivity to PX is high enough.As we know,methanol can be produced from coal and China is a coal-rich country,i.e.,the cost of methanol in China should be reasonable.Therefore,the key point for the process of toluene alkylation to PX is the high selectivity.
The toluene methylation to PX is a typical acid-catalyzed carbenium ion reaction,and the product distribution largely depends on reaction conditions and properties of the catalyst[7-10].ZSM-5 and its analog silicalite-1,sharing the same MFI crystal structure,are well known shape-selective zeolite catalysts[11-17].Although ZSM-5 has micropore dimensions(diameter:~0.55 nm),close to the size of PX,the alkylation of toluene with methanol on unmodified ZSM-5 zeolite catalyst can only produce xylene isomers with the thermodynamic equilibrium composition(23.6%PX,52.4%MX and 24.0%ortho-xylene(OX)).The main reason is that the isomerization of xylenes occurs on the surface acid sites of ZSM-5,turning PX produced in pores into MX and OX[18].To improve the selectivity of ZSM-5 in the toluene methylation,many modification methods have been intensively investigated.The PX selectivity of ZSM-5 catalysts enhanced from 24%to N90%by some well-known methods such as the hydrothermal synthesis of silicalite-1 shell[19-26],the chemical liquid or vapor deposition of TEOS on ZSM-5[27-33],the oxide impregnation such as MgO,P2O5,rare-earth oxide(e.g.La2O3),B2O3,CaO[34-45]as well as pre-coking[46]and treating with steam[47].Among the above methods,the hydrothermal synthesis of silicalite-1 shell on the surface of ZSM-5 is the most attractive method.Silicalite-1 crystals cannot only passivate the surface acid sites of ZSM-5,but also have the same structure and micropore dimensions with crystals of ZSM-5.Therefore,the silicalite-1 shell on the ZSM-5 surface is also expected to extend the diffusion path length of xylenes without narrowing the pore opening size.
The toluene alkylation on double structure ZSM-5 catalysts covered with a silicalite-1 shell was first reported by Lee et al.in 1993[48].However,the improvement of the selectivity to PX was very limited.The selective formation of PX was reported in 2001 by Nishiyama et al.in the disproportionation oftoluene using a particle catalystsurrounded with a permselective membrane(denoted as silica-alumina@silicalite)[49].The permselective membrane was composed of a silicalite-1 polycrystalline shell.The silicalite-1 coatings on catalyst particles enhanced the PX selectivity due to selective removalof PX through the membrane of silicalite-1.Although the PX selectivity of silica-alumina@silicalite was high,the toluene conversion over the catalyst with the coating significantly decreased from 1.5%to 0.08%because the membrane thickness of silicalite-1 was high(~40 μm).In addition,the catalyst activity of silica-alumina was low.To solve these problems,Nishiyama et al.developed a novel composite catalyst(silicalite/H-ZSM-5)in 2005(i.e.,H-ZSM-5@silicalite-1).A thin silicalite-1 layer was grown on the ZSM-5 zeolite crystal of a few micrometers;a high PX selectivity of 98.9%in xylene isomers was obtained on the H-ZSM-5@silicalite-1 catalysts[19].The PX selectivity was further increased to 99.9%by coating polycrystalline silicalite-1 layers after a repeated synthesis[20-22].Okamoto et al.reported that a high PX selectivity of 96%was obtained overthe ZSM-5@silicalite-1 catalystprepared in the presence of fluoride ion in the synthesis solution for both core and shell[23].Yin et al.reported that the HZSM-5@silicalite-1 catalyst exhibited para-selectivity of N 76%in the alkylation of toluene with methyl bromide using small ZSM-5 crystals as core crystals(0.85 μm)and the PX selectivity was increased to 91%using large ZSM-5 crystals(12 μm)as core crystals.The core-shell composite was not a single crystal[24].Very recently,Wang et al.reported that the HZSM-5@silicalite-1 catalyst showed an increased PX selectivity of N 88%in the alkylation of toluene with methanol[26].
As described above,different PX selectivity levels were reported over ZSM-5@silicalite-1 catalysts prepared by different groups.To obtain the highest PXselectivity for the alkylation oftoluene with methanol,a further investigation for the preparation of ZSM-5@silicalite-1 core-shell catalysts is highly desired.
