Low dose oral haloperidol does not prolong QTc interval in older acutely hospitalised adults: a subanalysis of a randomised double-blind placebo-controlled study
2018-08-17EdmJMSchrijverMaaikeVerstraatenPetervandeVenPierreBetAstridvanStrienCareldeCockPrabathWBNanayakkaraonbehalfofallHARPOONInvestigators
Edmée JM Schrijver, Maaike Verstraaten, Peter M van de Ven, Pierre M Bet, Astrid M van Strien,Carel de Cock, Prabath WB Nanayakkara*; on behalf of all HARPOON Investigators
Low dose oral haloperidol does not prolong QTc interval in older acutely hospitalised adults: a subanalysis of a randomised double-blind placebo-controlled study
Edmée JM Schrijver1, Maaike Verstraaten2,*, Peter M van de Ven3, Pierre M Bet4, Astrid M van Strien5,Carel de Cock2, Prabath WB Nanayakkara1*; on behalf of all HARPOON Investigators
1Department of Internal Medicine, VU University Medical Centre, Amsterdam, the Netherlands2Department of Cardiology, VU University Medical Centre, Amsterdam, the Netherlands3Department of Epidemiology and Biostatistics, VU University Medical Centre, the Netherlands4Department of Clinical Pharmacology and Pharmacy, VU University Medical Centre, the Netherlands5Department of Geriatric Medicine, Jeroen Bosch Hospital, ‘s-Hertogenbosch, the Netherlands
Haloperidol is the most frequently prescribed antipsychotic for delirium symptoms. The risk of QTc prolongation often raises concerns, although the effect of haloperidol on QTc interval has not yet been investigated in a randomised placebo-controlled fixed-dose study.A subanalysis of a randomised double-blind placebo-controlled study was conducted to evaluate the effect of prophylactic haloperidol 1 mg or placebo 1 mg orally twice-daily (maximum of 14 doses) on QTc interval in patients aged 70 years and over. Bedside, 12-lead ECGs were recorded before, during and after the one-week intervention period. Automatic QTc measurements were obtained in addition to manual measurements of QT and RR intervals, blinded for treatment status. Manual measurements were corrected (QTc) using Bazett (QTc-B), Framingham (QTc-Fa), Fridericia (QTc-Fi) and Hodges (QTc-H) methods. Mixed model analyses were used to test for differences in longitudinal course of QTc between patients receiving haloperidol and placebo.ECG recordings of 72 patients (haloperidol= 38) were analysed, 45.8% male. Median (range) haloperidol serum concentration on day 4 was 0.71 (0.32–1.82) µg/L (= 23). Longitudinal course of mean QTc did not significantly differ between treatment arms for any of the automatic or manually derived QTc values.Low dose oral haloperidol did not result in QTc prolongation in older acutely hospitalised patients. Results may not be generalizable to patients with existing ECG abnormalities such as atrial fibrillation.
