Antioxidative and antiapoptotic effects of (+)-clausenamide on acetaminophen-induced nephrotoxicity in mice
2018-09-03HongMinYuMinWangZongChaoYuYiFangLiChunXinHuangFangXuanHanFanNaLiuRongRongHe
Hong-Min Yu, Min Wang, Zong-Chao Yu, Yi-Fang Li, Chun-Xin Huang, Fang-Xuan Han*, Fan-Na LiuRong-Rong He*
1Anti-stress and Health Research Center and Guangdong Engineering Research Center of Chinese Medicine & Disease Susceptibility, Guangdong, China. 2College of Pharmacy, Jinan University, Guangdong, China. 3Department of Pharmacy,Hainan General Hospital, Hainan, China. 4Department of Nephrology, the First Affiliated Hospital of Jinan University,Guangdong, China.
Background
Acetaminophen (APAP) is a widely used analgesic and antipyretic drug and is safe at the dosages used for treatment [1]. In clinical use, APAP can be formulated as various dosage forms and is present in many compound preparations. However, it is liable to cause poisoning because of the overdoses during use [2]. Overdoses of APAP are commonly related to liver and kidney damage in both humans and animals [3,4]. Although APAP-induced liver damage has been studied accurately,further research into the extrahepatic toxicity of APAP has not been detailed well in the literature [5]. Moreover,renal insufficiency occurs in nearly 1–2% of patients with APAP overdoses [6,7].
There are several potential mechanisms of renal toxicity according to both animal and human data. The underlying mechanisms involved the cytochrome P-450 pathway and the enzymes prostaglandin synthetase and N-acetylglucosamine deacetylase [8]. The most probable mechanism of APAP-induced nephrotoxicity involves the reactive toxic metabolite N-acetyl-p-benzoquinone imine(NAPQI), which is formed by the action of cytochrome P-450. The oxidizing metabolite NAPQI is subjected to one-electron reduction and then re-oxidized during cellular metabolism, and this process is coupled with the generation of superoxide [9,10]. At a curative dose of APAP, the amount of formed NAPQI is comparatively small, and the formed NAPQI is detoxified by bonding covalently with glutathione (GSH). On the other hand,overdoses or long-term use of APAP will cause extreme accumulations of NAPQI that will exhaust cellular GSH and induce oxidative stress, which will result in renal injury [11-15]. Thus, we assumed that oxidative damage caused by reactive oxygen species (ROS) might be involved in APAP-induced renal injury, and available antioxidative agents might be able to reduce the adverse effects. However, thus far there have been no drugs for the specific treatment of APAP-induced renal failure [16].Thus, the search for alternative, safe, and therapeutically effective compounds for treating APAP-induced renal toxicity is essential.
Clausenamide is a pyrrolidone derivative and racemic drug isolated from the leaves of Clausena lansium (Lour.)Skeels which has four chiral centers and 16 possible stereoisomers [17]. Clausenamide has exhibited antioxidative, hepatoprotective, antidementia, and antiapoptotic effects in various experimental systems[17-19]. (+)-clausenamide ((+)-CLA, i.e., 3R, 4S, 5S,6R-clausenamide) and (-)-clausenamide (3S, 4R, 5R,6S-clausenamide), which may be separated by enantiomeric separation or asymmetric synthesis, have different functions depending on their chirality [20]. A study showed that (+)-CLA, but not (-)-clausenamide,could markedly increase the content of GSH and the activity of glutathione S-transferase in mice [21].(+)-CLA can significantly enhance the expression of Bcl-2 protein and inhibit apoptosis. This may also be the mechanism whereby brain cells were protected from ischemic damage by treatment with clausenamide [22].However, the protective capacity of (+)-CLA against nephrotoxicity caused by APAP has not been investigated.The aim of the present study was to evaluate the protective effect of (+)-CLA against APAP-induced nephrotoxicity, with an emphasis on antioxidative and antiapoptotic effects, and to elucidate the mechanisms of the protective effect of (+)-CLA.
