pH药物控释的氮掺杂碳点载运阿霉素用于光热与化疗的协同治疗
2018-10-12谢安建沈玉华
杨 正 谢安建 沈玉华
(安徽大学化学与化工学院,现代生物制造协同创新中心,合肥 230601)
0 Introduction
Cancer,especially themalignant tumor,which is one of the severe threats to anthropic health,is an arduous challenge till to now[1].Heretofore, massive methods have been developed to cure cancer,such as radiation therapy,surgical therapy,and chemotherapy[2].Although accompanying with the successful cancer ablation, the insurmountable defects of these treatments such as low selectivity,huge invasion,and serious side effects,abate heavy pains of patients suffering from cancer.To overcome the fatal disease,photo-thermal therapy(PTT)basing on the principle of heating target cells has become a feasible alternative for the cancer treatment recently[3].Near infrared(NIR)light-induced PTT which could cure tumor in deeper tissues is an emerging therapy in anticancer domain[4].Two ranges of wavelengths are called bio-windows benefitting for PTT,which are 650~950 nm and 1 000~1 350 nm respectively[5].Comparing to traditional cancer treatments,PTT destroys tumor selectively by extracorporeal laser irradiation,demonstrating that PTT is a noninvasive therapy with high selectivity.In past decades,massive amounts of optical absorbing materials for PTT have been developed,such as gold nanomaterials[7],carbon nanoparticles[8],and other metal-recombined nanocomposites[9]. However,the reasonable combination of PTT and other therapies to cure cancer more effectively is still main barrier in synergistic cancer treatment.
For solving the above-mentioned challenges,the rapid development of nanotechnology becomes a key avenue.Lately,fluorescent N-CDs possessing high water-solubility,facile surface modification,limited toxicity,excellent biocompatibility and extraordinary photo-stability,have been a kind of quite momentous nanomaterials in biomedical field[10].On account of aforementioned features,N-CDs could convert the absorbed NIR light into heat,proving to be an ideal PTT agent.However,augmenting photo-thermal effect and drug delivery capacity of N-CDs for enhanced synergetic effect between PTT and chemotherapy is still an insurmountable problem.
In this paper,we synthesized the N-CDs by a facile one-step hydrothermal process.Even more important,the synthesized N-CDs show a broad and forceful absorption band in the range of visible to NIR light(400~800 nm).Utilizing this property of N-CDs,we successfully prepared a simple and low-cost photothermal agent with high-performance for PTT in cancer therapy.Using the N-CDs as anti-cancer drug carrier,the Dox-loaded N-CDs could achieve the combined PTT and chemotherapy synergistically.
1 Experimental
1.1 M aterials and characterization
Critic acid,urea,and dimethyl sulfoxide(DMSO)were purchased from Aladdin Industrial Inc.3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT),high glucose dulbecco′s modified eagle′s medium (DMEM),phosphate buffer solution(PBS),and fetal bovine serum (FBS),were obtained from Sinopharm Chemical Reagent Co.,Ltd.Penicillin,streptomycin,pancreatin solution(25%(w/w)),hoechst 33342,and propidium iodide (PI),were purchased from Sangon Biotech Inc.All the agents were analytical pure and used as received without further purification.The experimental water were double distilled water(18.0MΩ·cm).
Morphological details of the obtained samples were recorded by Transmission electron microscopy(TEM)at an accelerating voltage of 100 kV(JEM-2100,Japan).Raman spectrum was studied using a Via-Reflex laser confocal Raman spectroscopy under a 785 nm laser excitation with the power of340mW.For investigating the crystallinity of samples,a DX-2700 X-ray diffractometer(Cu Kα source,λ=0.154 056 nm,40 V,100 mA)was used to obtain the power X-ray diffraction(XRD)patterns over the 2θrange of 10°to 70°.The fourier transform infrared (FT-IR)spectra was observed by a NEXUS-870 FT-IR spectrometer using the KBr pellet technique.An ESCALAB250 spectrometer with Al Kα source(hν=1 486.6 eV,150 W,1×10-6Pa)was used to analyze the element distribution of samples by X-ray photoelectron spectroscopy.The UV-Vis spectra were obtained using a Shimadzu UV-3600.The photothermal effects of NCDs dispersions were studied using a Fluke Ti32 infrared thermography camera to obtain the thermographies.