J Geriatr Cardiol 2018; 15: 401407. doi:10.11909/j.issn.1671-5411.2018.06.003
Haloperidol; Prolongation; QTc interval; The aged
1 Introduction
Haloperidol is the most frequently prescribed antipsychotic drug for the treatment of hyperactive-type delirium worldwide with prescription rates of over 5% in older surgical patients.[1]The recommended starting dose is mainly based on consensus and is 0.5 to 1.0 mg orally for older patients.[2,3]Haloperidol is associated with QT interval prolongation which generally raises concerns because of the potential risk for torsade de pointes (TdP) and sudden death.[4,5]In the Rotterdam Study, QTc [QT interval corrected for heart rate (HR)] prolongation was associated with an approximately twofold increased risk for cardiac and all-cause mortality in a cohort of older adults.[6,7]Despite the potential risk of QTc prolongation and related mortality risk, current guidelines do not agree on the requirements for monitoring QTc interval in patients prescribed haloperidol. In the guideline published in 1999 by the American Psychiatric Association, ECG monitoring is advised for all patients receiving antipsychotic medications for delirium,[8]while other guidelines do not advice ECG monitoring at all.[9,10]A Dutch guideline states that ECG monitoring in older patients is not necessary except when haloperidol is administered intravenously for doses greater than 2 mg.[11]Others endorse ECG evaluation in the presence of cardiac risk factors including age ≥ 65 years, electrolyte disturbances, known cardiac disease, and concomitant use of other medications that may prolong QTc interval.[12]Despite these recommendations, a retrospective study of agitated hospitalised patients (nearly 50% were age ≥ 65 years) receiving a mean cumulative dose of 5 mg () per 24 h showed that an ECG was recorded in only 65.5% before, and in 39.3% after haloperidol administration.[13]Data from primary care practices in the United Kingdom showed that less than 2% of patients with a new haloperidol prescription and who had least one additional risk factor for QT prolongation received an ECG upon treatment initiation.[14]Altogether, recommendations are sketchy and surveys evaluating clinicians’ approaches towards QTc monitoring emphasize a wide range of interpretations in daily practice.[2]
In older patients with multiple comorbidities, haloperidol is mostly prescribed with caution, orally and at low doses, while studies investigating the effect of haloperidol on QTc duration in this population are scarce.[15,16]In one retrospective cohort study, QTc duration upon haloperidol use increased in patients with normal baseline QTc and decreased in patients with prolonged baseline QTc, while mean QTc duration in the overall study group did not change.[16]This raised the following research questions: does low dose oral haloperidol prolong QTc interval in older acutely hospitalised patients compared to placebo, and are QTc interval changes related to haloperidol plasma levels?
2 Methods
2.1 Study population and design
For this subanalysis, we used data of participants from a multicentre randomised double-blind placebo-controlled clinical trial described in detail previously (Haloperidol prophylaxis in older emergency department patients, HARPOON study).[17]The study population consisted of patients presenting in the emergency department (ED), age ≥ 70 years, acutely admitted for a medical or surgical specialty, and at risk of developing delirium during admission. Exclusion criteria included QTc > 500 ms, potassium level < 3.0 mmol/L, concurrent use of QT prolonging medications in case of QTc > 450 or 460 ms for men and women respectively, recent myocardial infarction, second or third degree heart block, and (history of) ventricular arrhythmia or TdP. Patients were randomised to receive prophylactic haloperidol 1 mg or placebo 1 mg twice a day to study the effect on delirium occurrence within the first seven days after initiation of study treatment (maximum of 14 doses). This study was performed according to the principles expressed in the declaration of Helsinki and approved by the Medical Ethics Committee of VU University Medical Centre, Amsterdam, the Netherlands. All subjects provided written informed consent before participation in the HARPOON study, registered with clinicaltrials.gov NCT01530308.
2.2 Data collection
Information on patient characteristics including age, medical history, home medication use, and serum potassium levels was collected at baseline. Blood samples were drawn at baseline and before the seventh haloperidol dose on day four. Serum haloperidol concentrations were quantified in a validated liquid Chromatography-Tandem Mass Spectrometry assay allowing quantification from 0.4 to 40 µg/L.[18]If no spectrometry signal was detected, serum concentration was considered 0 µg/L. Serum concentrations exceeding 0 µg/L but below 0.4 µg/L (< 0.4 µg/L) were considered 0.4 in the analysis.
A 12-lead ECG was recorded at a paper speed of 25 mm/s before, on day 2 (after two doses), day 4 (after 6 doses), and following day 7 (after 14 doses) of the study intervention period. Subjects selected for the present study all had ECGs at baseline, on day 2, and at least one other time point. Exclusion criteria were less than three ECGs that allowed accurate QT interval assessment, specific cardiac arrhythmias (mostly atrial fibrillation), bundle branch block, and pacemaker beats.
