石墨相氮化碳光催化灭活水中多重耐药菌研究
2018-10-29孙迎雪常学明殷秀峰陆松柳胡洪营
齐 菲,孙迎雪*,常学明,殷秀峰,陆松柳,胡洪营
石墨相氮化碳光催化灭活水中多重耐药菌研究
齐 菲1,孙迎雪1*,常学明1,殷秀峰1,陆松柳2,胡洪营3
(1.北京工商大学环境科学与工程系,北京 100048;2.启迪水务集团有限公司,上海 200072;3.清华大学环境学院,环境模拟与污染控制国家重点联合实验室,国家环境保护环境微生物利用与安全控制重点实验室,北京 100084)
研究了光辐照和基于石墨相氮化碳(g-C3N4)光催化对二级出水中1株四环素和氨苄西林抗性多重耐药菌CGMCC 1.1595的灭活效果.结果表明,汞灯辐照功率(100/300/500W)和辐照强度越高,其灭活效率相对越高,在500W汞灯60min辐照条件下,紫外长波(UVA)-可见光(300~579nm)对该多重耐药菌的灭活率为0.41log;基于g-C3N4的光催化对其灭活率为1.31log.相比未加g-C3N4催化剂的光辐照灭菌,在UVA-可见光条件下g-C3N4对其灭活率的贡献为61%~69%;在可见光条件下g-C3N4对其灭活率的贡献达到60%~79%.在UVA-可见光g-C3N4光催化灭活CGMCC 1.1595反应体系中,活性氧自由基和电子空穴的活跃程度为:∙OH>∙O2->H2O2>h+>1O2,∙OH为该光催化体系的主要活性物质,其次是∙O2-和H2O2.
光照;石墨相氮化碳(g-C3N4);多重耐药菌;消毒;可见光
抗生素的大量生产和广泛使用,导致我国每年约有5.40万t抗生素随污水进入城市污水处理厂[1-4],污水中相对较高抗生素水平导致细菌产生抗生素抗性[5-6],有些抗生素抗性菌(ARB)含有多种抗性基因(即多重耐药细菌),将严重破坏水环境中微生物群落结构和功能,对水生生态系统安全甚至人类健康构成严重的威胁[7-8].
消毒工艺是饮用水处理、污水再生处理或排放水体控制微生物安全的重要环节,对灭活ARB发挥着重要作用[9-10].太阳光消毒由于利用清洁能源,近年来引起了消毒领域的广泛关注[11-12],其机理主要是利用热量和紫外光子的协同作用[13].对可见光响应的石墨相氮化碳(g-C3N4)是强化太阳光利用的新型半导体光催化材料,研究发现基于g-C3N4的光催化氧化可有效去除水中有机污染物以及使细菌和病毒失活[14-16].g-C3N4为二维层状结构,具有独特的电子能带结构,层内C原子和N原子以sp2杂化方式结合在一起,形成类苯环的双层结构,具有大量的π键存在[17-18].g-C3N4的导带为-1.12eV,价带为1.57eV[19],具有2.7eV的禁带宽度,存在于最高占据分子轨道(HOMO)和最低未占分子轨道(LUMO)之间,可以吸收波长约小于475nm的太阳光[20-21]. g-C3N4还具有优良的化学稳定性和热力学稳定性、结构和性能易于调控等优势[22-23],在水处理领域有良好的应用前景.
本研究选取1株对四环素和氨苄西林有多重耐药性的大肠杆菌为研究对象,研究光辐照和基于g-C3N4的光催化对该菌株的灭活效果和耐药性的影响,并探讨其光催化消毒机理.
1 材料与方法
1.1 实验水样
实验用多重耐药性大肠杆菌CGMCC 1.1595 (简称CGMCC 1.1595),购自中国普通微生物菌种保藏管理中心,该菌株携带质粒,且质粒上携带四环素耐药基因和内酰胺类耐药基因bla,可表达四环素和氨苄西林抗性[24].
1.2 光催化反应样品的制备
将该多重耐药菌接种于含有16mg/L四环素和32mg/L氨苄西林[25]的50mL营养肉汤培养基中(g/L:蛋白胨10,牛肉粉3,氯化钠5,pH=7.2),37℃过夜培养16h[26].将所得菌液离心(6000r/min,10min,4℃),倒掉上清液,用无菌磷酸盐缓冲溶液(PBS,pH=7.4)涡旋振荡,充分去除培养基和抗生素,反复洗涤2次,最后将菌液悬浮于150mLPBS中,用麦氏比浊法获得1.5×108CFU/mL(0.5麦氏)的菌液.加入无菌保存的g-C3N4光催化剂,菌液中的催化剂浓度为5g/L,在无菌黑暗条件下超声20min,使催化剂均匀悬浮于菌液中,以在光催化剂表面达到吸附-解吸平衡[27-28],然后分装到已灭菌的反应试管中,每只无菌试管的反应体系为20mL.
