桑肠杆菌菌株的富硒特性及其喷施对玉米籽粒的硒素强化
2018-09-03尹佳茗袁红莉谭伟明
乔 虹,尹佳茗,姜 峰,袁红莉,谭伟明
桑肠杆菌菌株的富硒特性及其喷施对玉米籽粒的硒素强化
乔 虹1,尹佳茗1,姜 峰1,袁红莉2,谭伟明1※
(1. 中国农业大学农学院,北京 100093;2.中国农业大学生物学院,北京 100093)
硒是人体必需微量元素之一,对新陈代谢过程有十分重要的促进作用,通过生物强化措施可以增加人体对硒素的补充。该研究利用从植物内生菌中筛选出的一株具有将无机硒转化成生物利用率更高的有机硒的菌株IX+2 2,测定其16S rRNA基因序列,鉴定该菌株为桑肠杆菌 ()。该文研究不同的硒含量、加硒时间与不同培养收集时间对菌株IX+2 2硒素转化率的影响。结果表明,培养基中硒含量越高,菌株IX+2 2胞内有机硒含量越高,硒素转化率越高,但达到某一个峰值后又开始下降;在对数生长期加硒有利于菌株IX+2 2的生长和对无机硒的转化;菌株培养24 h时收集硒素转化效果最好,继续培养菌体生长减弱。同时针对菌株对玉米籽粒富硒的强化效应做了进一步验证。该菌株对玉米籽粒富硒的强化作用表明:同等亚硒酸钠用量条件下,亚硒酸钠喷施处理后玉米籽粒中硒含量达到228.58g/kg,而富硒微生物菌剂处理后籽粒中硒含量达到378.89g/kg,是前者的1.66倍,富硒IX+2 2菌剂的富硒效果优于喷施亚硒酸钠溶液。该研究结果可为玉米籽粒的硒素强化提供理论支撑。
菌;硒;作物;菌株鉴定;富硒特性;硒素强化效应
0 引 言
硒是人类,动物和微生物的重要微量元素。在人体内主要以有机硒的形式存在,包括硒代氨基酸、硒蛋白、甲基硒、硒多糖、核酸硒等多种形式[1-2]。体内的有机硒能够清除自由基、抗氧化、增强人体免疫力及抗癌等作用[3-7]。人体自身无法合成硒,必须通过饮食从外部摄入,然而我国超过72%的地区缺硒严重,主要作物中的含硒量无法满足人类对硒的参考摄入量[8]。因此,富硒食品受到了广泛的关注[9]。
硒在动植物体内的抗氧化反应中有重要的作用[10]。在适当浓度下,硒可以改善抗氧化酶的活性,如过氧化氢酶,超氧化物歧化酶和过氧化物酶等,从而减轻植物叶绿体中的氧化应激反应[11-14]。另外,作物可以利用硒肥促进其生长。外源施硒能促进多种植物的光合色素含量,如:高粱、大麦、酢浆草和莴苣等[15-18]。同时,增施硒肥能有效促进小麦、马铃薯等作物产量的增加[19-20]。
在农业生产中,通过土壤施硒、硒拌种、叶面喷施硒肥等方法是改善作物中硒含量的解决方案之一[21]。土壤施硒肥不易操作,易过量而导致环境污染;硒拌种与叶面喷施硒肥更简单实用。然而,无机硒具有高毒性,吸收后容易在人体内积累[22]。另一方面微生物与硒的生化循环有着重要的联系,微生物能够改变硒的存在形式、增加硒的挥发作用、维持硒的循环。因此,利用微生物将毒性大的无机硒转化为生物利用率更高的有机硒,是安全且高效的生物富硒途径,也受到了国内外学者的普遍重视。徐巧林等[23]对微生物富硒现状研究表明细菌对硒元素有较强的富集作用,可将无机硒转化为有机硒。徐春兰[24]对产硒多糖菌种筛选和鉴定发现,Z0206菌株随着培养基中无机硒浓度的增加,菌体逐渐变红,经鉴定为肠杆菌属。Pieniz等[25]对肠球菌转化无机硒的功能进行了分析,随着培养基中无机硒浓度的增加,硒的生物富集增加并且在24 h后达到了240 mg/L。刘波[26]对富硒纳豆芽孢杆菌的选育试验得出,利用纳豆芽孢杆菌将无机硒转化为有机硒后,进行富硒纳豆的发酵,有助于人体对硒的摄取。宋照军[27]对乳酸菌富硒技术进行了初步研究,通过探讨多种乳酸菌富硒能力,以寻求获得富硒能力优良的乳酸菌菌株和富硒条件,以进一步研究开发富硒活性乳酸菌功能食品,维持人类健康。本研究利用从植物内生菌中分离筛选得到的一株具有将无机硒转化成有机硒的菌株IX+2 2,探究培养基中硒含量、亚硒酸钠添加时间、培养时间等因素对其硒素转化效率的影响,并就菌株对玉米籽粒富硒强化效应做了进一步的验证,为建立安全、高效的富硒谷物生产技术提供依据,具备广泛的实用性。