2.3 QTc interval measurements
Automated values for HR, QT, and QTc derived from the proprietary algorithms contained in the ECG machines were recorded. ECGs were analysed independently by two investigators blinded for treatment status. QT and RR intervals were manually measured on paper recordings from ECG machines with a 0.5-mm scale precision ruler and tangent method: the end of the T wave was determined by extrapolating its slope to baseline.[19]To correct QT interval for HR, the preceding RR interval was determined. QT and RR intervals were measured in a single lead of each ECG, preferably lead II or V2. If one of these was found not to be the best lead, measurements were taken in another lead (mostly V3 to V6).[20]The mean of three beats, or two if there were no more evaluable beats in the single best lead, was used to determine mean QT and RR intervals. If mean manual QT or RR measurements differed more than 10 ms between the investigators,[15]measurements were re-evaluated by a third investigator, an experienced cardiologist who was considered the gold standard. Manual QT interval measurements were corrected for HR using four different correction formulas. Although Bazett formula is the most widely used, it is known to be inaccurate for low and high HR. Since the presence of abnormal HR is highly likely among acutely ill patients, three other well-recognized formulas to correct QT interval for HR were also applied (Table 1).[21,22]

Table 1. QT correction methods.
Manual derived RR (in seconds) and QT (in milliseconds, for Framingham in seconds) interval values were used. HR = 60/RR (in seconds). HR: heart rate in beats per minute.
QTc interval > 500 ms or change from baseline > 60 ms can be considered thresholds for clinically relevant safety concerns.[23]
2.4 Data analysis
Statistical Package for the Social Sciences (IBM SPSS, version 22.0 for Windows) was used for the data analysis in this study. Descriptive statistics were used to describe patient characteristics and compute mean ECG values at each time point. Independent samples-test and chi-square test were used to test for differences in baseline characteristics between groups.
Longitudinal course of QTc interval (and similarly RR and QT) over time was compared between haloperidol and placebo group using mixed models with fixed effects for group and time point (as a categorical variable) and a random effect for subject. First, a model with main effects for group and time point and their interaction term was fitted. When the interaction was found to be significant, we concluded that the between-group differences in means QTc interval changed over time. In case the interaction was not significant, a simpler model including only main effects of time point and group was fitted in order to test for a difference in means between groups when averaged over the follow-up. Association between change in the QTc interval from baseline and serum haloperidol levels on day 4 for the haloperidol group were quantified by means of Pearson’s correlation. A two-sided significance level of 5% was used in all analyses.