1.3 g-C3N4的制备与性能
基于三聚氰胺(C3H6N6)的g-C3N4常用作模拟可见光条件下的光催化剂.以C3H6N6和三聚氰酸(C3H3N3O3)混合物为原料制备g-C3N4[27]:将5g三聚氰胺和5g三聚氰酸(质量比1:1)粉末放入带盖氧化铝坩埚中,使用马弗炉进行加热,以2.3℃/min升温至550℃(过程约4h),保持4h后自然冷却.冷却后放入研钵中充分研磨,得到淡黄色粉末,待用.通过紫外-可见漫反射光谱分析得到该g-C3N4光响应波长范围约为477nm以下,禁带宽度为2.61eV;BET比表面积为42.3m2/g;扫描电镜(SEM)和透射电镜(TEM)表征如图1所示,g-C3N4光催化剂是具有多孔和较为清晰的多层层状结构.

图1 g-C3N4光催化剂的SEM和TEM图
1.4 光辐照反应
使用光化学反应仪(XPA-7,南京胥江机电厂)进行光辐照实验,通过循环水和排风扇进行温度控制,反应温度设为25℃,反应仪中心部位配有汞灯(功率:100/300/500W;波长范围:265.2~579nm).配备300和400nm滤波片用于模拟UVA.利用紫外辐照计(UV-A,北京师范大学机电厂,测定范围:0.1~199.9× 103μW/cm2)和可见光辐照计(FZ-A,北京师范大学机电厂,测定范围:0.1~199.9×103μW/cm2)测定光辐照强度.将准备好的样品放入光化学反应仪外围的固定装置中进行光催化实验,并进行磁力搅拌.反应时间为60min,每隔10min取样一次.
1.5 耐药菌的检测和灭活率的计算
采用平板法测定样品中的耐药菌.首先在培养皿(Ф90mm)中加入25mL的营养琼脂培养基(g/L:蛋白胨10,琼脂15,氯化钠5,牛肉膏粉3,pH=7.2),待凝固.将光辐照反应后的菌液进行梯度稀释,取100μL梯度稀释后的菌液进行平板涂布,每组3个平行样品,然后将其倒置放于37℃培养箱中培养24h,计菌落数.用单位体积水样的菌落形成单位(CFU/mL)表示.
灭活率= log10(0/N) (1)
式中,0为光辐照前水样中耐药菌的菌落数;N为光辐照后水样中耐药菌的菌落数.
1.6 耐药性分析
抗生素耐药性是指细菌在抗生素存在条件下的生存和生长能力[29],根据美国临床和实验室标准协会制定的抗菌药物敏感性试验执行标准,采用药敏纸片扩散法考察该多重耐药菌对四环素和氨苄西林耐药性的变化[25].
上述的营养琼脂平板上挑取单菌落悬浮于生理盐水中,充分震荡,用麦氏比浊法获得1.5× 108CFU/ mL(0.5麦氏)的菌液.吸取100μL菌液加入到制备好的麦康凯琼脂培养基(g/L:酪蛋白水解物17.5,淀粉1.5,牛肉浸粉5,琼脂12.5,pH=7.2)平板表面,并涂布均匀.盖上皿盖,置于室温无菌条件下干燥3~5min,稍干后再用无菌镊子分别将四环素和氨苄西林药敏纸片贴放于琼脂平板表面,每组3个平行样品.(35±2)℃孵育16~18h,用游标卡尺量取四环素和氨苄西林药敏纸片周围的抑菌圈直径,根据抑菌圈直径的大小,判断CGMCC 1.1595对四环素和氨苄西林的耐药、中介和敏感程度.肠杆科细菌对四环素耐受性的判定标准是:抑菌圈直径>15mm为敏感,抑菌圈直径=12~14mm为中介,抑菌圈直径<11mm为耐药;对氨苄西林耐受性的判定标准是:抑菌圈直径>17mm为敏感,抑菌圈直径=14~16mm为中介,抑菌圈直径<13mm为耐药[25].
2 结果与讨论
2.1 光辐照对四环素和氨苄西林抗性大肠杆菌的消毒效果

图2 光辐照对四环素和氨苄西林抗性大肠杆菌的影响
实验采用不同功率(100,300,500W)的汞灯,利用300nm截止滤波片得到UVA-可见光(波长介于300~579nm),测得其中UVA光强分别为3.73,7.70, 15.70mW/cm2,可见光强分别为13.40,20.90, 39.20mW/cm2,UVA-可见光对该多重耐药菌的灭活效果如图2(a,b)所示.可以看出,随着辐照时间的延长,该多重耐药菌的浓度逐渐降低,光辐照60min时,100,300,500W汞灯对该多重耐药菌的灭活率分别为0.23,0.34,0.41log,菌液浓度由1.45×108, 1.68×108,1.68×108CFU/mL降至8.39×107,7.52×107, 6.45×107CFU/mL.且当500W汞灯(UVA光强和可见光强分别为15.70,39.20mW/cm2),辐照60min时对该多重耐药菌的灭活率最高.