1 材料与方法
1.1 供试菌株与材料
供试菌株:桑肠杆菌IX+2 2,菌株培养于牛肉膏蛋白胨固体培养基中。
供试玉米品种:郑单958,于中国农业大学吴桥实验站(37°41′N,116°37′E)进行田间试验。实验站土壤硒含量为54.14g/kg,属于贫硒土壤。
菌株种子液的制备:将活化的菌株IX+2 2挑取单菌落接种于已灭菌100 mL牛肉膏蛋白胨液体培养基中,于28 ℃、160 r/min振荡培养24 h备用。
亚硒酸钠母液的制备:将亚硒酸钠配制成100 g/L的溶液后过已灭菌0.22m膜,4 ℃保存备用。
1.2 富硒菌株的筛选及鉴定
1.2.1 富硒菌株的筛选
从植物材料中分离到有硒素转化能力的内生菌株;经复筛后,IX+2 2菌株对无机硒有明显的转化作用,确定该菌株为下一步试验菌株。
1.2.2 富硒菌株的16S rRNA基因序列分析及系统发育树构建
细菌总DNA制备采用水煮法[28]:用无菌枪头挑取少量单菌落菌体重悬于20L无菌ddH2O中,100 ℃水浴10 min,立即于冰上静置,之后于4 ℃、12 000 r/min条件下离心5 min,置于冰上,取1L上清液作为DNA模板。阴性对照以同体积无菌ddH2O为模板,在相同条件下进行PCR扩增。PCR扩增采用的引物为通用引物27F:5′-AGAGTTTGATCCTGGCTCAG-3′和1492R:5′-TACCTTGTTACGACTT-3′。
16S rRNA基因的PCR扩增程序为:94 ℃预变性5 min;94 ℃变性45 s、58 ℃复性45 s、72 ℃延伸2 min,30个循环;72 ℃末端延伸10 min,扩增结束后,对PCR产物进行琼脂糖凝胶电泳。由北京华大怡和生物科技有限公司进行序列测定,将获得的测序结果在GenBank上进行BLAST比对分析,并利用PHYLIP邻位相连法构建系统发育树。
1.2.3 IX+2 2菌株的富硒能力
将1 mL菌株种子液加入亚硒酸钠浓度分别为0、40、90、120、200、300、400g/L的牛肉膏蛋白胨液体培养基中,28 ℃振荡培养24 h,收集菌体,检测胞内有机硒含量。以不含硒的培养基为对照。
1.3 样品硒含量的测定
IX+2 2菌体细胞内总硒含量测定:将收集的IX+2 2菌体破碎后加入10 mL高氯酸与硝酸的混合酸(体积分数1:4),冷消化一定时间后再于电热板上加热,直至液体体积剩余2 mL,冷却,加入5 mL盐酸,继续加热至溶液变清亮,冷却,将其定容至50 mL容量瓶中,充分震荡混匀。利用AFS-920双道原子荧光光度计以及100 mg/L亚硒酸钠标准溶液逐级稀释的标准曲线,计算总硒含量。
无机硒含量测定参照GB1903.21-2016:将收集的IX+2 2菌体破碎后,置于50 mL容量瓶中,加水定容后摇匀。将定容后的溶液5 000 r/min离心10 min,吸取10 mL试样消化液,加入盐酸2 mL、铁氰化钾溶液1 mL,混匀。利用AFS-920双道原子荧光光度计,测定菌体胞内无机硒含量。
有机硒含量利用差减法,计算出菌株胞内有机硒的含量。
有机硒含量=总硒含量-无机硒含量 (1)
1.4 菌株IX+2 2硒素转化率影响因素分析
1.4.1 培养基中硒含量对硒素转化率的影响