Materials and Methods
Chemical and Reagents
(+)-Clausenamide was provided by department of medicinal chemistry, Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College (Beijing, China). Mitochondria isolation kit was purchased from Beyotime Biotechnology. Anti-Bcl-2(2870s), anti-Bax (2772s), anti-caspase-3 subunit (9665s),anti-cleaved caspase-3 subunit (9664s), anti-β-actin primary antibodies (AP0060) were purchased from Abcam (MA, USA), anti-Cyt C (4272s), anti-VDAC(4661s) and anti-rabbit HRP secondary antibody(PDR007) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). APAP was purchased from Sigma Aldrich Co. (St. Louis, MO, USA).GSH, MDA, CAT and SOD kits were provided by Nanjing Jiancheng Bioengineering Institute.
Animals
Specific-pathogen-free female C57BL/6 mice (5 weeks old) were obtained from the Center of Experimental Animals of Southern Medical University (Guangzhou,Guangdong, China, Approval ID: SCXK 2013-0002). The animals were housed in plastic cages with corn straw bedding material and maintained in a comfortable environment of 23±2°C and a relative humidity of 50±10% with cycles of equal light and dark. Animal experiments conformed to the Animal Care and Use Committee of Jinan University (Approval No:20150310001), as well as the National Institute of Health’s Guide for the Care and Use of Laboratory Animals (7th edition, USA).
After acclimatization in center of Experimental Animals of Jinan university, mice were divided randomly into the following four groups (n = 6): control group,APAP group, high dose of (+)-clausenamide (HCLA)group, low dose of (+)-clausenamide (LCLA) group.APAP was dissolved in 0.9% sodium chloride and(+)-clausenamide was suspended in 5% Tween-80.Control group and APAP group were received saline solution (0.1 mL/10 g/day) as the vehicle control. HCLA and LCLA groups were respectively administered orally with a high dosage of CLA (100 mg/kg/day) and low dosage of CLA (50 mg/kg/day) for 5 continuous days[23]. All groups except control group were administered with APAP (600 mg/kg) by intraperitoneal injection 15 h later after the last (+)-CLA administration [24]. Mice were anesthetized using diethyl ether vapor to obtain serum and renal tissues for further experiments.
Serum biochemistry
All mice were scarified after 6 h of APAP intraperitoneal injection. The serum was obtained from heart and centrifugated at a speed of 4500 rpm for 10 min and maintained at 4°C. Serum creatinine and blood urea nitrogen (BUN) were determined by automatic biochemical analyzer in clinical laboratory of The First Affiliated Hospital of Jinan University.
The biochemical estimations
The kidneys were carefully separated from the fat and connective tissue. The tissues were rinsed with cold saline. Kidney tissues were mixed with 0.1 M phosphate buffer salt and homogenized at a speed of 1000 rpm with a homogenizer (Remi Equipment Pvt. Ltd., Remi Motors Ltd., Mumbai, India) on ice for 60 s. Supernatant of tissue homogenate was employed to estimate the concentrations of malondialdehyde (MDA) and GSH as well as the activities of superoxide dismutase (SOD) and catalase(CAT). The levels of total protein, MDA and GSH concentrations, SOD and CAT activities in kidney homogenate were determined as previously reported[25-28].
Histopathological examination
Kidney tissues were stored in 4% paraformaldehyde for three days. The specimens were dehydrated in 70% ethyl alcohol overnight by oscillation, 80% ethyl alcohol for 12 h, 90% ethyl alcohol for 2 h, 95% ethyl alcohol for 2 h,100% ethyl alcohol for 30 min, 100% ethyl alcohol for 30 min and transfered into xylene for 30 min (three times).The processes of infiltration and impregnation were carried out by treating with paraffin wax three times, each time for 1 h. Tissue specimens were cut into sections of 5µm thickness and stained with hematoxylin and eosin(H&E). Histopathologic changes were observed using ×20 objectives [29].
Western blotting analysis
For western blotting analysis, the frozen kidney tissues were lysed by RIPA buffer (Beyotime, Shanghai, China)and separated using SDS-PAGE. Subsequently, the proteins resolved in the gel and transferred to the nitrocellulose membrane (Millipore, MA, USA), and blocked in blocking buffer (150 mM NaCl in 10 mM Tris containing 5% non-fat dry milk, pH 7.5) at room temperature for 1 h. Western blots were visualized using the ECL system (Fdbio Science, Hangzhou, China) and Quantity One software (Bio-Rad, USA) was used to analyze the intensity of individual bands. The following antibodies were used in western blotting analysis: B-cell lymphoma 2 (Bcl-2) rabbit pAb (1:1000),Bcl-2-associated X protein (Bax) rabbit pAb (1:1000),caspase-3 rabbit pAb (1:1000), cleaved caspase-3 rabbit pAb (1:1000), cytochrome c (Cyt C) mouse mAb (1:1000)and voltage-dependent anion channels (VDAC) mouse mAb (1:1000) and β-actin rabbit pAb (1:3000). Protein concentration was determined by BCA Protein Assay Kit(Pierce, Rockford, IL).