采用400nm截止滤波片分析可见光(波长介于400~579nm)辐照对该多重耐药菌灭活率的影响.100,300,500W汞灯辐照得到可见光光强分别为15.20,22.34,36.66mW/cm2,同时测得UVA光强分别为0.51,1.03,1.32mW/cm2,可见光辐照对该多重耐药菌的灭活效果如图2(c,d)所示,光辐照反应60min时,100,300,500W汞灯对该多重耐药菌的灭活率分别为0.07,0.13,0.14log,菌液浓度由1.78×108, 1.78× 108,1.82×108CFU/mL分别降至1.51×108, 1.31×108, 1.33×108CFU/mL.在可见光辐照条件下,功率较高的500W汞灯(可见光强为36.66mW/cm2)光辐照60min,对该多重耐药菌相对较高,达到0.14log.
UVA-可见光辐照在100,300,500W条件下对该多重耐药菌的灭活率是可见光辐照的3.29、2.62和2.93倍.100,300,500W汞灯UVA-可见光辐照下的UVA光强度分别是可见光辐照下的7.31、7.48和11.89倍,可以判断UVA-可见光辐照对该多重耐药菌的灭活率UVA起主要作用,在UVA-可见光辐照60min时,100,300,500W汞灯的UVA对该多重耐药菌的灭活率的贡献分别为70%、79%和66%.相比可见光,UVA能够穿透细菌细胞膜和细胞质,使细菌DNA相邻的2个胸腺嘧啶共价结合形成二聚体,破坏DNA和RNA中的嘧啶与嘌呤碱基[30],从而破坏DNA和RNA的构型,使DNA损伤干扰其正常复制,导致细菌死亡[31].此外,UVA还会对细胞产生生物学效应[32].因此,UVA可直接破坏抗性基因灭活耐药菌.
2.2 基于石墨相g-C3N4的光催化消毒效果
实验采用不同功率(100,300,500W)的汞灯,反应体系中加入g-C3N4光催化剂(剂量为5g/L[27]),光催化对该多重耐药菌的灭活效果如图3(a,b)所示.在0~60min的反应过程中,随着光催化时间的延长,该多重耐药菌的浓度逐渐降低,反应60min时,100,300, 500W汞灯UVA-可见光对该多重耐药菌的灭活率分别为0.75,0.87,1.31log,菌液浓度由1.98×108, 1.88×108,1.90×108CFU/mL降至3.51×107, 2.44×107, 9.33×106CFU/mL.且UVA-可见光光催化反应60min时,较高功率的500W汞灯对该多重耐药菌的灭活率达到最高.
采用400nm截止滤波片分析可见光光催化对多重耐药菌的灭活率,其实验结果如图3(c,d)所示,在该光催化条件下,反应60min时,100,300,500W汞灯对该多重耐药菌的灭活率分别为0.33,0.32, 0.42log,菌液浓度由1.57×108,1.82×108,1.57× 108CFU/mL降至7.20×107,8.63×107,6.10×107CFU/ mL,且汞灯功率和光强越高,对该多重耐药菌的灭活率相对越高.
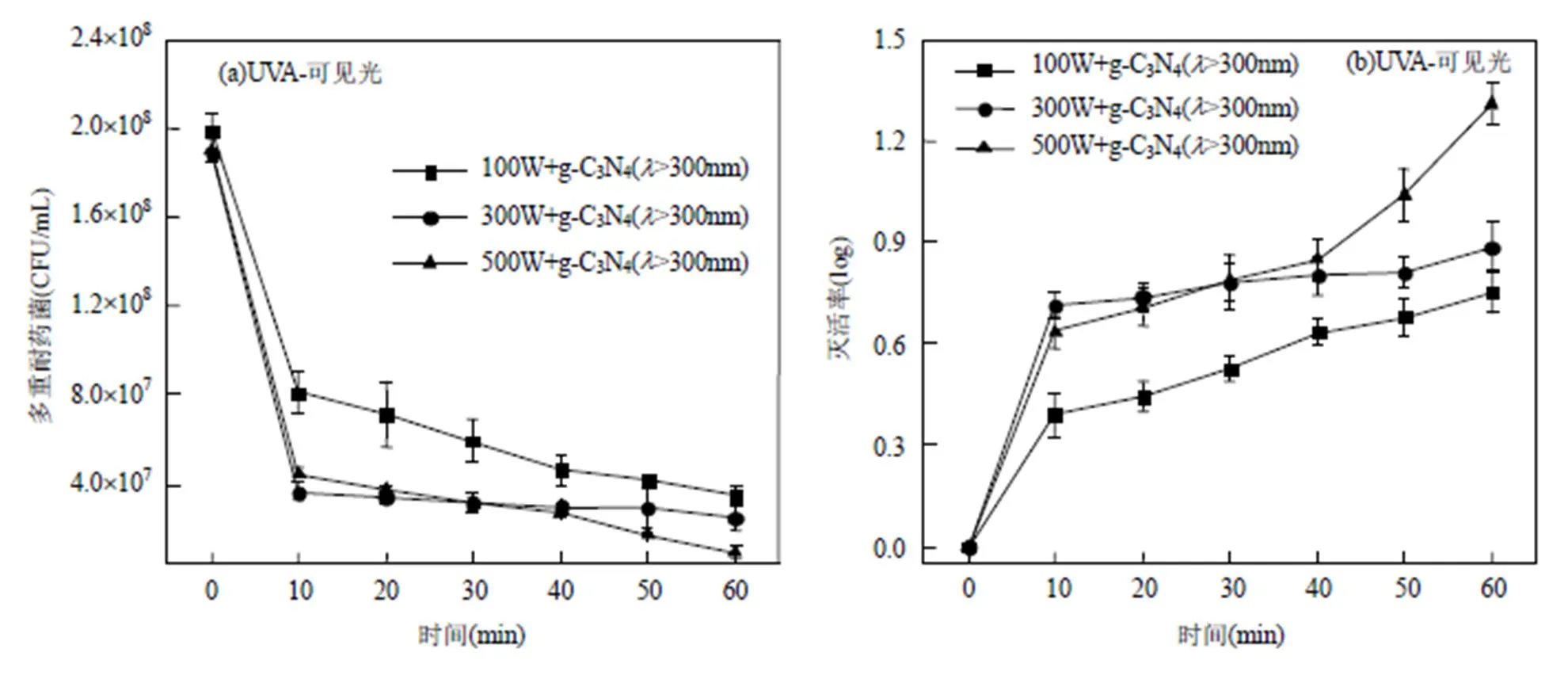

图3 光催化对四环素和氨苄西林抗性大肠杆菌的影响