Rimer etal.successfully synthesized ZSM-5@silicalite-1 core-shellsingle crystals with an epitaxial shell of thickness tunable from 5 to 20 nm in the solution with a relatively high water content(H2O/SiO2=500)at100°C for 24 h[50].Ourgroup found thathighly b-oriented continuous MFI films from b-oriented MFI seed monolayers could be obtained in the synthesis solution of 0.2TPAOH:1TEOS:1500H2O at 150°C for 4 h[51].It was deduced that twin growth can be decreased due to the low nucleation rate under a low supersaturation degree.Inspired by these two results,the effects ofwatercontentin the synthesis solution,the synthesis time,the core ZSM-5 crystal amount and the Si/Al ratio on the catalytic performance of ZSM-5@silicalite-1 core-shell catalysts in the alkylation of toluene with methanol were investigated in this study.The physicochemical properties of the catalysts were characterized by XRD,the nitrogen adsorption,NH3-TPD and FTIR techniques.
2.Experimental
2.1.Reagents
ZSM-5(Zeolyst international company,CBV8024,Si/Al=40 and CBV28024,Si/Al=140),tetraethylorthosilicate(TEOS,98 wt%,Acros Organics),aluminum isopropoxide(Sinopharm Chemical Reagent Co.,Ltd.),urea(CO(NH2)2,Sinopharm),tetrapropylammonium hydroxide(TPAOH,25 wt%,SACHEM.INC.),tetrapropylammonium bromide(TPABr,99 wt%,OURCHEM),and triisopropylbenzene(TiPB,95 wt%,ShanghaiMacklin BiochemicalCo.,Ltd.)were employed withoutfurther purification.The deionized water was made in the laboratory.Toluene,methanol,para-xylene(PX),ortho-xylene(OX),meta-xylene(MX)and 1,2,4-Trimethyl benzene were purchased from Sinopharm.
2.2.Synthesis of ZSM-5(Si/Al=80)
ZSM-5 crystals with a Si/Al ratio of 80 were synthesized by a slight modification procedure reported in the literature[52].For a typical synthesis,26.16 g TPAOH was mixed with 3.16 g urea and 43.32 g water under stirring.After a 1 h stirring,21.36 g TEOS and 0.19 g aluminum isopropoxide were added into the solution under stirring,respectively.The mixture was further aged at room temperature for 24 h,and the resultant gel with a molar ratio of 0.32TPAOH:1SiO2:35H2O:0.5urea:0.0045Al2O3,was transferred into a Te flon-lined autoclave for crystallization at 175°C for 48 h.The as-synthesized product was collected by centrifuging and washing repeatedly with DI water until the pH value was about 7.Then it was dried at 100 °C for 12 h and calcined at 500 °C for 12 h to remove the organic template.Finally,ZSM-5 crystal powder with a Si/Al ratio of~80 with a yield of~88%was obtained.
2.3.Hydrothermal synthesis of ZSM-5@silicalite-1
A typical synthesis of ZSM-5@silicalite-1(ZS)core-shell crystals(e.g.,ZS-W250-24h)was carried out as follows.3.52 g of TPAOH was mixed with 94.80 g H2O,and then 4.60 g of TEOS was added in the mixture.The obtained solution with a molar composition of 0.2TPAOH:1TEOS:250H2O was aged at atmospheric temperature for 4 h,and then 0.20 g of ZSM-5 was added into the solution.The solution was transferred into a stainless steel autoclave with a Te flon-liner and the crystallization was carried out at 175 °C and 10 r·min-1for 24 h.Then the autoclave was immediately cooled using tap water and the crystalline product was filtered and washed with DI water repeatedly to neutral.Then the powder was dried overnight at 60°C for 10 h and calcined at 500°C for 12 h to remove the organic template.
2.4.Characterization
X-ray diffraction(XRD)patterns of powder samples were obtained using a Rigaku Ultimate IV X-ray diffractometer(40 kV,40 mA)with CuKα(λ=0.15406 nm)radiation.Scanning electron microscopy(SEM)experiments were carried out on a Hitachi SU-1510 electron microscope.The thermogravimetric analysis(TGA,Pyris1,PerkinElmer,USA)experiments were carried out in N2at a heating rate of 15 °C·min-1from 50 °C to 700 °C.TiPB cracking reactions were employed at 400 °C and under atmospheric pressure,and the WHSVTiPBwas 1.7 h-1.The nitrogen adsorption isotherms at the liquid N2temperature were measured using Micromeritics ASAP 2020M.