3 Results
For the present subanalysis, 72 subjects were included, of which 38 were assigned to haloperidol (Figure 1, Table 2). Baseline characteristics are given in Table 1 and were not significantly different between the haloperidol and placebo group (> 0.05 for all).
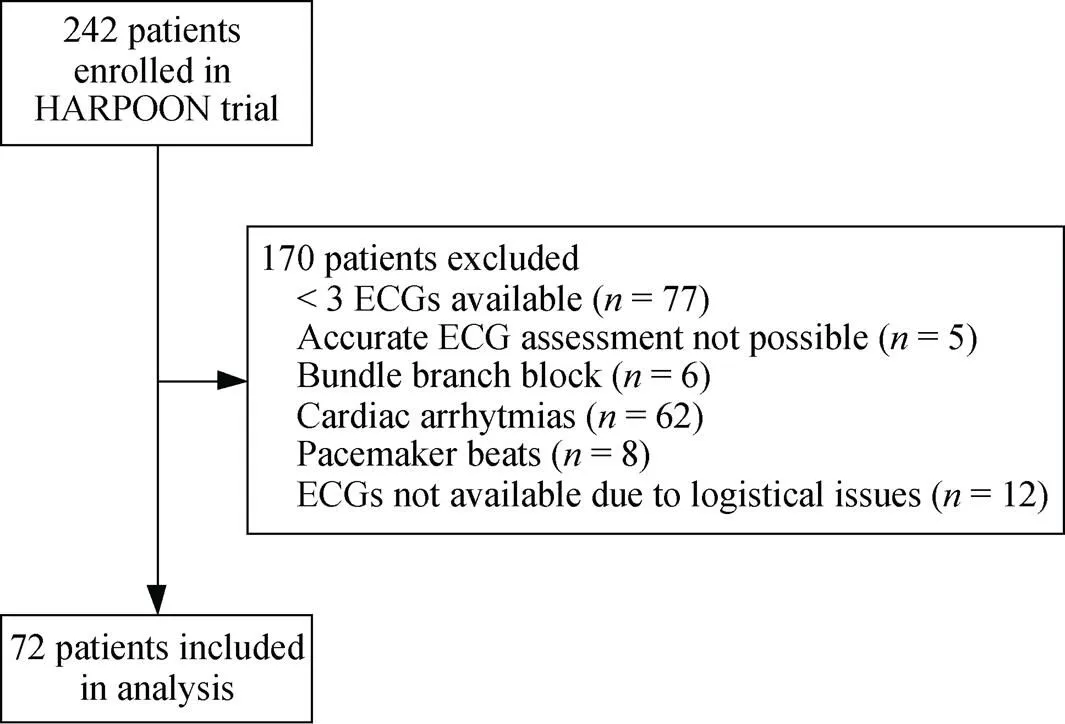
Figure 1. Study flowchart—cardiac arrhythmias mostly included atrial fibrillation.

Table 2. Baseline characteristics.
Values are reported as(%) or mean ±SD. There were no significant differences in baseline characteristics between groups. Medical history based on medical chart review and Charlson Comorbidity Index (ICD-10 codes).[34]Reference range potassium 3.5–5.0 mmol/L.BMI: body mass index; HR: heart rate.
At baseline, haloperidol serum concentrations were 0 µg/L in all patients with blood samples drawn at baseline (= 57), except for one patient in the placebo group who had a concentration exceeding 0 µg/L but below 0.4 µg/L. On day 4, median (range) haloperidol serum concentration in the haloperidol group in patients who had blood samples drawn at baseline and day 4 (= 23) was 0.71 (0.32–1.82) µg/L; three patients (13.0%) had levels ≤ 0.4 µg/L. Overall, haloperidol serum concentrations in the placebo group were 0 µg/L in patients who had blood samples drawn on day 4 (= 26).

Table 3. Results on QTc interval measurements, and between-group differences for change in QTc over time.
Values reported as mean ± SD, unless otherwise noted. ECG values were calculated using descriptive statistics. Mean of between-group differences over time was calculated using mixed models. Significance level< 0.05.Number of subjects. Baseline: Hal= 38, Pla= 34 or= 33 (QTc-aut); day 2: Hal= 38, Pla= 34; day 4: Hal= 35 or= 34 (QTc-aut), Pla= 32; day 7–8: Hal= 17, Pla= 14; after: Hal= 10 or= 9 (QTc-aut), Pla= 4. aut: automated; B: Bazett; Fi: Fridericia; Fa: Framingham; H: Hodges; Hal: haloperidol; Pla: placebo; QTc: QT interval corrected for heart rate.
There were no significant interactions between treatment group and time for any of the manual or automated variables, indicating that changes in mean QTc were not different in the treatment groups (Table 3). For the haloperidol group, a moderate positive correlation between change in QTc from baseline and serum haloperidol concentrations on day 4 was found for Bazett (= 0.50,= 23;= 0.016), Framingham (= 0.46,= 23;= 0.026), and Fridericia (= 0.48,= 23;= 0.022) correction methods. This was not the case for automated QTc (= 0.07,= 22;= 0.753) or Hodges formula (= 0.32,= 23;= 0.315).
There was a consistent downward trend in mean QTc towards the end of the intervention period across all manual correction methods in the placebo arm, but not in the haloperidol arm.