在g-C3N4光催化消毒体系中,UVA-可见光(100、300和500W)对该多重耐药菌的灭活率是可见光灭活的2.27、2.72和3.19倍,且在光催化反应60min时,UVA在100,300,500W条件下对该多重耐药菌灭活率的贡献分别为56%、63%和69%;可见光的贡献为44%、37%和31%.相比不加g-C3N4催化剂的光辐照灭活体系,在UVA-可见光(100、300和500W)光催化条件下,g-C3N4对该多重耐药菌灭活率的贡献分别为69%、61%和69%;在可见光光催化条件下,g-C3N4对其灭活率的贡献分别为79%、60%和66%.
加入g-C3N4光催化剂促进了光辐照对该多重耐药菌的消毒效果.这是由于g-C3N4的2.61eV的带隙使其能够响应吸收波长小于475nm的蓝紫光能量[33],受可见光激发可以产生电子跃迁,在催化剂内部生成光生电子和空穴,能够在催化剂表面发生氧化还原反应.其中光生空穴具有极强的氧化能力[34].同时,还能与水反应生成活性氧(ROS),ROS可通过破坏细菌的细胞壁和细胞膜导致细菌丧失呼吸活性和细菌细胞内的成分泄漏,从而达到除菌的效果[35].ROS具有的强氧化能力,可以诱导DNA氧化损伤[36].而空穴在光照条件下能够氧化破坏细菌细胞,并且其较大的表面积有利于与ARB充分接触,增加灭活几率,提高灭活率[22].
2.3 消毒前后的耐药性分析
抑菌圈直径越大,抗生素耐药菌对该抗生素的耐药性越低.图4为光辐照和光催化对四环素和氨苄西林抗性大肠杆菌0~60min的耐药性影响.

图4 光辐照/光催化对四环素和氨苄西林抗性大肠杆菌的耐药性影响
从图4(a)中可以看出,不同功率(100,300,500W) UVA-可见光辐照条件下,不同辐照时间该多重耐药菌四环素的抑菌圈直径无明显变化,且均小于12mm,氨苄西林药敏纸片周围没有形成抑菌圈,这说明该光辐照条件不能影响四环素和氨苄西林抗性大肠杆菌的耐药性.
从图4(b)中可以看出,不同功率(100,300,500W)的UVA-可见光g-C3N4光催化消毒条件下,该多重耐药菌四环素的抑菌圈直径也无明显变化,且均小于12mm,氨苄西林药敏纸片周围没有形成抑菌圈,这说明该光催化条件不能影响四环素和氨苄西林抗性大肠杆菌的耐药性,相关深入研究有待后续开展.
2.4 活性氧(ROS)对四环素和氨苄西林抗性大肠杆菌的影响
在光反应体系加入g-C3N4光催化剂后,能够产生电子空穴(h+)和ROS,ROS通常包括:超氧阴离子自由基(∙O2-)、羟基自由基(∙OH)、单线态氧(1O2)和过氧化氢(H2O2)[37],是激发氧化反应的必要条件[38].由于ROS可与2',7'-二氯二氢荧光素(DCFH,非荧光)快速反应生成2',7'-二氯荧光素(DCF,荧光),通过测定DCF的荧光强度确定光催化剂ROS的浓度,其浓度越高,光催化剂性能越好[39].为了考察g-C3N4光催化对该多重耐药菌的灭活机理,在光催化体系中分别添加50mmol/L叔丁醇(TBA)、50mmol/L草酸铵(AO)、50mmol/LL-组氨酸(L-H)、10U/mL超氧化物歧化酶(SOD)和1000U/mL过氧化氢酶(CAT),TBA、AO、L-H、SOD和CAT分别为∙OH、h+、1O2、∙O2-、和H2O2的淬灭剂,用抑制率(未加活性组分时耐药菌的降解量0减去加入活性组分抑制后耐药菌的降解量a,然后乘以100/0;%)表示该活性组分的在多重耐药菌的灭活过程中的活跃程度[27,40].