Statistical analysis
These studies complied with the recommendations on experimental design and analysis in pharmacology.Results are expressed as means ± SD. At least five different experiments were conducted to obtain the data.The statistical differences between the groups were estimated (SPSS, Version 15, USA) using ANOVA for multiple comparisons with Tukey post-hoc test to determine statistical significance. Survival curves were assessed using Kaplan-Meier method and the group differences were estimated using the log-rank test of GraphPad Prism 5 (GraphPad Software, CA, USA).Differences were considered significant when the P value was less than 0.05. All statistical tests were two-sided.
Results
Effects of (+)-CLA on renal function
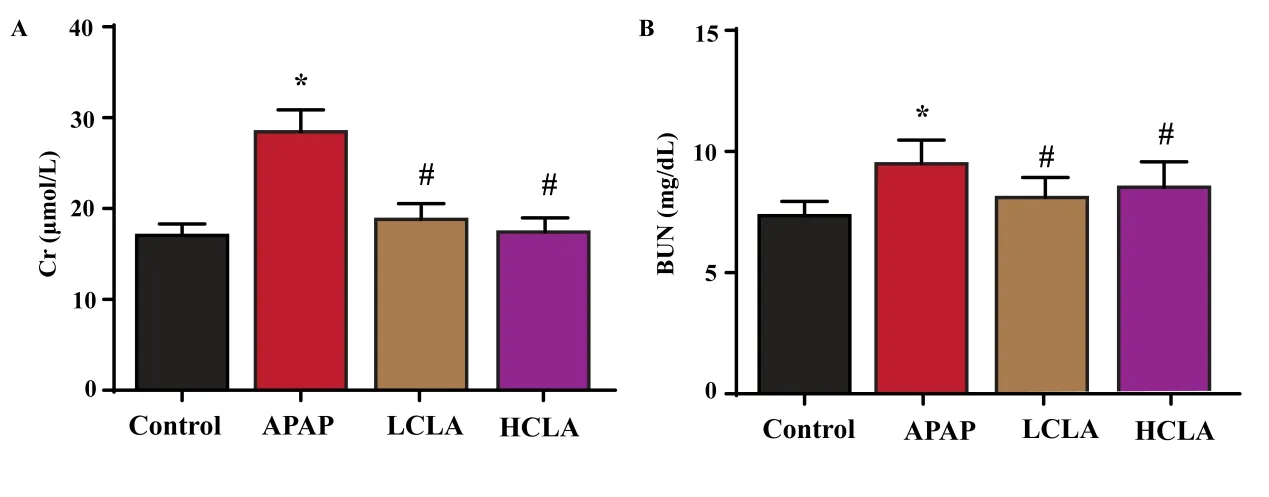
Figure 1 Effects of (+)-CLA on renal function. The levels of Cr (A) and BUN (B) in kidney tissue. The data were expressed as mean ± S.D (n = 6). Data were thought to a statistical significance with *P < 0.05 vs control group; #P< 0.05 vs APAP group.
As shown in Figure 1A and 1B, serum creatinine and BUN levels were increased significantly (P < 0.05) in the APAP-treated group compared with the control group.However, the levels of serum BUN and creatinine were significantly (P < 0.05) decreased in groups treated with HCLA (100 mg/kg/day) and LCLA (50 mg/kg/day)compared with APAP group..
Effects of (+)-CLA on renal histopathology
As shown in Figure 2A, the structure of kidney tissue in the control group was normal. However, kidney tissues from mice treated with APAP showed disordered arrangement of renal cells, collapses and flattens in the epithelial cell of renal tubule and felling off in brush border of lumen surface (Figure 2B). Although these changes were also observed in the HCLA and LCLA group (Figure 2C and 2D), the incidence and severity of histopathologic lesions were significantly lower than those in the APAP group.