The acid strength and amount in the catalystwere measured by NH3temperature-programmed desorption(TPD,PX200,Tianjin Golden Eagle Technology Co.LTD.,China).0.3 g of the sample was first pretreated by heating at 550 °C for 1 h,and then cooled to 100 °C.The NH3(10 ml·min-1)gas was pulled in for 30 min.The physically adsorbed NH3was removed by flushing with helium gas at 100°C for 1 h,and then,the chemically adsorbed NH3on the catalyst was desorbed by heating to 700 °C with a heating rate of 10 °C·min-1.The desorbed NH3gas was monitored by a thermal conductivity detector.The amount of acid sites was then quantitatively determined by titration using an ammonia pulse method.
2.5.Alkylation of toluene with methanol
The alkylation of toluene with methanol reaction was carried out at atmospheric pressure using a fixed-bed tubular quartz reactor with an inner diameter of 8 mm and a length of 50 cm.0.30 g of the catalyst(20-40 mesh)was loaded into the central zone of the reactor between two quartz wool layers.The zeolite particles were pressed into a pellet withoutany binder and then the pellet was crushed into small particles.The final catalyst particles were obtained between two mesh sieves(20 and 40 mesh).The toluene and methanol(molarratio 1:1)mixture was pumped into a preheater(100°C)and fed into the reactor with N2(10 ml·min-1).The reactor was preset400 °C,and the weighted hourly space velocity of toluene(WHSVToluene)was 1.0 h-1.The products from the reactor were analyzed online using a GC-2010 gas chromatograph(GC)equipped with a flame ionization detector(FID)and an AT-FFAP column(30 m and 0.50 μm).The selectivity and conversion at the reaction time of 3 h were presented.
3.Results and Discussion
3.1.Effects of water amount in the synthesis solution
The water content in the synthesis solution is a key factor on the nucleation and crystallization rate in the zeolite synthesis.Textural properties of ZSM-5(Si/Al ratio of 140,from Zeolyst Co.)and ZSM-5@silicalite-1(ZS)core-shell catalysts synthesized in the synthesis solution with different water contents are listed in Table 1.As we can see,the amount of H2O(x)ranged from 125 to 1000 in the synthesis solution with a molar ratio of 0.2TPAOH:1TEOS:xH2O.The surface area(SBET)for all ZS samples remained ~360 m2·g-1,slightly lower than that of parent ZSM-5(~393 m2·g-1),while the micropore surface areas(~320 m2·g-1)were slightly higher than that of parent ZSM-5(~306 m2·g-1).The pore volumes of ZS samples(~0.19 cm3·g-1)were slightly lower than that of parent ZSM-5(0.21 cm3·g-1),while the micropore volumes(~0.14 cm3·g-1)were slightly higher than that of parent ZSM-5(0.10 cm3·g-1).This indicates that the crystallinity of ZS samples was slightly higher than that of parent ZSM-5 and the silicalite-1 shells on ZSM-5 crystals did not blocked the pore mouths[18].

Table 1 Textural properties of ZSM-5 and ZSM-5@silicalite-1(ZS)synthesized in the synthesis solution with different water contents
As shown in Table 2,the PX selectivity of ZS core-shell catalysts(78.3%-91.6%)was much higher than that of parent ZSM-5(37.3%)in the alkylation of toluene with methanol,indicating the passivation of acid sites on the external surface of parent ZSM-5 by the silicalite-1 shell.The ZS-W250-24h catalyst(H2O/SiO2=250,synthesis time=24 h)had the highest PX selectivity(91.6%)among all ZS core-shell catalysts synthesized with different water amounts in the synthesis solutions.For the ZS-W125-24h sample,both the PX selectivity and toluene conversion of the catalyst were less than those of ZS-W250-24h.This is probably due to the faster crystallization rate of the synthesis solution with H2O/SiO2=125 than thatwith H2O/SiO2=250,resulting in more free-existing silicalite-1 crystals or a thicker silicalite-1 shell instead of uniformly covering the ZSM-5 surface.A non-uniform thicker silicalite-1 shell mightresult in a lower PX selectivity and lower toluene conversion.When the amount of H2O(x)in the solution was increased from 250(ZS-W250-24h)to 2000(ZS-W2000-24h),the PX selectivityobviously decreased from 91.6%to 78.3%,while the toluene conversion showed no obvious change.These results indicate that the H2O amount in the solution higher than 250 might lead to a lower crystal growth.As a result,the ZSM-5 external surface could not be covered by silicalite-1 shell completely[50].Therefore,the optimal water amount in the synthesis solution is H2O/SiO2=250 and this was used for the following experiments.