在UVA-可见光(汞灯100/300/500W)g-C3N4光催化剂体系中,反应时间分别为10,60min的ROS和电子空穴的淬灭实验结果如图5(a,b)所示.加入TBA淬灭∙OH后,反应时间10,60min,对该多重耐药菌灭活程度的抑制率分别为32.28%~38.73%和31.59%~39.58%;加入AO淬灭h+后,对其灭活程度的抑制率分别为18.24%~24.46%和22.28%~27.51%;加入L-H淬灭1O2后,对其灭活程度的抑制率分别为17.75%~18.88%和20.69%~24.17%;加入SOD淬灭∙O2-后,对其灭活程度的抑制率分别为21.88%~ 31.84%和24.03%~34.10%;加入CAT淬灭H2O2后,对其灭活程度的抑制率分别为23.79%~30.12%和20.55%~29.53%.
综上可知,ROS和电子空穴对该多重耐药菌灭活过程的活跃程度为:∙OH>∙O2->H2O2>h+>1O2,这是因为光激发能引起导带上电子与价带上空穴的空间电荷分离,产生光生电子空穴对.电子具有还原性,空穴具有氧化性,空穴与水中的OH-离子生成氧化性很高的∙OH,∙OH能够氧化损伤细菌DNA结构中的鸟嘌呤[41].鸟嘌呤易于氧化形成鸟嘌呤基团,其不稳定并且易于与空气中的O2和H+结合形成R- OOH并产生8-羟基-2'-脱氧鸟苷[42],由于∙OH的作用,8-羟基-2'-脱氧鸟苷被氧化成CO2和H2O,细菌失去活性[43].同时,∙OH还具有破坏微生物屏障、蛋白质酶和核酸的能力,导致细菌死亡[44].
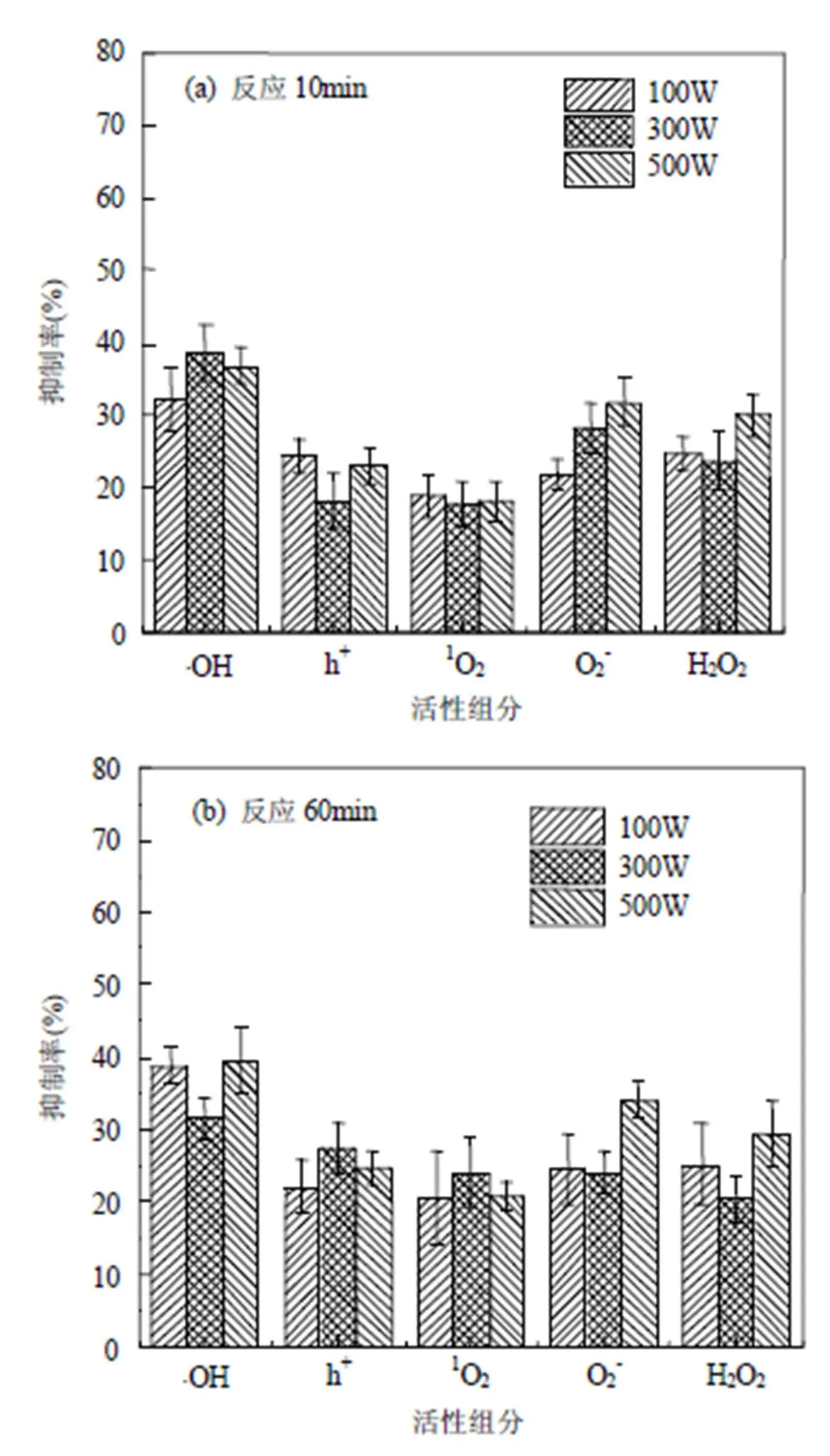
图5 活性物质对光催化反应的抑制率
Fig.5 Inhibitory rate of active substances on photocatalytic reaction
3 结论
3.1 光辐照(汞灯100,300,500W)对具有四环素和氨苄西林抗性多重抗性的大肠杆菌的灭活效率随着汞灯功率和强度的增大而提高.UVA-可见光(300~579nm)光辐照60min时,100,300,500W汞灯(其中UVA光强分别为3.73,7.70,15.70mW/cm2,可见光强分别为13.40,20.90,39.20mW/cm2)对其灭活率分别达0.23,0.34,0.41log,其中UVA对灭活率的贡献为66%~79%,可见光的贡献率仅为21%~34%.
3.2 基于石墨相g-C3N4的光催化消毒对该多重耐药菌的灭活率有明显提高,UVA-可见光在光催化反应60min时,100,300,500W汞灯对该多重耐药菌的灭活率分别为0.75,0.87,1.31log,其中可见光对其灭活效率的贡献达到31%~44%.相比未加g-C3N4催化剂的光辐照灭菌,在UVA-可见光条件下g-C3N4对灭活率的贡献为61%~69%,在可见光条件下g-C3N4对灭活率的贡献达到60%~79%.
3.3 在光催化反应中,ROS和电子空穴对CGMCC 1.1595灭活过程中的活跃程度为:∙OH> ∙O2->H2O2>h+>1O2,∙OH为主要催化活性物质,对该多重耐药菌的灭活起主要作用,淬灭∙OH后,对反应的抑制率最高(31.59%~39.58%).
[1] Chen C, Li J, Chen P, et al. Occurrence of antibiotics and antibiotic resistances in soils from wastewater irrigation areas in Beijing and Tianjin, China [J]. Environmental Pollution, 2014,193(2):94-101.
[2] Zhang Q Q, Ying G G, Pan C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science & Technology: 2015,49(11):6772-6782.