4 Discussion
In this sub-study, we analysed ECG data from a randomised placebo-controlled trial and found that a fixed low dose of oral haloperidol 1 mg twice per day did not result in significant QTc prolongation in older acutely hospitalised adults with multiple comorbidities and polypharmacy. We found moderate correlations between serum haloperidol concentration and QTc lengthening for certain QTc correction methods. Although QTc values exceeded the prolongation threshold in some patients, no episodes of TdP were observed.
In this sub-study, we analysed data from prospectively collected ECGs of older patients that were acutely hospitalised through the ED, 30.6% underwent surgery during admission. Baseline ECGs were recorded in the ED or within the first 24-h of admission, prior to initiation of prophylactic treatment with haloperidol or placebo. Other studies that previously aimed at investigating the effect of in-hospital haloperidol use on QTc changes were either of retrospective design (average dose 2.6, range 0.5 to 10, mg/day administered orally in 75% of the patients),[16]focused particularly on hip-fracture patients without a standardised dosing strategy (median oral dose 1, interquartile range 0.78 to 1.29, mg/day),[15]or on critically-ill patients receiving intravenous dosages (4 mg/day).[24]Although study design and populations were considerably different, the overall conclusions of these studies were in line: mean QTc did not prolong upon in-hospital low-dose haloperidol use, and no episodes of TdP were observed. One of these studies found that only normal QTc interval at baseline (male ≤ 430 ms, female ≤ 450 ms), but not borderline (male 431–450 ms, female 451–470 ms) or abnormal (male > 450 ms, female > 470 ms) QTc, was significantly associated with QTc prolongation during haloperidol use (mean QTc increase 23.1 ± 45.5 ms).[16]Although significant, the clinical relevance of these observations may be questioned, given that the mean increase in QTc duration tended to be less than the common threshold for potential dangerous prolongation of > 60 ms over baseline. Also, the proportion of patients experiencing abnormal QTc intervals during haloperidol use were similar in the normal and borderline baseline QTc group (23%) and even lower (9%) in the group with abnormal baseline QTc.[16]Altogether, it seems that the QTc prolonging effect upon haloperidol use was predominantly observed because of stratification of patients based on their baseline QTc, in the overall study population no change was found.[16]
We applied four different correction methods to calculate QTc from manual QT and RR interval measurements. Bland-Altman plots (Y axis showing the difference between automated QTc and QTc from any correction method, X axis representing the average of these two measurements; data not shown) showed that automated QTc values were generally higher, and the mean bias was lowest for QTc calculated using Bazett formula (QTc-B): 6.6 ± 39.7 ms; QTc calculated using Framingham formula (QTc-Fa): 23.8 ± 37.5 ms; QTc calculated using Fridericia formula (QTc-Fi): 24.3 ± 39.4 ms; QTc calculated using Hodges formula (QTc-H): 24.1 ± 39.1 ms). Previous studies comparing the four correction methods used in our study also found that Bazett formula provides higher QTc values, particularly for HR above 60 beats/min.[25,26]
In daily clinical practice, automated QTc intervals are most likely used for clinical decision making, since this is less time consuming than manual measurements from paper recordings. Because Bazett correction method may be inaccurate at abnormal HR, over- and underestimating at high and low HR respectively, and most ECG machines use this formula, verification of automated QTc values by visual inspection of paper ECG recordings and additional manual measurements are recommended.[27]Unfortunately, most physicians are unable to correctly evaluate the QTc interval.[28]Assuming that automated QTc values derived with Bazett formula overestimate true QTc values at higher HR,[26]they may therefore be used as routine screening for QTc prolongation when HR ≥ 60 beats/min. Subsequently, a cardiology specialist may be consulted to determine whether there truly is an increased risk.