将1 mL IX+2 2菌株种子液接入到硒含量分别为0、20、30、45、60、75、100、150、200、250g/L的牛肉膏蛋白胨液体培养基中,在28 ℃,160 r/min振荡培养24 h,测定培养液OD600,离心收集菌体,检测胞内有机硒含量每个处理设3个重复,计算平均值,研究硒含量对硒素转化率的影响。
1.4.2 亚硒酸钠添加时间对硒素转化率的影响
将1 mL IX+2 2种子液接入牛肉膏蛋白胨液体培养基中,分别在菌体培养0、3、6、9、12 h加入一定量的亚硒酸钠母液,使培养基中硒含量为75g/L,于28 ℃、160 r/min振荡培养24 h后,测定培养液OD600,离心收集菌体,检测胞内有机硒含量。
1.4.3 培养时间对硒素转化率的影响
将1 mL IX+2 2菌株种子液接入硒含量为75g/L的牛肉膏蛋白胨液体培养基中,分别在培养12、24、36、48 h时收集菌体,测定培养液OD600,离心收集菌体,检测胞内有机硒含量。
1.5 IX+2 2对玉米富硒的强化效应研究
于中国农业大学吴桥实验站进行。播种时间为2014年5月1日,收获时间为2014年9月29日。具体试验步骤如下:
施用方法:将IX+2 2菌株种子液接入亚硒酸钠浓度为400g/L的牛肉膏蛋白胨液体培养基中,于28 ℃,160 r/min震荡培养24 h后,制备成IX+2 2菌株菌剂,于玉米拔节期叶面喷施。硒矿粉于播种前施入土壤中。亚硒酸钠配制成溶液后,于玉米拔节期叶面喷施。
试验设计:在总结前人研究的基础上,田间试验设5个处理,分别为CK;A1:硒矿粉75 g/m2;B1:亚硒酸钠10 mg/m2;C1:富硒IX+2 2菌剂3.3 mg/m2(培养基中亚硒酸钠用量5 mg);C2:富硒IX+2 2菌剂10 mg/m2(培养基中亚硒酸钠用量10 mg)。采用完全随机区组设计,每个处理小区15 m2,重复4次,每小区喷施500 mL。玉米成熟后,取风干籽粒磨碎成粉末,按照1.3节测定籽粒硒含量。
2 结果与分析
2.1 IX+2 2菌株鉴定
对IX+2 2菌株16S rRNA基因PCR扩增产物进行琼脂糖凝胶电泳检测,结果显示阴性对照未出现任何条带,表明整个PCR操作过程中未有外源DNA污染(图1)。

图1 菌株IX+2 2 16S rRNA基因PCR扩增琼脂糖凝胶电泳图
16S rRNA基因PCR扩增的片段长度为1 500 bp,在GenBank数据库的BLAST比对结果显示,其与菌株桑肠杆菌(GL890774)的16S rRNA基因序列同源性达99.45%。对测得的基因型序列进行系统发育分析(图2)。发现,菌株IX+2 2与肠杆菌属()聚到了一个簇,亲缘关系相近。确定菌株为肠杆菌属()。表1为IX+2 2菌株16S rRNA基因片段BLAST比对结果。

图2 菌株IX+2 2 16S rRNA基因的系统发育树

表1 IX+2 2菌株16S rRNA基因片段BLAST比对结果
2.2 菌株IX+2 2富硒能力研究
将菌株种子液加到不同亚硒酸钠浓度的牛肉膏蛋白胨液体培养基中培养24 h后,对菌株的生长量和胞内硒含量进行测定后发现(图3),菌株IX+2 2富硒能力随亚硒酸钠浓度的增加呈上升趋势,在硒浓度低于120g/L时,菌体胞内有机硒含量缓慢上升到17.8g/L,随后亚硒酸钠浓度增加,菌体胞内有机硒含量迅速增加;在亚硒酸钠浓度为400g/L时,胞内有机硒含量达到最大值141.68g/L。菌株在亚硒酸钠浓度0~400g/L的条件下均可生长,表明该菌株耐硒能力较强。
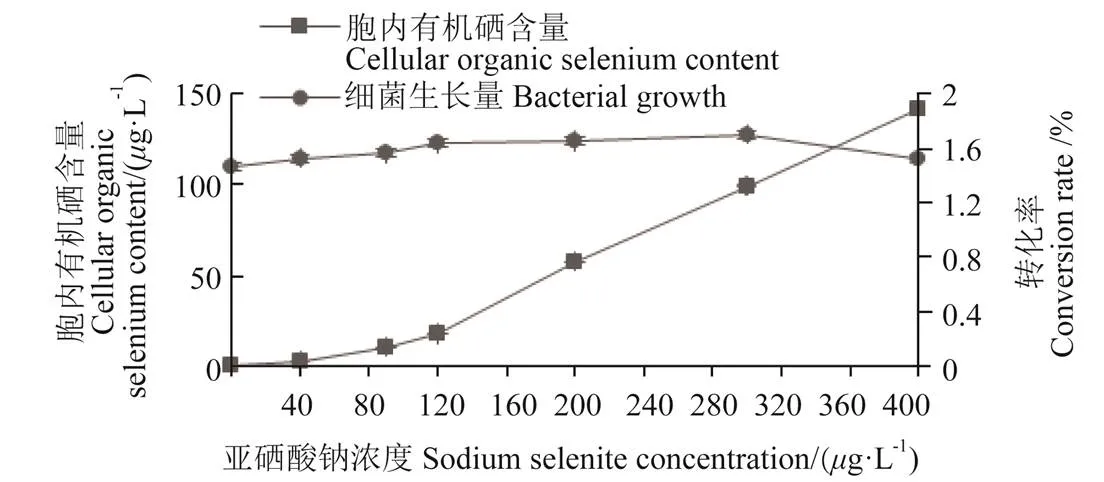
注:转化率为有机硒转化率,下同。
2.3 菌株IX+2 2硒素转化率影响因素探究
2.3.1 培养基中硒含量对硒素转化率的影响