[3] Zhao X, Wang J H, Zhu L S, et al. Environmental analysis of typical antibiotic-resistant bacteria and ARGs in farmland soil chronically fertilized with chicken manure [J]. Science of the Total Environment, 2017,593–594:10-17.
[4] Sun W, Qian X, Gu J, et al. Mechanism and Effect of Temperature on Variations in Antibiotic Resistance Genes during Anaerobic Digestion of Dairy Manure [J]. Scientific Reports, 2016,6:30237-30245.
[5] Fang H, Han Y L, Yin Y M, et al. Variations in dissipation rate, microbial function and antibiotic resistance due to repeated introductions of manure containing sulfadiazine and chlortetracycline to soil [J]. Chemosphere, 2014,96(2):51-56.
[6] Grenni P, Ancona V, Caracciolo A B. Ecological effects of antibiotics on natural ecosystems: A review [J]. Microchemical Journal, 2018, 136:25-39.
[7] Collignon P. Antibiotic resistance: are we all doomed? [J]. Internal Medicine Journal, 2015,45(11):1109-1115.
[8] Pawlowski A C, Wang W L,Koteva K, et al. A diverse intrinsic antibiotic resistome from a cave bacterium [J]. Nature communications, 2016,7:13803-13812.
[9] Dodd M C. Potential impacts of disinfection processes on elimination and deactivation of antibiotic resistance genes during water and wastewater treatment [J]. Journal of Environmental Monitoring Jem, 2012,14(7):1754-1771.
[10] Moreira N F F, Narciso-Da-Rocha C, Polo-López M I, et al. Solar treatment (H2O2, TiO2-P25 and GO-TiO2, photocatalysis, photo-Fenton) of organic micropollutants, human pathogen indicators, antibiotic resistant bacteria and related genes in urban wastewater [J]. Water Research, 2018,135:195-206.
[11] Rizzo L, Fiorentino A, Anselmo A. Effect of solar radiation on multidrug resistant E. coli strains and antibiotic mixture photodegradation in wastewater polluted stream. [J]. Science of the Total Environment, 2012,s427–428(8):263-268.