1.2 Synthesis of N-CDs
Using a reported facile one-step hydrothermal treatment procedure,we synthesized the N-CDs[11].Briefly,1.0 g ofcitric acid and 0.7 g ofureawere added into 30 mL of DIwater.Then themixed solution was sonicated to be transparent solution.After that,the asprepared solution was poured to a 50.0 mL Teflonlined stainless autoclave with sealing tightly and heated to 180℃with maintaining for 5 h by hydrothermal reaction.After naturally cooling down to room temperature,the hyaline solution with brown and yellow color was dialyzed in a dialysis bag (3.5 kDa)over night.Finally,the dialyzed suspension was frozen in ultra-low temperature freezer(-86℃)for 10 min and then was subjected to freeze-drying to obtain the black-green solid powders,i.e.,N-CDs.
1.3 Photo-thermal effect of N-CDs
For measuring the PTT effect of the N-CDs,2 mL of the various samples dispersions with different concentrations (0.5,1.0,2.0,5.0 and 10.0 mg·mL-1)were added in a small glass bottle and then irradiated with an 650 nm NIR laser at regular time intervals,respectively.The temperatures of dispersions were recorded by the Fluke Ti32 thermal infrared camera immediately after irradiation every 1 min.The equal volume of water was used as controls.All data were acquired from three independent experiments to ensure the reliability of the data.
1.4 Drug loading and release of N-CDs-Dox nanocomposites
For loading N-CDswith Dox,N-CDs(20mg)were added to Dox solution (150 μg·mL-1,20 mL)under sonicating.Stirring by amechanical agitator for 24 h,then the hybrid nanocomposites were dialyzed in a dialysis bag (3.5 kDa)over night.The final products were frozen in ultra-low temperature freezer(-86℃)for 10 min and then were subjected to freeze-drying.To calculate the efficacy of Dox loading,1 mg of NCDs-Dox powders were dispersed in 5 mL deionized water.The absorption peak at 510 nm was used for validating the presence of Dox and estimating the Dox loading efficacy by comparison with the standard curve.
In order to investigate the drug release in different environments,2 mg of N-CDs-Dox powders were added to 1 mL of PBS solution at pH values of 5.0,6.5 and 7.4 in dialysis bags respectively.Then the dialysis bags were put into 9 mL of PBS solution with the same pH value respectively.At different time points during 0 to 72 h,1mL of dialyzed PBSsolution was collected and analyzed by UV-Vis spectrophotometer at 510 nm to calculate the drug releasing efficiency according to the standard curve of Dox solution.After determination,the collected solutionwas put back to original solution for continuous dialysis.
1.5 In vitro photo-thermal effect
The as-prepared N-CDs and N-CDs-Dox nanocomposites were dispersed in DMEM medium to form mixed dispersions with different concentrations of 1.0,2.0,5.0,7.5 and 10.0 mg·mL-1.The HeLa cells suspended in DMEM medium was seeded into 96-well plates at a density of 5×103each well(200 μL)and incubated for 24 h.After washing each well by PBS for 3 times,the cellswere incubated with only 100μL of DMEM medium(control),and with 100μL of DMEM medium containing the samples of N-CDs and N-CDs-Dox nanocomposites(0.5,1.0,2.0,5.0,7.5 and 10.0mg·mL-1,respectively)for 24 h,respectively.The DMEM without samples was used as control group at the same condition.After washing each well by PBS for 3 times,20μL of MTT and 100μL of DMEM medium were added into each well with sequential incubation for 4 h.As contrast,the irradiated groups were taken out with the irradiation of 650 nm laser (1 W·cm-2)for 10 min and then incubated for 24 h.100μL of DMSO solution was then added for dissolving the insoluble formazan crystals.To investigate the selectivity of the prepared N-CDs normal cells,293T cells were used as control incubating at the same conditions,following with the equal post-treatments.The optical density of samples was measured using an Elisa reader at 490 nm to calculate the cell viability by the formula:
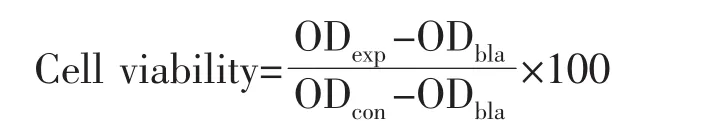
Where ODbla,ODexp,and ODconare the optical densities of blank, experimental and control groups,respectively.