将1 mL IX+2 2种子液接入到不同硒含量的牛肉膏蛋白胨液体培养基中培养24 h后,对菌株IX+2 2的生长量、胞内硒含量进行测定并计算有机硒转化率(表2)发现,菌株IX+2 2在硒含量为250/L时仍可生长;随着培养基中硒含量的增加,胞内有机硒含量逐渐增加,但是有机硒转化率呈现先升高后降低的趋势。在硒含量为200/L时,转化率最高,达到70.84%(<0.05)。当硒含量为250g/L时,菌株细胞内有机硒含量达到最大值,为163.45g/L(<0.05),但菌株生长明显受到抑制,转化率也随之降低,说明培养基中硒含量在适宜范围才会表现较高硒素转化能力[29]。
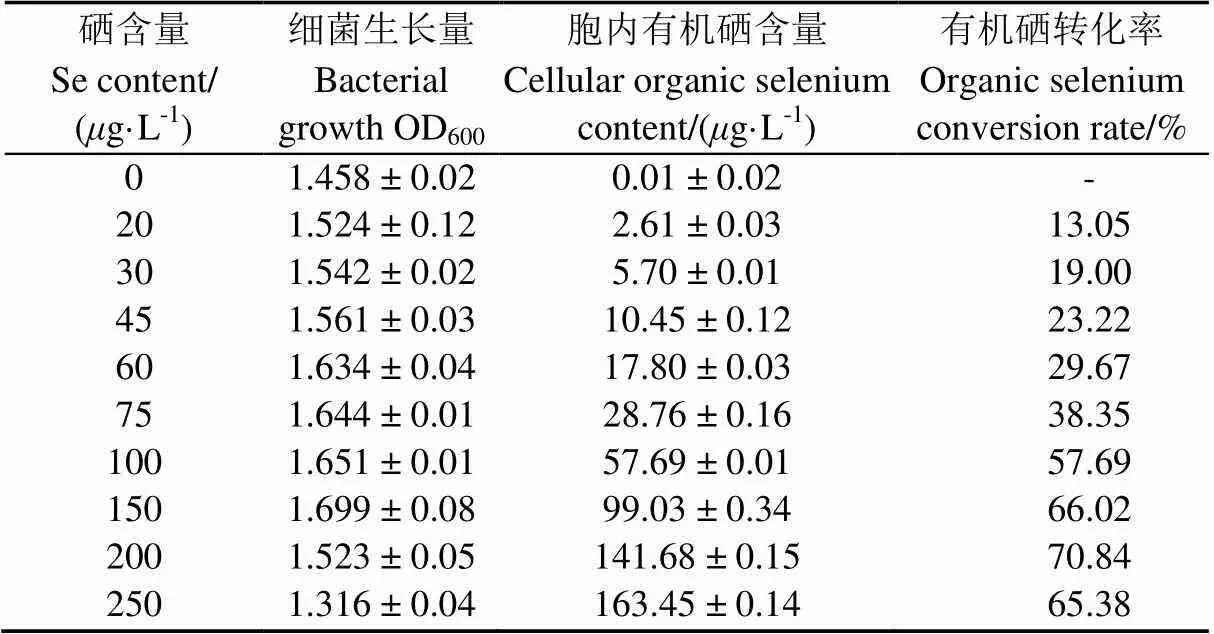
表2 硒含量对菌体富硒效果的影响
注:硒素转化率=胞内有机硒含量/培养基中硒含量×100%
Notes: Organic selenium conversion=cellular organic selenium content/selenium content in the medium ×100%
2.3.2 亚硒酸钠添加时间对硒素转化率的影响
在本研究中,亚硒酸钠添加时间可以影响菌株的硒素转化率,分别在菌体培养0、3、6、9、12 h添加亚硒酸钠,使培养基的硒含量为75g/L,培养24 h后,菌株IX+2 2的生长量和胞内有机硒含量见图4,在0~12 h时间范围内亚硒酸钠添加时间对菌株IX+2 2的生长情况影响较小,但是菌株的富硒效率随添加时间呈先上升后下降趋势,在菌株培养3 h时添加亚硒酸钠,菌株胞内有机硒含量迅速上升到49.93g/L,硒素转化率达到最高值66.57%。此后随着培养时间增加,菌体胞内有机硒含量逐渐减少,硒素转化率也逐渐降低至40.20%。
菌株硒素转化率以培养3 h时添加亚硒酸钠最高,可能是由于此时菌株生长处于对数期,菌体生长旺盛[30]。
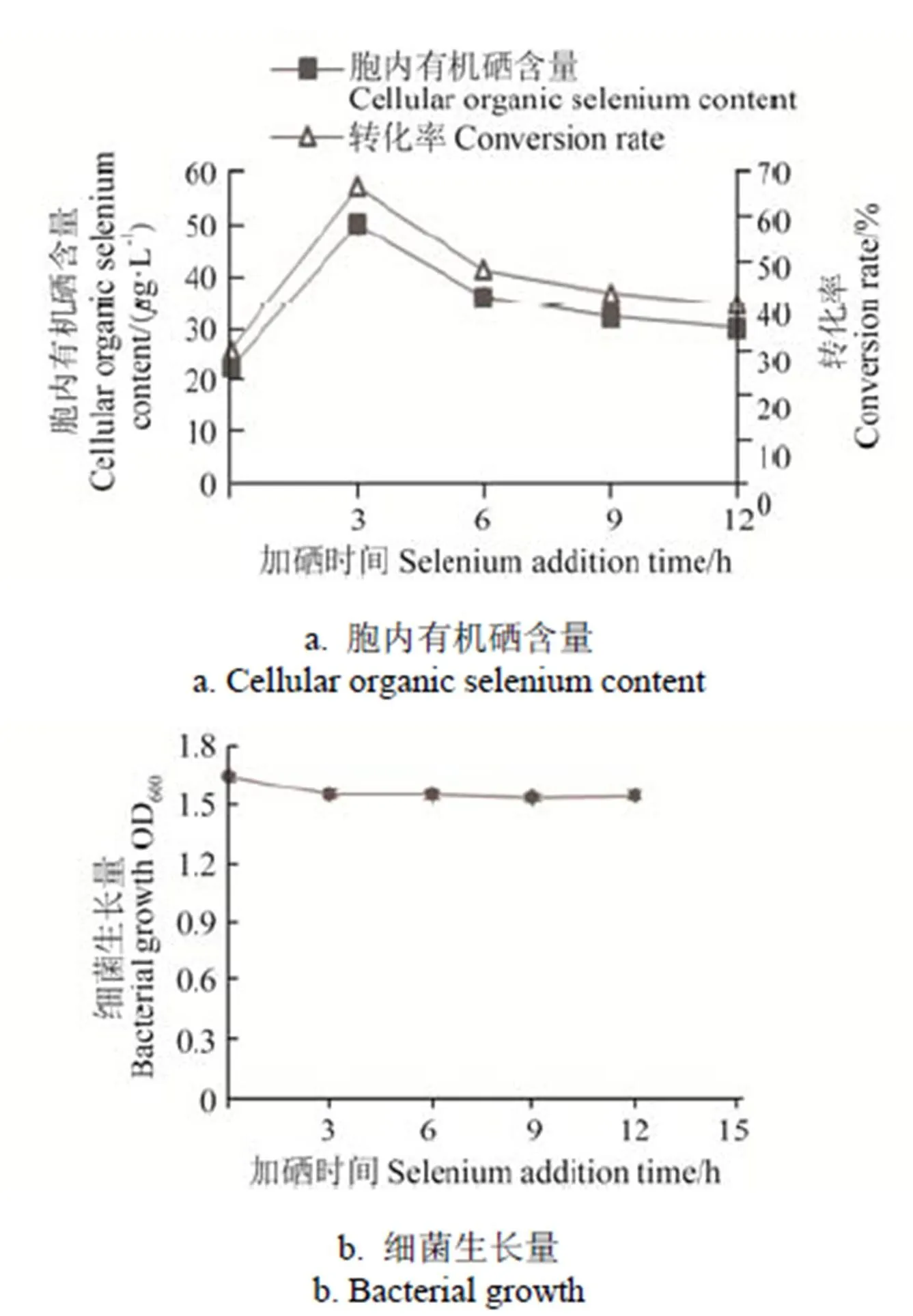
图4 亚硒酸钠添加时间对IX+2 2菌株富硒效率的影响
2.3.3 培养时间对硒素转化率的影响
在培养12、24、36、48 h时收集菌体,菌株IX+2 2的生长量和胞内有机硒含量见图5。由图5可知,菌株IX+2 2随着培养时间的增加,硒转化率先升高后降低,在培养24 h时转化效率最高,为72.10%,随着培养时间增加,菌体生长减弱及部分菌体自溶,转化效率也随之下降。
2.4 IX+2 2对玉米籽粒富硒的强化效应研究
对比硒矿粉、亚硒酸钠和富硒IX+2 2菌剂不同硒源处理对玉米籽粒富硒效果的影响见图6。由图6可知,以富硒IX+2 2菌剂作为硒源时,玉米籽粒中硒含量最高,为378.89g/kg,达到富硒谷物的标准。从富硒效率看,硒矿粉富硒效率最低,富硒IX+2 2菌剂富硒效率最高。同等施加量下,喷施亚硒酸钠玉米籽粒中硒含量为228.58g/kg,与喷施低浓度IX+2 2菌剂富硒效果差异不显著,富硒IX+2 2菌剂为硒源比普通喷施硒含量高1.66倍。
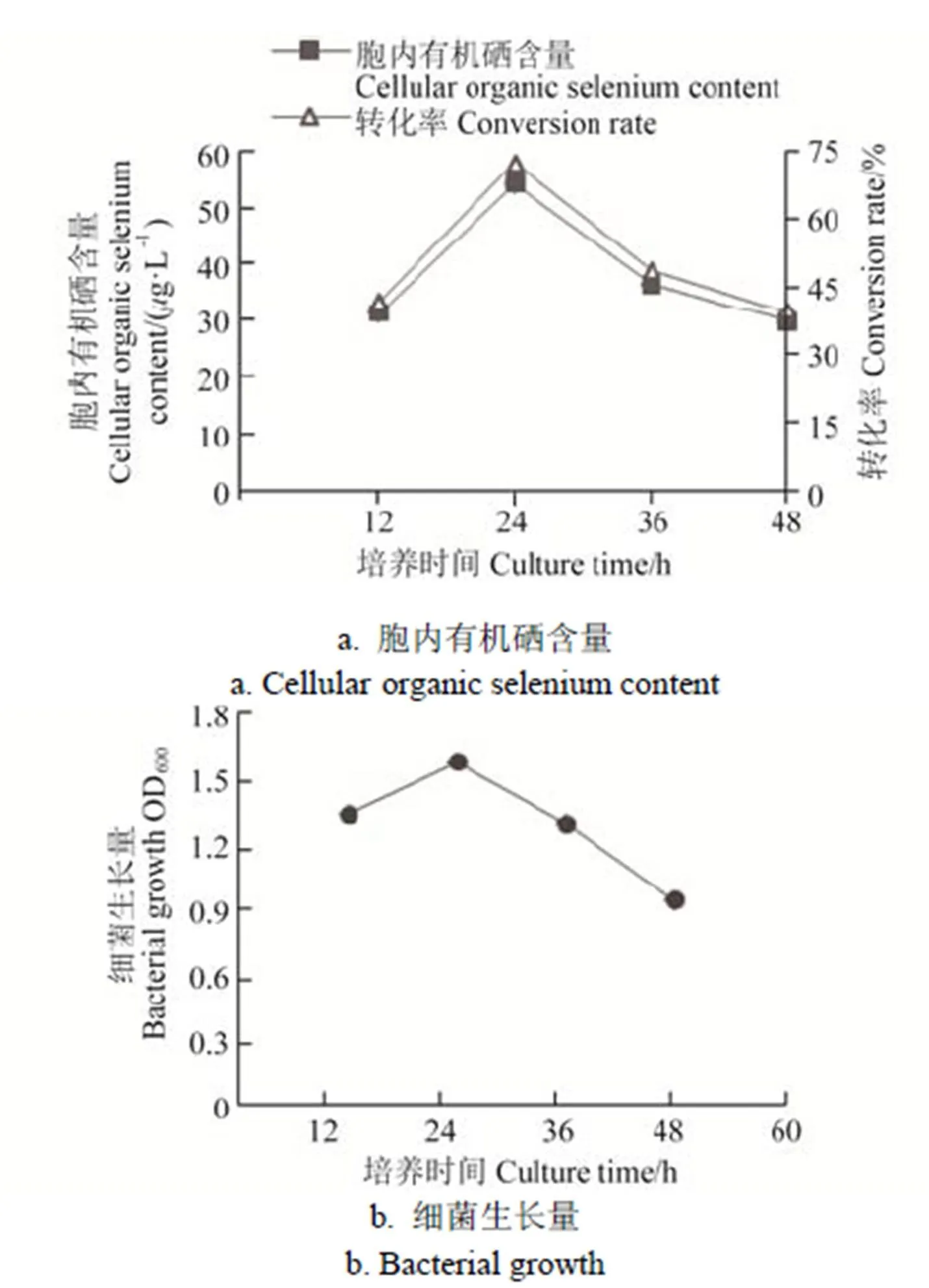
图5 培养时间对IX+2 2菌株富硒效率的影响

注:A1:硒矿粉75 g·m-2;B1:亚硒酸钠10 mg·m-2;C1:富硒IX+2 2菌剂3.3 mg·m-2;C2:富硒IX+2 2菌剂10 mg·m-2。
3 讨 论
本研究表明该菌株有较好的耐硒能力。培养基中亚硒酸钠浓度0~400g/L的条件下均可生长。当培养基中亚硒酸钠浓度为400g/L时,胞内有机硒含量达到最大值141.68g/L。王浩阳[31]研究了967株耐硒菌表明,不同亚硒酸盐浓度处理下,肠杆菌属为耐硒优势种群。