[12] Kadir K, Nelson K L. Sunlight mediated inactivation mechanisms of Enterococcus faecalis and Escherichia coli in clear water versus waste stabilization pond water [J]. Water Research, 2014,50(1):307-317.
[13] Mcguigan K G, Conroy R M, Mosler H J, et al. Solar water disinfection (SODIS): a review from bench-top to roof-top [J]. Journal of Hazardous Materials, 2012,235-236(20):29-46.
[14] Li G, Xin N, Chen J, et al. Enhanced visible-light-driven photocatalytic inactivation of Escherichia coli, using g-C3N4/TiO2, hybrid photocatalyst synthesized using a hydrothermal-calcination approach [J]. Water Research, 2015,86:17-24.
[15] Li Y, Zhang C, Shuai D, et al. Visible-light-driven photocatalytic inactivation of MS2by metal-free g-C3N4: Virucidal performance and mechanism [J]. Water Research, 2016,106:249-258.
[16] Fresno F, Portela R, Suárez S, et al. Photocatalytic materials: recent achievements and near future trends [J]. Journal of Materials Chemistry A, 2014,2(9):2863-2884.
[17] Thomas A, Fischer A, Goettmann F, et al. ChemInform Abstract: Graphitic Carbon Nitride Materials: Variation of Structure and Morphology and Their Use as Metal-Free Catalysts [J]. Journal of Materials Chemistry, 2009,40(9):4893-4908.
[18] Xie L, Ni J, Tang B, et al. A self-assembled 2D/2D-type protonated carbon nitride-modified graphene oxide nanocomposite with improved photocatalytic activity [J]. Applied Surface Science, 2018, 434:456-463.
[19] Nowotny J, Alim M A, Bak T, et al. Defect Chemistry and Defect Engineering of TiO2-Based Semiconductors for Solar Energy Conversion [J]. Chemical Society Reviews, 2015,44(23):8424-8442.
[20] Maeda K, Wang X, Nishihara Y, et al. Photocatalytic Activities of Graphitic Carbon Nitride Powder for Water Reduction and Oxidation under Visible Light [J]. Journal of Physical Chemistry C, 2009, 113(12):4940-4947.
[21] Tian Y, Zhou F, Zhan S, et al. Mechanisms on the enhanced sterilization performance of fluorocarbon resin composite coatings modified by g-C3N4/Bi2MoO6, under the visible-light [J]. Journal of Photochemistry & Photobiology A Chemistry, 2018,350:10-16.
[22] Song Y, Tian J, Gao S, et al. Photodegradation of Sulfonamides by g-C3N4, under Visible Light Irradiation: Effectiveness, Mechanism and Pathways [J]. Applied Catalysis B Environmental, 2017,210: 88-96.
[23] Wang Y, Shi Z, Fan C, et al. ChemInform Abstract: Synthesis, Characterization, and Photocatalytic Properties of BiOBr Catalyst [J]. Cheminform, 2013,44(19):224-229.
[24] Huang J J, Hu H Y, Wu Y H, et al. Effect of chlorination and ultraviolet disinfection on tetA-mediated tetracycline resistance of Escherichia coli [J]. Chemosphere, 2013,90(8):2247-2253.
[25] CLSI, 2013. Clinical and Laboratory Standards Institute.
[26] 席劲瑛,黄晶晶,胡洪营,等.污水处理厂二级出水中四环素抗性菌的生长特性与耐药性 [J]. 环境科学学报, 2014,34(7):1724-1729.
[27] Zheng Q, Durkin D P, Elenewski J E, et al. Visible-Light-Responsive Graphitic Carbon Nitride (g-C3N4): Rational Design and Photocatalytic Applications for Water Treatment [J]. Environmental Science & Technology, 2016,50(23):12938-12948.
[28] Cao Y, Xing Z, Li Z, et al. Mesoporous black TiO2-x/Ag nanospheres coupled with g-C3N4nanosheets as 3D/2D ternary heterojunctions visible light photocatalysts [J]. Journal of Hazardous Materials, 2018, 343:181-190.
[29] Pruden A. Balancing water sustainability and public health goals in the face of growing concerns about antibiotic resistance [J]. Environmental Science & Technology, 2014,48(1):5-14.
[30] Mckinney C W, Pruden A. Ultraviolet Disinfection of Antibiotic Resistant Bacteria and Their Antibiotic Resistance Genes in Water and Wastewater [J]. Environmental Science & Technology, 2012,46(24): 13393-13400.
[31] Horai Y, Ando Y, Kimura S, et al. Mutation spectrum resulting in M13mp2phage DNA exposed to N-nitrosoproline with UVA irradiation [J]. Mutation Research, 2017,821:1-4.
[32] Giannakis S, López M I P, Spuhler D, et al. Solar disinfection is an augmentable, in situ -generated photo-Fenton reaction-Part 1: A review of the mechanisms and the fundamental aspects of the process [J]. Applied Catalysis B Environmental, 2016,199:199-223.
[33] Wang X, Blechert S, Antonietti M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis [J]. Acs Catalysis, 2012, 2(8):1596–1606.