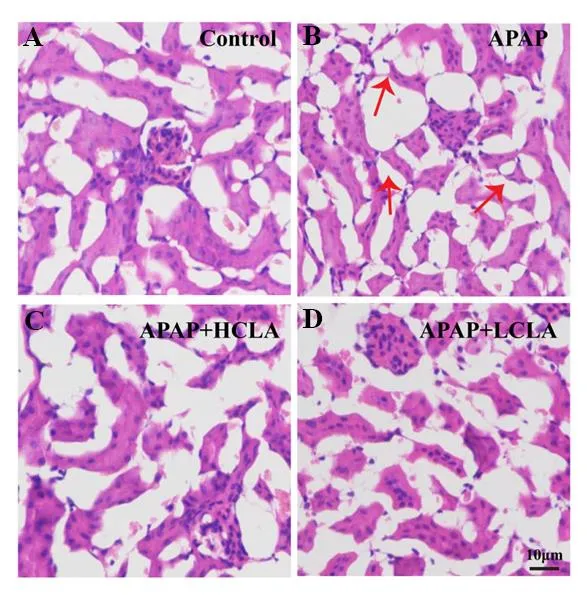
Figure 2 Effects of (+)-CLA on renal histopathology. In APAP group, flat multifocal tubular epithelial cells were pointed out by red arrow. The bar was 10 μm.
Effects of (+)-CLA on antioxidative status
As shown in Figure 3A, renal GSH level was decreased significantly (P < 0.05) in the APAP-treated group when compared with the control group. The level of GSH was significantly (P < 0.05) increased by treatment of HCLA(100 mg/kg/day) and LCLA (50 mg/kg/day) compared with APAP mice.
In addition to the GSH level, the activities of SOD and CAT enzymes in renal tissue were also determined. As shown in Figure 3B and 3C, the group treated with APAP alone showed significantly (P < 0.05) lower renal SOD and CAT activities compared with the control group.Administration of (+)-CLA at both dosages significantly(P < 0.05) increased renal SOD and CAT activities compared with the APAP group.
Effects of (+)-CLA on oxidative stress
As shown in Figure 3D, administration of APAP (600 mg/kg) for 6 h caused a significant (P < 0.05) increase in MDA content. In contrast, administrations with HCLA(100 mg/kg/day) and LCLA (50 mg/kg/day) significantly(P < 0.05) prevented APAP-induced MDA increases, with greater effects demonstrated in HCLA group.
Effects of (+)-CLA on Bcl-2, Bax, Caspase-3, Cleaved Caspase-3 and Cytochrome c protein expression
Results of protein expression of Bcl-2, Bax, caspase-3,cleaved caspase-3, cytochrome c in cytoplasm, and cytochrome c in mitochondria are shown in Figure 4.Administration of APAP caused a significant (P < 0.05 or P < 0.01) increase in Bax, caspase-3, cleaved caspase-3 and cytoplasm cytochrome c expression levels in renal homogenate. The expression levels of Bcl-2 and mitochondrial cytochrome c in the APAP group were reduced significantly compared with the control group. In contrast, administrations with HCLA and LCLA significantly (P < 0.05 or P < 0.01) prevented the increase of APAP-induced Bax, caspase-3, cleaved caspase-3 and cytoplasm cytochrome c expression levels,and the decrease of Bcl-2 and mitochondrial cytochrome c expression.

Figure 3 Effects of (+)-CLA on antioxidative status. The levels of GSH (A), SOD (B), CAT (C) and MDA (D) in kidney tissue were shown. The data were expressed as mean ± S.D (n = 6). Data were thought to a statistical significance with *P < 0.05 vs control group; #P < 0.05 vs APAP group.

Figure 4 Effects of (+)-CLA on Bcl-2, Bax, Caspase-3, Cleaved Caspase-3 and Cytochrome c protein expression.(A) Western blotting analysis of apoptosis pathway proteins in kidney tissue. (B) The relative intensities of apoptosis pathway proteins were quantified using Image J densitometry software. The data were expressed as mean ± S.D (n =3). Data were thought to a statistical significance with **P < 0.01, *P < 0.05 vs control group; ##P < 0.01, #P < 0.05 vs APAP group.