Table 2 Catalytic performances of ZSM-5 and ZSM-5@silicalite-1(ZS)synthesized in the synthesis solution with different water contents
3.2.Effect of the ZSM-5 adding amount in the solution
The effects of adding amount of parent ZSM-5 crystals on the catalytic performance of ZS core-shell catalysts in the alkylation of toluene with methanol were investigated.Different amounts of ZSM-5 crystals were added in the synthesis solution with a molar ratio of 0.2TPAOH:1TEOS:250H2O and the synthesis was carried out at 175°C and 10 r·min-1for 24 h.Table 3 shows thatthe PX selectivity obviously decreased as the adding amount of ZSM-5 crystals in the synthesis solution increased.When the mass ratio of ZSM-5/SiO2was 0.08,the PXselectivity ofthe catalystwas more than 92%,butthe toluene conversion was only 17.1%in this condition.The PX selectivity of the catalyst was still higher than 91%with the toluene conversion of 22.1%when the ZSM-5/SiO2mass ratio was 0.15.The conversion was lower than that of parent ZSM-5(34.9%).When the mass ratio was increased to 2.0,the PX selectivity decreased to only~45%.Therefore,the amount of ZSM-5 added to the synthesis solution has a significant effect on the catalytic performance.We speculate that adding more ZSM-5 into thesolution would decrease the possibility of the silicalite-1 formation on the surface of ZSM-5 crystal,which results in an insufficient shell coating.Therefore,the increase of PX selectivity became slight with the increase of ZSM-5 adding amount.On the other hand,as the adding amount of ZSM-5 was too low,extra silicalite-1 crystals were produced in the final catalyst,which results in the decrease of the catalytic activity.Therefore,the best mass ratio of ZSM-5/SiO2in the synthesis solution was 0.15.

Table 3 Catalytic performance ofthe ZSM-5@silicalite-1 catalysts with differentamounts ofZSM-5(Si/Al=140)in the solution①

Fig.1.Catalytic performances of the ZSM-5@silicalite-1 catalysts synthesized with different synthesis times in the synthesis solution 0.2TPAOH:1TEOS:250H2O.The mass ratio ZSM-5(Si/Al=140)/SiO2 is 0.15.
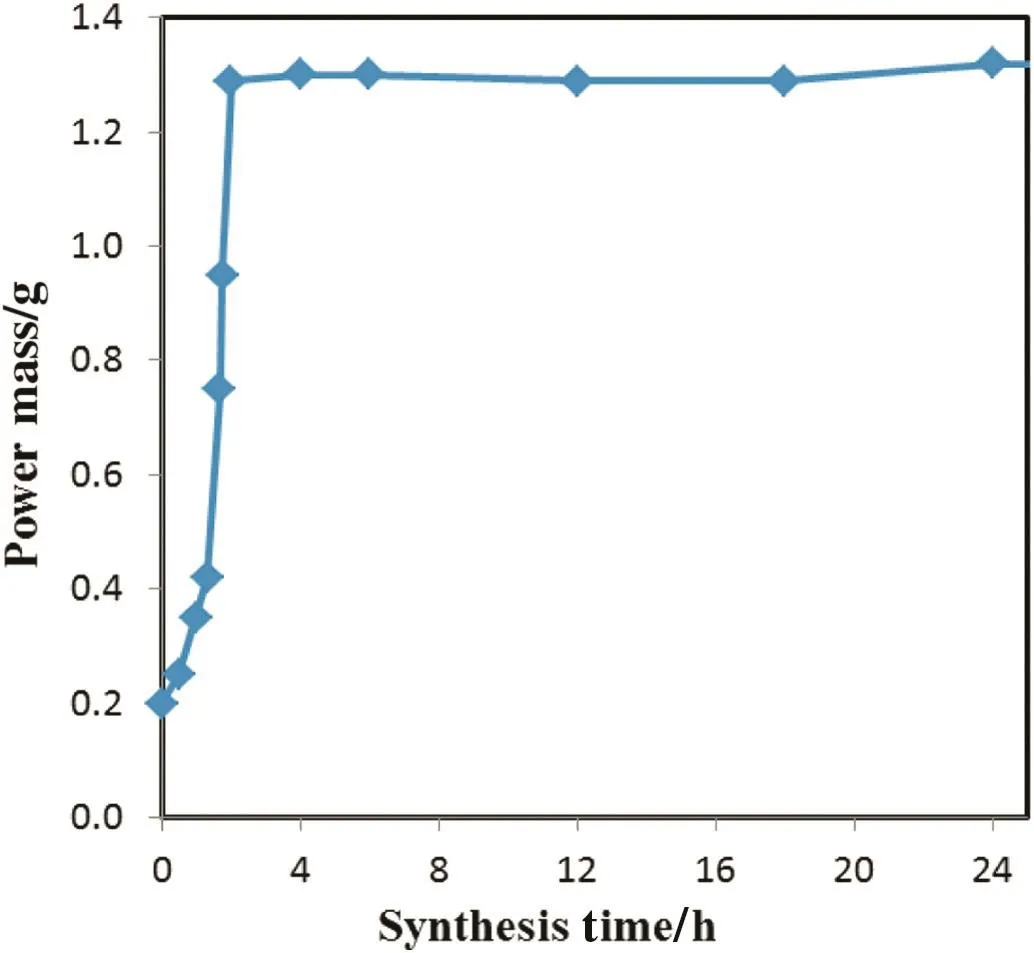
Fig.2.The change of the catalyst weight with the synthesis time.The synthesis solution was 0.2TPAOH:1TEOS:250H2O,the mass ratio of ZSM-5 to SiO2 from TEOS is 0.15,and the crystallization was at 175 °C and 10 r·min-1.
3.3.Effect of the synthesis time