对菌株IX+2 2硒素转化率的影响因素进行探究,硒含量、加硒时间、培养时间等因素会对其硒素转化率产生影响。培养基中硒含量对菌株的硒素转化率呈单峰曲线,在适宜范围内表现较高硒素转化能力。IX+2 2菌株在培养基硒含量为200g/L时,其硒素转化效果最好,转化率达到70.84%。培养基中硒含量继续增加,菌株硒素转化率下降。这与杨丽华等[29]和Zhang等[32]报道的结果一致。相同硒含量采用不同的添加时间会产生不同的结果。在培养3 h,菌株生长处于对数期时加入亚硒酸钠,硒素转化效果最好,转化率可以达到66.57%。此后随着培养时间增加,硒素转化率也逐渐降低。吴竞对比啤酒酵母不同添加时间得出,在对数期8 h添加亚硒酸钠的有机硒转化率高于其他一次性添加方式的转化率[30]。
对比不同硒源对玉米籽粒的富硒强化效应,有机硒处理较亚硒酸钠有较好的富硒效果。同等亚硒酸钠用量条件下,喷施亚硒酸钠籽粒中硒含量达到228.58g/kg,而施加富硒IX+2 2菌剂籽粒中的硒含量达到378.89g/kg。李玉梅[33]以酵母硒作为有机硒硒源,叶面喷施后对水稻籽粒的硒含量进行检测,结果表明喷施有机硒120 g/hm2时,大米中硒含量达0.74 mg/kg。植物对有机硒的吸收和利用机理的研究较少。硒代蛋氨酸是谷物籽粒中的主要存在形态,不同种类的植物对有机硒的吸收利用率普遍高于硒酸盐和亚硒酸盐[34]。邓坤[35]研究水稻根系对硒代蛋氨酸的吸收转运机制表明当对植物外源同时供应硒代蛋氨酸和蛋氨酸时,蛋氨酸能显著影响植物对硒代蛋氨酸的吸收,因此,推测植物根系可能是通过蛋氨酸转运蛋白来吸收利用硒代蛋氨酸。研究发现,喷施较高浓度IX+2 2菌剂玉米籽粒中的硒含量达到378.89g/kg,达到富硒谷物的标准[36]。相比于硒矿粉及喷施亚硒酸钠,富硒IX+2 2菌剂不仅可以提高富硒效率,而且将大部分具有毒性的无机形态的硒被转化成有机硒,为建立安全、高效的富硒谷物生产技术提供了依据。
4 结 论
1)本研究从植物内生菌中分离筛选到一株能够将无机硒转化为有机硒的细菌,经分子生物学鉴定为桑肠杆菌()。按1%接种量,培养在亚硒酸钠浓度0~400g/L的牛肉膏蛋白胨液体培养基中,菌株均可生长,表明该菌株耐硒能力较强。
2)桑肠杆菌IX+2 2菌株硒素转化率影响因素探究
研究表明硒含量、加硒时间、培养时间等因素会对菌株IX+2 2的硒素转化率产生影响。培养基中硒含量在适宜范围内表现较高硒素转化能力。相同硒含量采用不同的添加时间会产生不同的结果,在菌株生长处于对数期时加入亚硒酸钠,硒素转化效果最好。培养时间增加,菌株生长逐渐变弱,硒素转化率也逐渐降低。
3)桑肠杆菌IX+2 2对玉米籽粒富硒强化效应研究同等亚硒酸钠用量条件下,富硒微生物菌剂处理后籽粒中硒含量是亚硒酸钠喷施处理后玉米籽粒中硒含量的1.66倍。富硒微生物菌剂处理较亚硒酸钠有更好的富硒效果。
[1] Brigelius-Flohé R. Tissue-specific functions of individual glutathione peroxidases[J]. Free Radical Biology & Medicine, 1999, 27(9/10): 951.
[2] Huang Z, Xiang J J, Guo B J. Progress in molecular biology research of selenoproteins[J]. Progress in Biochemistry and Biophysics, 2001, 28(5): 642-645.