The median haloperidol plasma concentration in our study was 0.71 (range: 0.32–1.82) µg/L. Previous studies in elderly patients have found comparable concentrations of 0.17–0.99 µg/L for haloperidol dose 1 to 2 mg/day,[29]and slightly higher concentrations of 0.13–4.11 µg/L when 0.3 to 5 mg/day was prescribed.[30]In relatively younger patients with schizophrenia or schizoaffective disorder, haloperidol 2.5 mg/day resulted in a mean plasma concentration of 1.58 µg/L (day 4 blood sampling five hours after morning dose, defined as Cmax), with a mean decrease in QTc interval from baseline of 1.2 (95% CI: –4.1 to 1.7) ms.[31]Escalating doses of haloperidol up to 30 mg/day only resulted in an average QTc increase from baseline of 7.2 (95% CI: 1.4–13.1) ms at a mean serum level of 16.1 µg/L.[31]We found moderate positive correlations between haloperidol serum concentration and change in QTc interval only for Bazett, Framingham, and Fridericia methods. When applying these three correction methods, a maximum of 24.8% in the variability of change in QTc intervals compared to baseline was accounted for by haloperidol serum concentration at day four. To our knowledge, this is the first study that attempted to correlate haloperidol serum levels with QTc prolongation.
Several limitations of our study have to be discussed. First, this study was a sub-analysis from a larger randomised controlled trial with subject selection based on ECG features which may have introduced selection bias. In addition, no separate a-priori power analysis was performed for this secondary analysis. Lack of significance may be due to low power and for this reason we have also included the 95% confidence intervals for the difference in means. These intervals give an estimated range in which the difference in QTc interval is likely to lie and their widths reflects the uncertainty. Second, ECG and blood sampling was performed prior to the next scheduled dose. The time elapsed between the antecedent dose and the time of data collection therefore varied between subjects, up to a maximum of 16 h (time between 8 pm and 12 am on the following day). Also, the amount of study medication received prior to the time of data collection may have differed between subjects due to the possibility of missed doses. The Tmax(time at which Cmaxis observed) of oral haloperidol is 2 to 6 hours.[32]We therefore did not capture the appropriate timeframe to establish the effect of maximum haloperidol serum concentration (Cmax) on QTc interval, if there was any. Furthermore, we only registered preadmission (home) medications and did not collect any data on medication changes during hospital admission. Therefore, we are not able to account for concomitant treatment with drugs that may have QTc prolonging potential. Also, in every individual there is an observed variability in QTc when all circumstances known to influence QTc are held constant, the so called ‘intrinsic QTc variability’.[33]Since we only registered ECG recordings once per day and at different time intervals, this may have contributed to variability in QTc measurements independent of extrinsic factors. On the other hand, the fact that we collected ECG data after multiple doses during a total intervention period of seven days is one of the strengths of our study. Generally, haloperidol administration for delirium treatment in older patient will not exceed the duration of one week. In our opinion, ECG monitoring in the current study therefore mimics true clinical practice. Third, the tangent method has less inter-reader variability but can provide shorter QT interval measurements compared with other methods and may be more inaccurate with unusual T wave morphology.[20]Although ECGs with flat T wave morphology were excluded and a cardiologist was appointed as gold standard when average measurements of the two primary observers differed more than 10 ms, the chosen method may have contributed to the observed differences in automated and manual QT interval measurements, the latter being generally shorter.
In conclusion, the results of our sub-analysis from a prospective double-blind placebo-controlled randomised study suggest that low dose oral haloperidol of 2 mg/day or less does not provide a risk of dangerous QTc prolongation in acutely hospitalised older patients with (near) normal ECGs. Although there was a moderate and significant association between haloperidol concentration at day 4 and QTc change between baseline and day 4 in the haloperidol group, only 24% of the variability in QTc change was explained by haloperidol concentration. Despite this association, there was no clinical significant difference between haloperidol an placebo group in mean QTc change over time. These results are in line with previous findings and current guideline recommendations. If there are no concomitant risk factors present based on patient medical records and baseline ECG, (continued) ECG monitoring is not strictly necessary when oral haloperidol up to 2 mg/day is prescribed.[11]More research is needed to evaluate if this is also applicable to older patients receiving higher dosages or with for example baseline QTc > 500 ms, prolonged baseline QTc and concomitant use of other QTc prolonging medications, atrial fibrillation, and other risk factors. Automated methods to calculate QTc tend to overestimate QTc interval duration and we believe they may therefore be used only as a routine warning mechanism. Manual measurement methods seem more precise, but have practical constraints and lack wide spread use in clinical practice.