Catalytic performances of the ZS catalysts synthesized with different times(sample ZS-W250-t,t is the synthesis time)in the synthesis solution with a molar ratio of 0.2TPAOH:1TEOS:250H2O are shown in Fig.1.ForparentZSM-5,the PXselectivity was only 37.3%,and the selectivity was higher than 60%when the silicalite-1 layer was synthesized for 1 h(sample ZS-W250-1h).The most excellent PX selectivity of the catalysts synthesized for only 1.5-2 h was~95.5%,and the PXselectivity slightly dropped to~91%and the toluene conversion kept~22.5%with the increase of the synthesis time(e.g.,sample ZS-W250-24h).As shown in Fig.2,the catalyst weight increased quickly when the synthesis time increased up to 2 h,while the catalyst weight was almost the same after the synthesis of longer than 2 h.This indicates that the crystallization was almostcompleted after the 2 h synthesis.The reason for the decrease of the PX selectivity of the catalyst synthesized for N 2 h is not very clear.We speculate that some silicalite-1 crystals covered on the ZSM-5 surface might be dissolved and crystallized on the surface of other silicalite-1 crystals due to the existence of dynamic balance between dissolution and crystallization.That is,some ZSM-5 crystals cannot be fully covered by silicalite-1 crystals,resulting in the decrease of PX selectivity.Therefore,we conclude that the synthesis time should not be too long,and the catalyst synthesized at 175°C for only 2 h is enough to achieve the excellent catalytic performance.This synthesis time is obviously shorter than other methods reported in the literature[23-25].Morphology of the ZSM-5 and ZS catalysts with different hydrothermal synthesis times are shown in Fig.3.The morphology of sample ZS-W250-1h in Fig.3b was similar to that of the parent ZSM-5 in Fig.3a,while some twin crystals and coffin crystals appeared(Fig.3c&d)when the synthesis time was≥2 h.This indicates that silicalite-1 crystals formed on the surface of ZSM-5 crystals.This is also verified by the powder XRD patterns of catalysts.As shown in Fig.4,the ZS catalysts produced with different synthesis times are pure MFI type zeolite like the parent ZSM-5 sample.The peak intension of ZS samples was similar to that of ZSM-5 sample,illustrating that all the samples were almost completely crystallized when the synthesis time was≥2 h[25].

Fig.3.SEM images of ZSM-5@silicalite-1 catalysts produced after different synthesis times,(a)ZSM-5,(b)1 h,(c)2 h and(d)24 h.

Fig.4.XRD patterns of the powder of(a)ZSM-5 and ZSM-5@silicalite-1 produced after different synthesis times,(b)2 h(ZS-W250-2h),(c)6 h(ZS-W250-6h),(d)12 h(ZSW250-12h)&(e)24 h(ZS-W250-24h).The mass ratio of ZSM-5/SiO2 is 0.15 and the crystallization was at 175 °C and 10 r·min-1.