[3] 仝宗喜,康世良,武瑞. 硒及硒蛋白生物学作用的研究进展[J]. 动物医学进展,2002,23(6):17-19. Tong Zongxi, Kang Shiliang, Wu Rui. Advances of researches on biological effects of selenium and selenoproteins[J]. Progress in Veterinary Medicine, 2002, 23(6): 17-19. (in Chinese with English abstract)
[4] Reid M E, Duffield-Lillico A J, Slate E, et al. The nutritional prevention of cancer: 400 mg per day selenium treatment[J]. Nutrition & Cancer, 2008, 60(2): 155-163.
[5] Cheng Y Y, Qian P C. The effect of selenium-fortified table salt in the prevention of Keshan disease on a population of 1.05 million[J]. Biomedical & Environmental Sciences Bes, 1990, 3(4): 422.
[6] Wallace K, Kelsey K T, Schned A, et al. Selenium and risk of bladder cancer: A population-based case-control study.[J]. Cancer Prevention Research, 2009, 2(1): 70-73.
[7] Clark L C. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: A randomized controlled trial[J]. Jama the Journal of the American Medical Association, 1997, 277(19): 1520.
[8] 石爱华,彭祚全,张妍艳,等. 我国富硒大米的研究与开发[J]. 微量元素与健康研究,2015,32(1):31-32.
[9] 杨旭,董文宾. 富硒食品的研究进展[J]. 食品安全质量检测学报,2017,8(6):2091-2097. Yang Xu, Dong Wenbin. Research progress of Se-enriched food[J]. Journal of Food Safety & Quality, 2017, 8(6): 2091-2097. (in Chinese with English abstract)
[10] Jing Dawei, Du Zhenyu, Ma Hailin, et al. Selenium enrichment, fruit quality and yield of winter jujube as affected by addition of sodium selenite[J]. Scientia Horticulturae, 2017, 225: 1-5.
[11] Vitova M, Bisova K, Hlavova M, et al. Glutathione peroxidase activity in the selenium-treated alga Scenedesmus quadricauda[J]. Aquatic Toxicology, 2011, 102(1/2): 87-94.