1.6 Fluorescent images
To further understand the therapeutic abilities of as-prepared N-CDs and N-CDs-Dox nanocomposites against cancer cells,the Hela cells were seeded into 6-well plates at a density of 5×103,incubating at 37℃under 5%(V/V)CO2atmosphere(95%(V/V)air)in the CO2constant temperature incubator for 24 h.Then 2 mL ofmedium with the sample concentration of 10.0 mg·mL-1was added into part of wells,using pure medium as control.When the incubation time lasting for 4 h,the irradiated groups were taken out and irradiated using 650 nm laser(1W·cm-2)for 10 min.After further 24 h incubation,PBS buffer(pH=7.4)was used to replace themedium,then 0.5 mL of Hoechst 33342 (10 μg·mL-1)and 0.5 mL of PI(10 μg·mL-1)were added into each well for 15 min.The final cells were washed twice with PBS and observed by an inverted fluorescence microscopy to obtain fluorescent images.
2 Results and discussion
2.1 M orphology,constituent,and m icrostructure of N-CDs
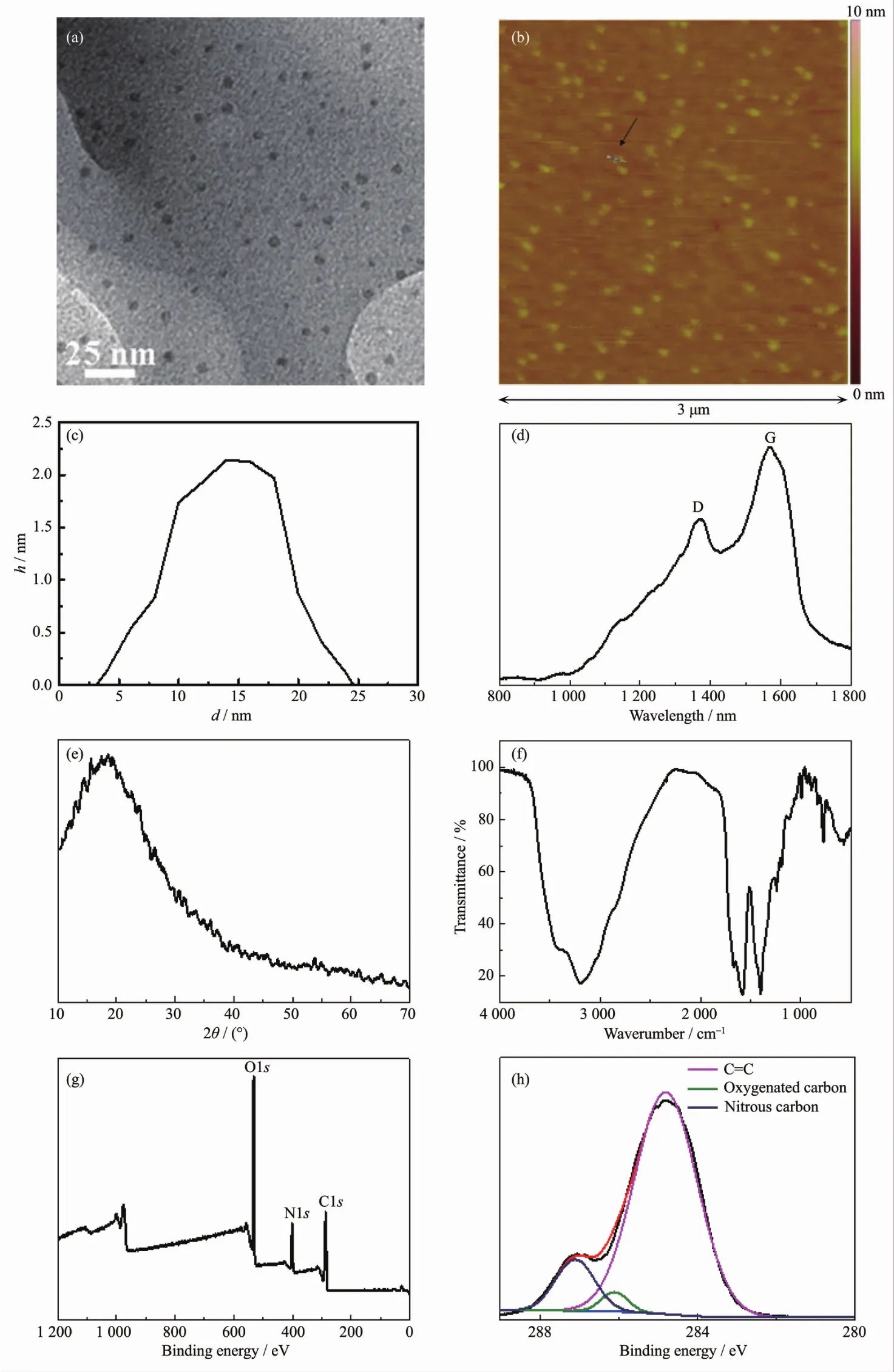
Fig.1 (a)TEM image,(b)AFM image,(c)height profile,(d)Raman spectrum,(e)XRD pattern,(f)FT-IR spectrum,(g)XPSsurvey spectrum,and(h)high-resolution spectrum of C1s of N-CDs