As shown in Fig.5,TG analysis profiles(TGA)of the coked catalysts after the reaction of 3 h revealed that the weight loss from the combustion of retained coke species significantly decreased from 4.6%on the parent ZSM-5 to only 1.5%on the ZS core-shell sample(ZS-W250-2h).This is attributed to thatthe growth ofsilicalite-1 shellon ZSM-5 passivated the surface acid sites and prevented the aromatic molecules from forming coke species on the external surface of the ZS core-shell catalyst[53].As a result,the ZS catalyst exhibited a much lower coke formation rate than the parent ZSM-5,which improved the catalytic performance and the stability of catalyst.

Fig.5.TGAcurves of(a)ZSM-5 and(b)ZS-W250-2h catalysts afterthe toluene methylation reaction for 3 h.

Fig.6.Time on stream of toluene conversion over parent HZSM-5(triangles)and ZS-W250-2h(squares).Reaction temperature=400°C and WHSVToluene=1.0 h-1.
The stability difference between the parent ZSM-5 and the core-shell ZS-W250-2h was also studied by a long time reaction.As shown in Fig.6,the ZS-W250-2h catalyst kept stable for more than 72 h of the reaction.For the parent ZSM-5,although the initial activity was higher than that of ZS-W250-2h,the deactivation was fast and the activity was lower than that of ZS-W250-2h after 24 h.Meanwhile,the regenerated ZS-W250-2h catalyst showed a stable activity for more than 80 h.Our results indicate that the stability of the ZS sample(e.g.,ZS-W250-2h)is better than the parent ZSM-5 zeolite.

Fig.7.Conversion of TiPB versus the time on stream over(a)ZSM-5 and(b)ZS-W250-2h catalysts.Reaction temperature=400°C and WHSVTiPB=1.7 h-1.
Triisopropylbenzene(TiPB)with three reactive isopropyl groups and a kinetic diameterof0.85 nmis larger than the pores ofMFItype zeolite(0.55 nm).So it can be used to confirm the surface passivation and pore properties of the zeolite catalyst[54].Fig.7 showed a significant reduction in the reactivity of TiPB over the ZS-W250-2h sample compared to parent H-ZSM-5.The TiPB conversion over the parent ZSM-5 catalyst was about 65%.However,the conversion was less than 1.5%over ZS-W250-2h,which indicates that the external surface acid sites of ZSM-5 were almost passivated by the hydrothermal synthesis process[50].
The results of temperature-programmed desorption of ammonia(NH3-TPD)profiles(Fig.8)showed that both the strong and weak acid sites of the ZS-W250-2h catalyst were decreased.The profile of the parent HZSM-5 revealed a clear peak at around 398°C that corresponds to the strong acid sites,but sample ZS-W250-2h showed a peak at around 383 °C which is 12 °C lower than the former.The strong acid sites(measured by area)for sample ZS-W250-2h were only 70%of parent ZSM-5[24].

Fig.8.NH3-TPD profiles of(a)ZSM-5 and(b)ZS-W250-2h catalysts.
From the above results of XRD,TiPB conversion and NH3-TPD profiles,we can conclude that the external acid sites of core ZSM-5 were passivated by silicalite-1 crystals and the acid sites in pores remained static.Therefore,the isomerization of PX to xylene isomers(MX and OX)can be largely prevented on the external surface of the catalyst,resulting in the high PX selectivity.

Table 4 Catalytic performances of the ZSM-5@silicalite-1 catalysts from ZSM-5 with different Si/Al ratios
3.4.Effect of the Si/Al ratio of ZSM-5
The effects ofthe Si/Alratio ofthe parentZSM-5 were also investigated in the synthesis solution with a molar ratio of 0.2TPAOH:1TEOS:250H2O.As shown in Table 4,the PX selectivity ofparent ZSM-5 increases with the increase of the Si/Al ratio and the PX selectivity of ZSM-5(Si/Al=140)is much higher than those of other two ZSM-5 catalysts with lower Si/Al ratios.The PX selectivity of ZS was only increased from 23.7%to 39.6%after a 2 h synthesis for the parent ZSM-5(Si/Al=40,from Zeolyst Co.),while the selectivity of ZS was more than 95%for the parent of ZSM-5(Si/Al=140)in the same synthesis condition.For the parent ZSM-5(Si/Al=80)which was synthesized in our laboratory,the PX selectivity of ZS was 67.7%which was between 39.6%and 95%.When the synthesis time was extended to 24 h,the PX selectivity of ZS also increased with the Si/Alratio ofparent ZSM-5.However,the PXselectivity of ZS catalysts(the Si/Al ratio of parent ZSM-5 lower than 80)slightly increased compared to the 2 h synthesis,while the PX selectivity of ZS catalyst(Si/Al=140)slightly decreased.The above results indicate that the growth of silicalite-1 crystals on the surface of ZSM-5 zeolites with lower Si/Al ratios is more difficult,resulting in the incomplete passivation of acid sites on the external surface of core ZSM-5.Another reason might be the re-incorporation of aluminum leached from core ZSM-5 with low Si/Alratios(e.g.,≤80)into the newly formed shelllayer.