Discussion
APAP is an antipyretic and analgesic drug that is commonly used in clinical practice. It has mild side effects and is safe to use at therapeutic doses. However,an overdose of APAP can cause acute liver and kidney injury, which can even lead to life-threatening liver and kidney failure in severe cases. Therefore, APAP-induced liver and kidney injury has become an essential model of drug-induced acute liver and kidney injury. Researchers have shown that acetylcysteine (NAC), curcumin, and arjunolic acid were used to prevent APAP-induced liver and kidney damage in animal models [3,14,30]. At present, NAC as a safe prodrug of GSH, is the only drug to treat APAP-induced hepatotoxicity in clinical practice.However, it has no obvious protective effect against acute renal injury caused by APAP and even aggravates its renal toxicity [31]. There have been virtually no data on the molecular mechanisms of APAP-induced nephrotoxicity.Therefore, the aim of the present study was to determine the mode of action of (+)-CLA as a preventive agent against APAP-induced renal injury and ascertain the molecular pathways involved in the renal pathophysiology of APAP.
BUN and creatinine levels have been proven to be sensitive indicators of the extent of renal damage [32,33].In the present study, BUN and creatinine levels were significantly increased in serum of APAP treated group,which demonstrated a deterioration in renal function.These alterations were closely correlated with the following histopathological findings: disordered arrangements of renal cells, the collapse and flattening of epithelial cells in renal tubules, and detachment of the brush border of the lumen surface [1]. These findings which were observed in the APAP group, might indicate impaired renal function. The administration of (+)-CLA modified serum BUN and creatinine levels in all mice exposed to APAP, which indicated the renoprotective activity of (+)-CLA against acute renal injury caused by APAP. This phenomenon was also confirmed by a histopathological examination, which showed a decrease in the incidence and severity of renal histopathological lesions.
Oxidative stress has been suggested to play a critical role in cellular toxicity, as well as in the pathophysiology of several diseases [34]. When the generation of ROS exceeds the availably antioxidative capacity, the free radicals can interact with endogenous macromolecules and alter the functions and even the integrity of cells. In the present study, high doses of APAP caused a significant increase in MDA levels and a decrease in GSH levels in mouse renal tissues, with simultaneous inhibition of the antioxidative activities of CAT and SOD.Lipid peroxidation is a well-established mechanism of cellular injury. Lipid hydroperoxides are byproducts of lipid peroxidation, and increases in levels of lipid peroxidation products are associated with the toxicity induced by a variety of chemical agents, including APAP.Lipid hydroperoxides can cause cellular injury by inactivating membrane enzymes and receptors and depolymerizing polysaccharides, as well as breaking protein crosslinks [35]. The overproduction of free radicals in the mice treated with APAP may have triggered lipid peroxidation and consequently increased MDA levels. This may also explain the decline in GSH levels that stored GSH might have been depleted by increased formation of free radicals. APAP also simultaneously decreased the activities of antioxidative enzymes such as CAT and SOD. The administration of(+)-CLA can significantly reverse the APAP-induced increase in MDA levels and the depletion of GSH, with restoration of the normal activities of CAT and SOD in renal tissues, which indicated the powerful antioxidative role of (+)-CLA.
Bcl-2 is a protein that acts to inhibit apoptosis without affecting cellular proliferation [36]. The Bax protein is a member of the Bcl-2 family that promotes apoptosis [37].Aberrations in the Bcl-2 family cause disordered homeostasis, which is a pathogenic event in disease [38].A Bcl-2 mutation plays a critical role in the pathogenesis of polycystic kidney disease in mice [39]. Changes in Bax and Bcl-2 expression have been described in various experimental renal models [40]. Here, the treatment of mice with APAP was shown to upregulate Bax expression and decrease Bcl-2 expression. However, treatment with(+)-CLA reversed the alterations in Bax and Bcl-2 levels.The intrinsic apoptosis pathway results in mitochondrial membrane destabilization, the release of cytochrome c into the cytoplasm, caspase-3 activation, and DNA fragmentation [41]. The results of the present research demonstrate that APAP reduces the mitochondrial membrane potential, which leads to the release of cytochrome c into the cytoplasm, and activates the expression of caspase-3, followed by the cleavage of poly(ADP-ribose) polymerase. (+)-CLA might have potential as a candidate agent against experimental APAP-induced nephrotoxicity via its antiapoptotic and antioxidative activities.