According to Fig.1a,TEM image of N-CDs investigates that the as-prepared N-CDs possess uniform size with the diameter about 7 nm[12].Further evidences coming from Fig.1b and 1c,it is seen that the synthesized N-CDs are dispersed uniformly on the substrate and possess regular shapes with a size of about 7.0 nm and a height of ca.2.2 nm,which is consistent with that of TEM image.As shown in Fig.1d,Raman spectroscopic analysis shows that the NCDs exhibit two bands around 1 352 and 1 597 cm-1relating to the D band of disordered carbon structure and the G band of graphitic layer of the N-CDs,respectively[12].Fig.1e is the XRD pattern of N-CDs showing that a broad diffraction peak emerges at 2θ ranging from 10°to 40°,which illustrates the N-CDs are comprised by disorder carbon structure mainly.The result coming from XRD pattern is corresponding to that of Raman spectrum of N-CDs.FT-IR spectrum(Fig.1f)reveals that the broad peak at 3 430 cm-1is related to the stretching vibration of C-OH and N-H,while the peak at 1 128 cm-1is corresponded to the asymmetric stretching vibration of C-NH-C[12].Meanwhile,the peak at 1 572 cm-1is attributed to the flexure vibration of N-H,alongwith the peaks at1 635 and 1 082 cm-1are belonged to the specific vibration of C=O[12].Based on upwards results,the surface of NCDs contain large amounts of hydroxyl groups,carbonyl groups,amino groups,and epoxy groups which are benefitting for solubility of N-CDs in water.