Our result about the effect of Si/Al ratio of core ZSM-5 is similar to that reported by Wang et al.[26].However,the PX selectivity(SPX)of ZS catalysts(e.g.SPX=53%for Si/Al=170;SPX=86%for Si/Al=250)in the literature,which was synthesized in a synthesis solution with a molar ratio of 17TEOS:14TPAOH:9500H2O at 170°C for 48 h,was much lower than that(SPX=95.5%for Si/Al=140)in this study.Zhang et al.reported that the PX selectivity of catalyst increased with the Si/Al ratio of ZSM-5(i.e.,SPXN 95%for Si/Al=40 and SPX=89.6%for Si/Al=12.5)for Methanol-to-Aromatics(MTA)reaction[55],their result is in accordance with ours.Therefore,the Si/Al ratio of ZSM-5 has an important in fluence on the catalytic performance,i.e.,a higher Si/Al ratio of ZSM-5 results in a higher PX selectivity.
However,Nishiyama et al.reported that the high PX selectivity of N 99%was obtained for all ZS catalysts with different Si/Al ratios(from 30 to 70)of core H-ZSM-5[22],which indicates that the Si/Al ratio in the range of 30-70 has no significant effect on the PX selectivity.The exact reason is not clear.With decreasing the contact time,the ZS catalyst showed gradual increase in the PX selectivity[26].The contact time used in the literature[22]might be very short,resulting in the high PX selectivity regardless of the difference of Si/Al ratio(30-70)of core ZSM-5.The crystal size of core ZSM-5 might be an important factor because very large ZSM-5 crystals(e.g.,7-10 μm)were used in the literature,which is much larger than those used in this study.We will investigate the effect of crystal size in the near future.
4.Conclusions
Herein,we have systematically investigated the effects of the Si/Al ratio of the core ZSM-5 and the hydrothermal synthesis conditions of ZSM-5@silicalite-1(ZS)core-shell catalysts,such as the water amount,the ZSM-5 adding amount in the synthesis solution and the hydrothermal synthesis time.The highest PX selectivity of 95.5%was obtained for the ZS catalyst prepared under the following conditions:the mass ratio ofZSM-5/SiO2was 0.15,the Si/Alratio ofcore ZSM-5 was 140,the molar ratio composition of synthesis solution was 0.2TPAOH:1TEOS:250H2O,and the synthesis was carried out at 175 °C and 10 r·min-1for only 2 h(i.e.,catalyst ZS-W250-2h);the corresponding toluene conversion was as high as 22.8%for the toluene alkylation with methanol.The high PX selectivity is mainly due to epitaxial growth of a silicalite-1 shell(without strong acid sites)on the ZSM-5 crystal surface without blocking pore openings and with little effect on the acid sites in pores.Our synthesis conditions can be employed to prepare core-shell ZS catalysts using the commercial ZSM-5 product(e.g.,Zeolyst).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Online measurement of solids motion in fluidized bed reactor with different distributor for Fischer-Tropsch synthesis☆
- Compressibility induced bubble size variation in bubble column reactors:Simulations by the CFD-PBE
- Meniscus behaviors and capillary pressures in capillary channels having various cross-sectional geometries☆
- Comparison of continuous homogenous azeotropic and pressure-swing distillation for a minimum azeotropic system ethyl acetate/nhexane separation☆
- Pressure relief-dipping-microwave assisted polymerization of melamine-L-aspartic acid resin at activated carbon for purification of L-threonine fermented crude product☆
- Conceptual design of an extractive distillation process for the separation of azeotropic mixture of n-butanol-isobutanol-water☆