1 Nijboer H, Lefeber G, McLullich A,. Haloperidol use among elderly patients undergoing surgery: a retrospective 1-year study in a hospital population.2016; 3: 83–88.
2 Morandi A, Davis D, Taylor JK,. Consensus and variations in opinions on delirium care: a survey of European delirium specialists.2013; 25: 2067–2075.
3. Nederlandse Vereniging voor Klinische Geriatrie (NVKG): Utrecht, Nederland, 2013.
4 Glassman AH, Bigger JT. Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death.2001; 158: 1774–1782.
5 Beach SR, Celano CM, Noseworthy PA,. QTc prolongation, torsades de pointes, and psychotropic medications.2013; 54: 1–13.
6 de Bruyne MC, Hoes AW, Kors JA,. Prolonged QT interval predicts cardiac and all-cause mortality in the elderly. The Rotterdam Study.1999; 20: 278–284.
7 Straus SM, Kors JA, De Bruin ML,. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults.2006; 47: 362–367.
8 American Psychiatric Association. Practice guideline for the treatment of patients with delirium.1999; 156(5 Suppl): S1–S20.
9 NICE clinical guideline 103. Delirium: diagnosis, prevention and management (2010). Evidence Update April 2012. http://www.nice.org.uk/guidance/cg103/evidence/evidence-update-134649181 (accessed Dec 6, 2017).
10 American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults.2015; 63: 142–150.
11 Kleijer BC, on behalf of the WKGF. Werkgroep Klinische Gerontofarmacologie (WKGF)-standpunt inzake het bepalen van het QT-interval bij het voorschrijven van antipsychotica bij ouderen. http://www.ephor.nl/media/1132/wkgf-standpunt- antipsychotica-en-qt-tijd-en-ecg-controle-2011.pdf (accessed Dec 6, 2017).
12 Shah AA, Aftab A, Coverdale J. QTc prolongation with antipsychotics: is routine ECG monitoring recommended?2014; 20: 196–206.
13 Muzyk AJ, Rivelli SK, Jiang W,. A computerized physician order entry set designed to improve safety of intravenous haloperidol utilization: a retrospective study in agitated hospitalized patients.2012; 35: 725–731.
14 Warnier MJ, Rutten FH, Souverein PC,. Are ECG monitoring recommendations before prescription of QT-prolonging drugs applied in daily practice? The example of haloperidol.2015; 24: 701–708.
15 Blom MT, Jansen S, de JA,. In-hospital haloperidol use and perioperative changes in QTc-duration.2015; 19: 583–589.
16 Blom MT, Bardai A, van Munster BC,. Differential changes in QTc duration during in-hospital haloperidol use.2011; 6: e23728.
17 Schrijver EJ, de Vries OJ, Verburg A,. Efficacy and safety of haloperidol prophylaxis for delirium prevention in older medical and surgical at-risk patients acutely admitted to hospital through the emergency department: study protocol of a multicenter, randomised, double-blind, placebo-controlled clinical trial.2014; 14: 96.
18 Kratzsch C, Peters FT, Kraemer T,. Screening, library- assisted identification and validated quantification of fifteen neuroleptics and three of their metabolites in plasma by liquid chromatography/mass spectrometry with atmospheric pressure chemical ionization.2003; 38: 283–295.
19 Postema PG, De Jong JS, Van der Bilt IA,. Accurate electrocardiographic assessment of the QT interval: teach the tangent.2008; 5: 1015–1018.