[34] Dong H, Guo X, Yang C, et al. Synthesis of g-C3N4, by different precursors under burning explosion effect and its photocatalytic degradation for tylosin [J]. Applied Catalysis B Environmental, 2018, 230:65-76.
[35] Nadtochenko V A, Rincon A G, Stanca S E, et al. Dynamics of E. coli, membrane cell peroxidation during TiO2, photocatalysis studied by ATR-FTIR spectroscopy and AFM microscopy [J]. Journal of Photochemistry & Photobiology A Chemistry, 2005,169(2):131-137.
[36] Li Y L, Wang J S, Yang Y L, et al. Seed-induced growing various TiO₂ nanostructures on g-C₃N₄ nanosheets with much enhanced photocatalytic activity under visible light [J]. Journal of Hazardous Materials, 2015,292:79-89.
[37] Cruz-Ortiz B R, Hamilton J W J, Pablos C, et al. Mechanism of photocatalytic disinfection using titania-graphene composites under UV and visible irradiation [J]. Chemical Engineering Journal, 2017, 316:179-186.
[38] He W, Kim H K, Wamer W G, et al. Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity [J]. Journal of the American Chemical Society, 2014,136(2):750-757.
[39] Lan Z J, Yu Y L, Yao J H,et al. The band structure and photocatalytic mechanism of MoS2-modified C3N4photocatalysts with improved visible photocatalytic activity [J]. Materials Research Bulletin, 2018, 102:433-439.
[40] 尹 竞,廖高祖,朱东韵,等.g-C3N4/石墨烯复合材料的制备及光催化活性的研究. [J]. 中国环境科学, 2016,36(3):735-740.
[41] Zheng L F, Dai F, Zhou B, et al. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu(II) ions: Mechanism and structure-activity relationship [J]. Food & Chemical Toxicology, 2008,46(1):149-156.
[42] Murakami K, Haneda M, Makino T, et al. Prooxidant action of furanone compounds: Implication of reactive oxygen species in the metal-dependent strand breaks and the formation of 8-hydroxy- 2′-deoxyguanosine in DNA [J]. Food & Chemical Toxicology, 2007,45(7):1258-1262.
[43] Wang H M, Liu Y J, Wang H X, et al. Stability and properties of the two-dimensional hexagonal boron nitride monolayer functionalized by hydroxyl (OH) radicals: a theoretical study [J]. Journal of Molecular Modeling, 2013,19(12):5143-5152.
[44] Tian Y, Zhou F, Zhan S, et al. Mechanisms on the Sterilization Performance of Fluorocarbon Resin Composite Coatings Enhanced by g-C3N4/TiO2[J]. Journal of Inorganic & Organometallic Polymers & Materials, 2017,27(1):353-362.
Graphite carbon nitride (g-C3N4) photocatalytic disinfection on a multidrug resistantstrain from secondary effluent.
QI Fei1, SUN Ying-xue1*, CHANG Xue-ming1, YIN Xiu-feng1, LU Song-Liu2, HU Hong-ying3
(1.Department of Environmental Science and Engineering, Beijing Technology and Business University, Beijing 100048, China;2.Tus-Water Group Limited, Shanghai 200072, China;3.State Key Joint Laboratory of Environmental Simulation and Pollution Control, State Environmental Protection Key Laboratory of Microorganism Application and Risk Control, School of Environment, Tsinghua University, Beijing 100084, China)., 2018,38(10):3767~3774
The inactivation effects of multi-drug resistant bacteriumCGMCC 1.1595against tetracycline and ampicillin from secondary effluent by light irradiation and photocatalysis with graphite carbon nitride (g-C3N4) were studied. The results showed that the higher irradiation power of the mercury lamp (100/300/500W) with higher irradiation intensity could lead to higher inactivation efficiency. Under the inactivation by 500W mercury lamp irradiation of 60min, the inactivation rate ofCGMCC 1.1595was 0.41log by ultraviolet A (UVA)-visible light (300~579nm) irradiation, and the inactivation rate was up to 1.31log by g-C3N4photocatalysis. The contribution of g-C3N4to the UVA-visible light inactivation was 61%~69% compared to that without g-C3N4catalyst, while the contribution of g-C3N4to the visible light inactivation was 60%~79%. The significance of the reactive oxygen species (ROS) and hole (h+) for the g-C3N4photocatalytic inactivation ofCGMCC 1.1595were also investigated, with the activity order as ∙OH>∙O2->H2O2>h+>1O2. Hydroxyl radical (∙OH) was a leading contributor to the irradiation, followed by ∙O2-and H2O2.
light irradiation;graphite carbon nitride (g-C3N4);multidrug resistant bacteria;disinfection;visible light
X505
A
1000-6923(2018)10-3767-08
齐 菲(1993-),女,北京人,北京工商大学硕士研究生,主要研究方向为水污染控制理论与技术.发表论文1篇.
2018-03-16
国家自然科学基金资助项目(21306003)
* 责任作者, 教授, sunyx@th.btbu.edu.cn