Conclusion
Overall, (+)-CLA exhibited a protective function against APAP-induced oxidative nephrotoxicity in mice. This research also proved that (+)-CLA displayed protective action against APAP-induced nephrotoxicity, which was achieved by limiting the expression of an apoptosis-associated protein. This study reveals that(+)-CLA might serve as a protective agent against varieties of kidney damage induced by oxidative damage and apoptotic activity.
1. Shin JY, Han JH, Ko JW, et al. Diallyl disulfide attenuates acetaminophen-induced renal injury in rats.Lab Anim Res 2016, 32(4): 200-207.
2. Jaeschke H. Acetaminophen: Dose-Dependent Drug Hepatotoxicity and Acute Liver Failure in Patients.Dig Dis 2015, 33(4): 464-471.
3. Ghosh J, Das J, Manna P, et al. Acetaminophen induced renal injury via oxidative stress and TNF-alpha production: therapeutic potential of arjunolic acid. Toxicology 2010, 268(1): 8-18.
4. Yoon E, Babar A, Choudhary M, et al.Acetaminophen-Induced Hepatotoxicity: a Comprehensive Update. J Clin Transl Hepatol 2016,4(2): 131-142.
5. Mazer M, Perrone J. Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J Med Toxicol 2008, 4(1): 2-6.
6. Prescott LF. Paracetamol overdosage.Pharmacological considerations and clinical management. Drugs 1983, 25(3): 290-314.
7. Belde, Mehmet, Demet. Acetaminophen induced nephrotoxicity in an adolescent girl. Turk Pediatri Arşivi 2011, 46(3): 343-345.
8. Bessems JG, Vermeulen NP. Paracetamol(acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 2001, 31(1): 55-138.
9. Cermik H, Taslipinar MY, Aydin I, et al. The relationship between N-acetylcysteine, hyperbaric oxygen, and inflammation in a rat model of acetaminophen-induced nephrotoxicity.Inflammation 2013, 36(5): 1145-1152.
10. Das J, Ghosh J, Manna P, et al. Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology 2010, 269(1): 24-34.
11. Wang EJ, Li Y, Lin M, et al. Protective effects of garlic and related organosulfur compounds on acetaminophen-induced hepatotoxicity in mice.Toxicol Appl Pharmacol 1996, 136(1): 146-154.
12. Schnellmann RG. Toxic responses of the kidney.Casarett & Doulls Toxicology 2001, 6(1): 491-514.
13. Li C, Liu J, Saavedra JE, et al. The nitric oxide donor,V-PYRRO/NO, protects against acetaminophen-induced nephrotoxicity in mice.Toxicology 2003, 189(3): 173-180.
14. Cekmen M, Ilbey YO, Ozbek E, et al. Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem Toxicol 2009,47(7): 1480-1484.
15. Bektur NE, Sahin E, Baycu C, et al. Protective effects of silymarin against acetaminophen-induced hepatotoxicity and nephrotoxicity in mice. Toxicol Ind Health 2016, 32(4): 589-600.
16. Mour G, Feinfeld DA, Caraccio T, et al. Acute renal dysfunction in acetaminophen poisoning. Ren Fail 2005, 27(4): 381-383.
17. Chu SF, Zhang JT. Recent advances in the study of(-)clausenamide: chemistry, biological activities and mechanism of action. Acta Pharm Sin B 2014, 4(6):417-423.
18. Lin TJ, Liu GT, Xiao-Jie LI, et al. Anti-lipid peroxidation and oxygen free radical scavenging activity of clausenamide. Chinese Journal of Pharmacology & Toxicology 1992, 6(2): 97-102.
19. Wu YQ, Liu GT. Protective effect of enantiomers of clausenamides on aflatoxin B1-induced damage of unscheduled DNA synthesis of isolated rat hepatocytes. Chin J Pharmacol Toxicol 2006, 20(5):393-398.
20. Wang XF, Sun YK, Sun K, et al. Review: Separation and Pharmacology of Chiral Compounds in Traditional Chinese Medicine. Analytical Letters 2017, 50(1):33-49.
21. Wu YQ, Liu LD, Wei HL, et al. Different effects of nine clausenamide ennatiomers on liver glutathione biosynthesis and glutathione S-transferase activity in mice. Acta Pharmacol Sin B 2006, 27(8): 1024-1028.