20 Kasamaki Y, Ozawa Y, Ohta M,. Automated versus manual measurement of the QT interval and corrected QT interval.2011; 16: 156–164.
21 Indik JH, Pearson EC, Fried K,. Bazett and Fridericia QT correction formulas interfere with measurement of drug- induced changes in QT interval.2006; 3: 1003–1007.
22 Funck-Brentano C, Jaillon P. Rate-corrected QT interval: techniques and limitations.1993; 72: 17B-22B.
23 International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs: E14. 2005. https://www.ich.org/fileadmin/Public_Web_Site/ICH_ Products/Guidelines/Efficacy/E14/E14_Guideline.pdf (accessed Feb 25, 2018).
24 Duprey M, Al-Qadheeb N, Roberts R,. 856: QTc internal prolongation with low-dose iv haloperidol: post hoc analysis of a placebo control trial [abstract].2016; 44: P290.
25 Luo S, Michler K, Johnston P,. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs.2004; 37 (Suppl): S81–S90.
26 Vandenberk B, Vandael E, Robyns T,. Which QT correction formulae to use for QT monitoring?2016; 5: e003264.
27 Charbit B, Samain E, Merckx P,. QT interval measurement: evaluation of automatic QTc measurement and new simple method to calculate and interpret corrected QT interval.2006; 104: 255–260.
28 Viskin S, Rosovski U, Sands AJ,. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one.2005; 2: 569–574.
29 van Strien AM, Vermeulen Windsant-van denTweel A, Leliveld-van den Heuvel M,. Correlation of haloperidol concentration in blood and cerebrospinal fluid: a pharmacokinetic study.2014; 34: 516–517.
30 Knol W, van Marum RJ, Jansen PA,. Parkinsonism in elderly users of haloperidol: associated with dose, plasma concentration, and duration of use.2012; 32: 688–693.
31 Miceli JJ, Tensfeldt TG, Shiovitz T,. Effects of oral Ziprasidone and oral Haloperidol on QTc interval in patients with schizophrenia or schizoaffective disorder.2010; 30: 127–135.
32 Kudo S, Ishizaki T. Pharmacokinetics of haloperidol: an update.1999; 37: 435–456.
33 Beasley CM Jr., Dmitrienko A, Mitchell MI. Design and analysis considerations for thorough QT studies employing conventional (10 s, 12-lead) ECG recordings.2008; 1: 815–839.
34 Charlson ME, Pompei P, Ales KL. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.1987; 40: 373–383.
35 Bazett HC. An analysis of time relations of the electrocardiogram.1920; 7: 353–370.
36 Sagie A, Larson MG, Goldberg RJ,. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study).1992; 70: 797–801.
37 Fridericia LS. Die systolendauer im elektrokardiogramm bei normalen menschen und bei herzkranken.1920; 53: 469–486.
*Current position: Oskarshamn Hospital, Oskarshamn, Sweden
Prabath WB Nanayakkara, Department of Internal Medicine, VU University Medical Centre, PO Box 7057, 1007 MB, Amsterdam, the Netherlands. E-mail: p.nanayakkara@vumc.nl
December 7, 2017
February 26, 2018
March 20, 2018
June 28, 2018
杂志排行
Journal of Geriatric Cardiology的其它文章
- “Malignant” right coronary artery presenting as an ST-segment elevation myocardial infarction—a case report
- Influenza vaccination in acute coronary syndromes patients in Thailand: the cost-effectiveness analysis of the prevention for cardiovascular events and pneumonia
- The trend of change in catheter ablation versus antiarrhythmic drugs for the management of atrial fibrillation over time: a meta-analysis and meta-regression
- Early mortality and safety after transcatheter aortic valve replacement using the SAPIEN 3 in nonagenarians
- Depression and chronic heart failure in the elderly: an intriguing relationship
- CIED implantation in elderly patients: a single-center experience