According to the XPS survey spectrum of N-CDs in Fig.1g,the as-synthesized N-CDs are combined by the elements of carbon,oxygen,and nitrogen primarily.As shown in Fig.1h,the characteristic peaks at 284.75,286.56 and 287.1 eV in the C1s spectrum,indicate the presence of varietal types of carbon bonds corresponding to sp2C=C,and C=O as well as C-N,respectively[12].The mentioned results demonstrated the obtained N-CDs are doped by nitrogen successfully,within amounts of hydroxyl and carbonyl groups on the surface which are consistent with the FT-IR spectrum completely.
2.2 Drug loading and release analysis
Further evidences coming from Fig.2a,the drug loading content achieves about 1 μg·mg-1,illustrating that the N-CDs are promising drug carriers.The loaded drug may be combined with the N-CDs by main electrostatic attraction,also π-π stacking,hydrophobic and van der Waals interactions[13].To investigate the drug loadingmechanism in depth,zeta potentials of N-CDs dispersion,Dox solution,and NCDs-Dox dispersion were obtained at pH=7.4 respectively (Fig.2b).ζpotential of N-CDs dispersion is about-24.10mV,showing the N-CDs are negatively charged materials.The Dox solution possesses the positively charged surface with theζpotential of ca.9.54mV.After drug loading process,the prepared NCDs-Dox dispersion shows theζpotential was about-10.25 mV,which was enlarged comparing to that of N-CDs dispersion.The result confirms that Doxloading of N-CDs is due to the charge interaction between Dox and N-CDs[14].The release behaviors(Fig.2c)of Dox from the N-CDs-Dox nanocomposites are studied in PBS buffer solution at different pH values of 5.5,6.5 and 7.4 to simulate the lysosome,tumor site,and physiological conditions respectively.It is seen that when the pH value is 7.4,only 10%Dox is released from the nanocomposites after 72 h.While the pH value decreases to 6.5,the cumulative amount of released Dox increases to about 30%after the same time.On the contrast,while the pH value lowers to 5.5,the amount of the released Dox from the nanocomposites reached as high as ca.98%within the same time.The kinetics of Dox release at low pH valuesmay be induced by the increase of H+in PBS buffer solution,which could weaken and destroy the charge interaction of Dox with N-CDs in the obtained nanocomposites[14].The above results indicate the fact that the release behavior of Dox from the N-CDs-Dox nanocomposites was pH dependent and the decrease of pH value could enhance the release rate of the Dox from the nanocomposites significantly.All the results mentioned above demonstrate that N-CDs are potential drug carriers which could achieve pH-controlled drug release at tumor site locally.

Fig.2 (a)UV-Vis spectra of Dox before and after loading in the N-CDs;(b)ζpotentials of N-CDs dispersion,Dox solution,and N-CDs-Dox dispersion at pH=7.4,respectively;(c)Release curve of Dox at different pH values from N-CDs-Dox nanocomposites
2.3 Photothermal effect of N-CDs
The photo-thermal effects of all samples were shown in Fig.3.Under continuous irradiation of 650 nm laser for 10min,the temperature elevation of pure water increased about 3℃,indicating the used laser could not induce obvious temperature elevation in pure water.However,the irradiated N-CDs dispersions(0.5,1.0,2.0,5.0 and 10.0 mg·mL-1,respectively)emerge remarkable temperature changes depending on concentrations of samples.The highest temperature enhancement of N-CDs dispersion (10.0 mg·mL-1)is about 10℃,demonstrating that the prepared N-CDs possess excellent photo-thermal ability.The above evidences indicate the synthesized N-CDs are suitable photo-thermal agents for PTT.