22. Jiang ZC, Bi GN, Shi SL. [Effect of clausenamide on the expression of Bcl-2 protein and apoptosis after focal cerebral ischemia/reperfusion in renovascular hypertensive rats]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2005, 17(5): 289-292.
23. Liu Y, Shi CZ, Zhang JT. Anti-lipid peroxidation and cerebral protective effects of clausenamide. Acta Pharmaceutica Sinica 1991, 26(3): 166-170.
24. Marques PE, Antunes MM, David BA, et al. Imaging liver biology in vivo using conventional confocal microscopy. Nat Protoc 2015, 10(2): 258-268.
25. Berton TR, Conti CJ, Mitchell DL, et al. The effect of vitamin E acetate on ultraviolet-induced mouse skin carcinogenesis. Mol Carcinogen 1998, 23(3):175-184.
26. Moron MS, Depierre JW, Mannervik B. Levels of glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979, 582(1): 67-78.
27. Mccord JM, Fridovich I. Superoxide Dismutase AN ENZYMIC FUNCTION FOR ERYTHROCUPREIN(HEMOCUPREIN). J Biol Chem 1969, 242(22):6049-6055.
28. Aebi H. Catalase in vitro. Method Enzymol 1984,105: 121-126.
29. Honmore V, Kandhare A, Zanwar AA, et al.Artemisia pallens alleviates acetaminophen induced toxicity via modulation of endogenous biomarkers.Pharm Biol 2015, 53(4): 571-581.
30. Şener G, ouml, ksel, et al. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice: a comparative study.J Pineal Res 2003, 35(1): 61-68.
31. Ucar F, Taslipinar MY, Alp BF, et al. The effects of N-acetylcysteine and ozone therapy on oxidative stress and inflammation in acetaminophen-induced nephrotoxicity model. Ren Fail 2013, 35(5): 640-647.
32. Palani S, Kumar RP, Kumar BS. Effect of the ethanolic extract of Indigofera barberi (L.) in acute acetaminophen induced nephrotoxic rats. New Biotechnol 2009, 25(6): S14.
33. Visnagri A, Kandhare AD, Chakravarty S, et al.Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions.Pharm Biol 2014, 52(7): 814-828.
34. Naguib YM, Azmy RM, Samaka RM, et al. Pleurotus ostreatus opposes mitochondrial dysfunction and oxidative stress in acetaminophen-induced hepato-renal injury. BMC Complement Altern Med 2014, 14(1): 494-505.
35. Gale SL, Burritt DJ, Tervit HR, et al. An investigation of oxidative stress and antioxidant biomarkers during Greenshell mussel (Perna canaliculus) oocyte cryopreservation.Theriogenology 2014, 82(6): 779-789.
36. Hockenbery D, Nuñez G, Milliman C, et al. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 384(6299):334.
37. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog,Bax, that accelerates programmed cell death. Cell 1993, 74( 4):609-619.
38. Korsmeyer SJ, Yin XM, Yang E, et al. Korsmeyer SJ.BCL-2 gene family and the regulation of programmed cell death. Cancer Res 1995, 59(7):1693s-1700s.
39. Veis DJ, Sorenson CM, Shutter JR, et al.Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 1993, 75(2): 229-240.
40. Hattori T, Shindo S, Kawamura H. Apoptosis and expression of Bax protein and Fas antigen in glomeruli of a remnant-kidney model. Nephron 1998,79(2): 186-191.
41. Naseri MH, Mahdavi M, Davoodi J, et al. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells.Cancer Cell Int 2015, 15(1): 55.
杂志排行
TMR Modern Herbal Medicine的其它文章
- A novel natural compound Shikonin inhibits YAP function by activating AMPK
- Neuroprotective Effect of Bu-Shen-Huo-Xue Extract against High Glucose-induced Apoptosis in PC12 Cells
- Pharmacological effects of Paeoniflorin and Albiflorin on IL-3, GM-CSF, IL-6 and TNF-α in the rats of syndrome of stagnation of liver qi and blood deficiency
- Effect of splitting combination of different components of Zhilong Huoxue Tongyu Capsule on vascular remodeling in hypertension
- Kangfuxin Fluid on the Treatment of Ulcerative Colitis with Retention Enema: a Systematic Review
- Evidence-based optimization of integrated traditional Chinese and Western medicine therapies for prevention and treatment of coronary heart disease: design and implementation