[12] Feng R W, Wei C Y. Antioxidative mechanisms on selenium accumulation inL., a potential selenium phytoremediation plant[J]. Plant Soil and Environment, 2012, 58(3): 105-110.
[13] Feng T, Chen S S, Gao D Q, et al. Selenium improves photosynthesis and protects photosystem II in pear (Pyrus bretschneideri), grape (Vitis vinifera), and peach (Prunus persica)[J]. Photosynthetica, 2015, 53(4): 609-612.
[14] Hartikainen H, Xue T L, Piironen V. Selenium as an anti-oxidant and pro-oxidant in ryegrass[J]. Plant and Soil, 2000, 225(1/2): 193-200.
[15] Akbulut M, Cakir S. The effects of Se phytotoxicity on the antioxidant systems of leaf tissues in barley (L.) seedlings[J]. Plant Physiology and Biochemistry, 2010, 48(2/3): 160-166.
[16] Djanaguiraman M, Prasad P V V, Seppanen M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system[J]. Plant Physiology and Biochemistry, 2010, 48(12): 999-1007.
[17] Kong L A, Wang M, Bi D L. Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress[J]. Plant Growth Regulation, 2005, 45(2): 155-163.
[18] Xue T L, Hartikainen H, Piironen V. Antioxidative and growth-promoting effect of selenium on senescing lettuce[J]. Plant and Soil, 2001, 237(1): 55-61.
[19] Yasin M, El-Mehdawi A F, Anwar A, et al. Microbial-enhanced selenium and iron biofortification of wheat (L.) applications in phytoremediation and biofortification[J]. International Journal of Phytoremediation, 2015, 17(4): 341-347.
[20] Turakainen M, Hartikainen H, Seppänen M M. Effects of selenium treatments on potato (L.) growth and concentrations of soluble sugars and starch[J]. Journal of Agricultural and Food Chemistry, 2004, 52(17): 5378-5382.
[21] 李圣男,岳士忠,乔玉辉,等. 中国富硒玉米的生产与富硒效应[J]. 中国农学通报,2014,30(30):6-10. Li Shengnan, Yue Shizhong, Qiao Yuhui, et al. Studies on Se-enriched maize production[J]. Chinese Agricultural Science Bulletin, 2014, 30(30): 6-10. (in Chinese with English abstract)
[22] Wang Qi, Yu Yao, Li Jixiang, et al. Effects of different forms of selenium fertilizers on Se accumulation, distribution, and residual effect in winter wheat-summer maize rotation system[J]. Journal of Agricultural and Food Chemistry, 2017, 65(6): 1116-1123.
[23] 徐巧林,吴文良,赵桂慎,等. 微生物硒代谢机制研究进展[J]. 微生物学通报,2017(1):207-216. Xu Qiaolin, Wu Wenliang, Zhao Guishen, et al. Selenium metabolism in microorganisms[J]. Microbiology China, 2017(1): 207-216. (in Chinese with English abstract)
[24] 徐春兰. Enterobacter cloacae z0206富硒多糖的制备、结构分析及其主要生物学功能研究[D]. 杭州:浙江大学,2008. Xu Chunlan, Preparation, Structural Analysis and Biological Function Research of Selenium-enriched Exopolysaccharide Produced by Bacterium Enterobacter cloacae Z0206[D]. Hangzhou: Zhejiang University. 2008. (in Chinese with English abstract)
[25] Pieniz S, Andreazza R, Pereira J Q, et al. Production of selenium-enriched biomass by enterococcus durans[J]. Biological Trace Element Research, 2013, 155(3): 447-454.
[26] 刘波. 富硒纳豆芽孢杆菌的选育试验[J]. 北方园艺,2009(1): 215-216. Liu Bo. The screening experiment of baccillus subtilis natto enriching selenium[J]. Northern Horticulture, 2009(1): 215-216. (in Chinese with English abstract)
[27] 宋照军. 乳酸菌富硒技术初步研究[J]. 食品科学,2004,25(9):137-140. Song Zhaojun. Technology study on enriching selenium(Se) in lactobacillus strains[J]. Food Science, 2004, 25(9): 137-140. (in Chinese with English abstract)
[28] 李海峰,李志建,屈建航. 高效聚磷鞘氨醇杆菌XF-5的分离与鉴定[J]. 河南农业科学,2012(9):68-72. Li Haifeng, Li Zhijian, Qu Jianhang. Isolation and identification of a high-efficient phosphate accumulatingstrain XF-5[J]. Journal of Henan Agricultural Sciences, 2012(9): 68-72. (in Chinese with English abstract)
[29] 杨丽华,代国庆,马兴. 酵母对无机硒的富集及其影响因素[J]. 食品工程,2006(4):37-39. Yang Lihua, Dai Guoqing, Ma Xing. Inorganic selenium concentrated in yeast and influencing factors[J]. Food Engineering, 2006(4): 37-39. (in Chinese with English abstract)
[30] 吴竞,王阳光,刘永杰,等. 不同酵母菌种富硒能力比较与发酵条件优化[J]. 畜牧与兽医,2012,44(1):15-18. Wu Jing, Wang Yangguang, Liu Yongjie, et al. Comparison on selenium-enriched ability of different strains of yeast and optimization of fermentation conditions[J]. Animal Husbandry& Veterinary Medicine, 2012, 44(1): 15-18. (in Chinese with English abstract)
[31] 王浩阳. 耐硒细菌的筛选与鉴定[D]. 北京:中国农业大学,2015. Wang Haoyang. Isolation and Identification of Selenium-tolerance Bacteria[D]. Beijing: China Agricultural University. 2015. (in Chinese with English abstract)
[32] Zhang B, Zhou K, Zhang J, et al. Accumulation and species distribution of selenium in Se-enriched bacterial cells of the Bifid bacterium animals[J]. Food Chemistry, 2009, 115(2): 727-734.