Fig.3 Photo-thermal effects of purewater,and N-CDs dispersions(0.5,1.0,2.0,5.0 and 10.0mg·mL-1,respectively)with a 650 nm laser irradiation for 10min
2.4 M TT assay

Fig.4 Viabilities of(a)HeLa and(b)293T cells incubated with different concentrations of N-CDs and N-CDs-Dox nanocompositeswithoutorwith irradiation(650 nm,1W·cm-2,10min)
Fig.4a shows the results of MTT assay using different concentrations of samples to evaluate the cytotoxicity to HeLa cells.The group which is incubated with N-CDs in dark shows high cell viabilities(all above 90%),illustrating the obtained N-CDs possess good biocompatibility.Meanwhile,N-CDs coincubated group shows the obvious apoptosis of HeLa cells after 10 min laser irradiating,with the cell mortalities about 27%,40%and 57%at the concentrations of 5.0,7.5 and 10.0 mg·mL-1respectively.The obtained results demonstrate that the prepared NCDs have excellent property in PTT.Comparing to NCDs-Dox co-incubated groups in dark,the irradiated N-CDs-Dox group exhibits serious photo-induced cell apoptosis with the cell viabilities of about 63%,32%and 18%at the concentrations of 5.0,7.5 and 10.0 mg·mL-1respectively,which are higher than those of irradiated N-CDs groups.The calculated experimental data performed that the drug loaded N-CDs own outstanding synergistic PTT and chemotherapy to HeLa cells,which are potential multifunctional drug carriers for cancer treatment.For comparison,Fig.4b illustrates the cell viabilities of 293T cells incubating at the same conditions to HeLa cells.The results of all groups are similar to those of HeLa cells with the uniform condition incubations,demonstrating that the obtained N-CDs do not possess prominent selectivity to normal cells and cancer cells.However,the outstanding photothermal ability of N-CDs is benefitting for synergetic therapy with chemotherapy to superficial tumors by the injection method,such as melanoma etc.,which could decrease the damage to normal cells aswell as improve the antitumor effect.
2.5 Fluorescent imaging
Base on the evidences shown in Fig.5,the nonirradiated groups of HeLa cells incubate with control,and N-CDs which are stained by Hoechst 33342 and PI,emitting blue fluorescence (a1,b1)and almost invisible red fluorescence(a2,b2)respectively,aswell as blue fluorescence (a3,b3)in the merged images,indicating that all the cells grow well without obvious cell apoptosis.The group in dark incubates with NCDs-Dox nanocomposites shows partial red fluorescence(c2),demonstrating that N-CDs-Dox nanocomposites could achieve chemotherapy due to the loaded drug without irradiation.The irradiated group of control shows blue fluorescence (d1)and negligible red fluorescence (d2),demonstrating that the mere laser irradiation could only induce negligible apoptosis of HeLa cells.Meanwhile,the group of N-CDs exhibits local red fluorescence (e2),illustrating the photothermal effect of N-CDs could kill cancer cells effectively.Moreover,when N-CDs-Dox nanocomposites co-incubated cells are irradiated by laser for 10 min,an intense red fluorescence (f3)could be seen,which shows that N-CDs-Dox nanocomposites caused a large amount of cell apoptosis with laser irradiation.The reason is that the PTT ability of NCDs and chemotherapy property of Dox in N-CDs-Dox nanocomposites realized excellent synergetic efficacy in cancer treatment.Therefore,the N-CDs are novel PTT agents which could be used as drug carrier to achieve combined PTT and chemotherapy.

Fig.5 Fluorescencemicroscopy images of HeLa cells incubated with(a,d)control(without samples),(b,e)N-CDs,and(c,f)N-CDs-Dox nanocomposites,(a~c)without laser irradiation and(d~f)with laser irradiation(650 nm,1W·cm-2)
3 Conclusions
In summary,the water-soluble N-CDs are synthesized and applied for in vitro photo-thermal ablation of cancer cells successfully.According to the experimental results,the as-prepared N-CDs possess good biocompatibility,and efficacious photo-thermal ability as well as favorable drug delivery property.Meanwhile,the N-CDs-Dox nanocomposites exhibit synergetic efficacy of cancer therapy owing to the chemotherapy induced by the loaded Dox and the photo-thermal effect attributing to N-CDs.This study paves a new way for the design and synthesis of other multi-functionalmaterials.