[33] 李玉梅,王根林,李艳,等. 水稻对有机态硒的吸收与积累[J]. 中国农学通报,2017(10):7-11. Li Yumei, Wang Genlin, Li Yan, et al. Organic selenium absorption and accumulation of rice[J]. Chinese Agricultural Science Bulletin, 2017(10): 7-11. (in Chinese with English abstract)
[34] Kikkert J, Berkelaar E. Plant uptake and translocation of inorganic and organic forms of selenium[J]. Archives of Environmental Contamination and Toxicology, 2013, 65(3): 458-465.
[35] 邓坤. 水稻根系吸收和转运硒代蛋氨酸的机制研究[D]. 洛阳:河南科技大学,2015. Deng Kun. Study on mechanism of uptake and translocation of selenomethionine by rice roots[D]. Luoyang: Henan University of Science and Technology. 2015.(in Chinese with English abstract)
[36] 彭祚全,张欣,牟敏,等. 富硒食品含硒量范围标准的研究[J]. 微量元素与健康研究,2013(1):41-43.
Se-enrichment characteristics ofand its Se strengthening effect on corn grain
Qiao Hong1, Yin Jiaming1, Jiang Feng1, Yuan Hongli2, Tan Weiming1※
(1.100193,; 2.100193,)
Selenium is an important trace element in humans, animals and microorganisms. In the human body, it is mainly in the form of organic selenium, which plays an important role in promoting the metabolic process. Organic selenium in the body can scavenge free radicals, resist oxidation, enhance human immunity and fight cancer. The body itself cannot synthesize selenium, and it must be consumed from the outside through diet. However, there are serious shortages of selenium in over 72% of regions in China, and the selenium content in the main crops cannot meet the human reference intake of selenium. Therefore, selenium-enriched foods have received extensive attention. Selenium plays an important role in antioxidant reactions in plants and animals. At appropriate concentrations, selenium can improve the activity of antioxidant enzymes, such as catalase, superoxide dismutase, and peroxidase, etc. in order to reduce oxidative stress in the plant chloroplast, and enhance plant growth. In addition, exogenous application of selenium can promote the photosynthetic pigment content of various plants. In this study, a strain IX+2 2 was previously selected from plant endophytes for transforming inorganic selenium into organic selenium with higher bioavailability. By determining its 16S rRNA gene sequence, the strain was identified as Enterobacter mori. This paper studied the effect of different selenium content, selenium addition time and different culture collection time on intracellular selenium content and selenium conversion rate of strain IX+2 2. The results showed that the selenium content in the medium had a great influence on the growth and selenium conversion ability of strain IX+2 2. With the increase of selenium content in the medium, the intracellular selenium content and selenium conversion rate gradually increased, but after reaching a certain peak, they begins to decline again. When the selenium content was 200g/L, the conversion rate was the highest, reaching 70.84%. When the selenium content was 250g/L, the selenium content in the cells reached the maximum, which was 163.45g/L, but the growth of the strain was significantly inhibited, and the conversion rate also decreased, It shows that the selenium content in the medium will show a high selenium conversion ability in a suitable range. The addition of selenium in the logarithmic phase favors the growth of strain IX+2 2 and the conversion of inorganic selenium. Sodium selenite addition time had little effect on the growth of strain IX+2 2 in the time range of 0-12 h, however, the selenium-enrichment efficiency of strains first increased and then decreased with the addition time. Sodium selenite was added to the strains at 3 h, and the intracellular selenium content of the strains rapidly increased to 49.93g/L, selenium conversion was the best, and the conversion rate could reach 66.57%. After that, with the increase of culture time, the intracellular selenium content of the cells gradually decreased, and the conversion rate of selenium also gradually decreased to 40.20%. The selenium conversion rate of the strain was highest when sodium selenite was added at 3 h, which may be due to the growth of the strain in logarithmic phase and the vigorous growth of the bacteria. When the strain was cultured for 24 h, the selenium conversion rate was best, and the growth of the cultured cells continued to decrease, with the increase of culture time, the selenium conversion rate of strain IX+2 2 increased first and then decreased. The highest transformation efficiency was 72.10% at 24 hours of culture. As the culture time increased, the growth of the bacteria decreased and some of the bacteria autolyzed, and the transformation efficiency also decreased. Simultaneously, the ability of the strain to strengthen selenium in corn kernel shows that the selenium transformation efficiency of the selenium enriched inoculate solution is better than application of sodium selenite with foliage spray. The corn kernel selenium content were noted 228.58g/kg when equal quantity of sodium selenite was applied with foliage spray while the microorganisms agent get to378.89g/kg, the latter was 1.66 times higher than the former.
bacteria;selenium; crops; bacteria identification;selenium rich characteristics;strengthening effect of selenium
2018-04-07
2018-05-27
公益性行业(农业)科研专项(201303106)
乔 虹,主要从事作物栽培与研究。Email:qiaohng@cau.edu.cn
谭伟明,副教授,博士生导师,主要从事作物生理与栽培研究。Email:tanwm@cau.edu.cn
10.11975/j.issn.1002-6819.2018.17.037
Q93
A
1002-6819(2018)-17-0284-07
乔 虹,尹佳茗,姜 峰,袁红莉,谭伟明. 桑肠杆菌菌株的富硒特性及其喷施对玉米籽粒的硒素强化[J]. 农业工程学报,2018,34(17):284-290. doi:10.11975/j.issn.1002-6819.2018.17.037 http://www.tcsae.org
Qiao Hong, Yin Jiaming, Jiang Feng, Yuan Hongli, Tan Weiming. Se-enrichment characteristics ofand its Se strengthening effect on corn grain[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(17): 284-290. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2018.17.037 http://www.tcsae.org
