Comparative study of the hemolytic and cytotoxic activities of nematocyst venoms from the jelly fish Cyanea nozakii Kishinouye and Nemopilema nomurai Kishinouye*
2018-08-02PANGMin庞敏XUJintao徐金涛LIUYunlong刘云龙ZHANGXuelei张学雷
PANG Min (庞敏) , XU Jintao (徐金涛) , LIU Yunlong (刘云龙) , ZHANG Xuelei (张学雷) ,
1 . Key Laboratary of Science and Engineering for Marine Ecology and Environment, First Institute of Oceanography, State Oceanic Administration, Qingdao 266003, China
2 . Laboratory of Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology,Qingdao 266200,China
3 . Qinhuangdao Marine Environmental Monitoring Central Station of SOA, Qinhuangdao 066000, China
4 Chinese Research Academy of Environmental Sciences, Beijing 100012, China
Abstract Two species of jelly fish, Cyanea nozakii Kishinouye and Nemopilema nomurai Kishinouye,have occurred off coastal areas of the northeastern China Sea, Yellow Sea, and Bohai Sea in recent years. They in fluence marine ecosystem safety and fishery production, and also pose a risk to human health. The current study examined the hemolytic and cytotoxic activities of crude venoms extracted from the nematocysts of C.nozakii and N. nomurai. The results showed that there were more nematocysts on tentacles from C. nozakii than on tentacles of the same length from N. nomurai. The protein concentration per nematocyst extracted from N. nomurai was higher than that from C. nozakii. Both nematocyst venoms showed dose- and timedependent hemolytic activity on erythrocytes from chicken, pigeon, and sheep, with sheep erythrocytes being the most sensitive, with EC 50 values of 69.69 and 63.62 μg/mL over a 30-min exposure with N.nomurai and C. nozakii nematocyst venoms, respectively. A cytotoxic assay of both jelly fish venoms on A431 human epidermal carcinoma cells resulted in IC 50 values of 68.6 and 40.9 μg/mL after 24-h incubation,respectively, with venom from C. nozakii showing stronger cytotoxic activity than that from N. nomurai.The results of current study indicate that nematocyst venom from C. nozakii had stronger hemolytic and cytotoxic activities than that from N. nomurai and, thus, C. nozakii might be more harmful to the health of humans and other species than are N. nomurai when they appear in coastal waters.
Keyword: Cyanea nozakii; Nemopilema nomurai; nematocyst venom; jelly fish; hemolytic activity;cytotoxicity
1 INTRODUCTION
Blooms of the jelly fish Cyanea nozakii Kishinouye( C. nozakii), a cnidarian of the Class Scyphomedusae,Order Semaeostomeae, Family Cyaneidae, have occurred off coastal areas of the Northeastern China Sea, Yellow Sea, and Bohai Sea since the late 20th century (Dong et al., 2006). In addition, blooms of the jelly fish Nemopilema nomurai Kishinouye( N. nomurai), a cnidarian of the Class Scyphomedusae,Order Rhizostomeae, Family Stomolophidae (Huang and Lin, 2012) have occurred in recent years in temperate China seas, including the northern East China Sea, Yellow Sea, and Bohai Sea (Dong et al.,2010). Both C. nozakii and N. nomurai, which are often abundant in late summer to early autumn from the Bohai Sea to the Yellow Sea, are the major species causing blooms in the northern China seas. Such blooms have deleterious effects on marine ecosystems as well as fishery production, and pose a risk to human health. Humans stung by either species suffer itching,edema, muscle aches, tightness of breath, hypotension,shock, and even death (Yu et al., 2005; Li et al., 2013,2014). These symptoms occur in response to the venom contained in nematocysts on the tentacles of the jelly fish. These venoms contain toxic polypeptides and/or proteins, which all increase the risk to human health during jelly fish blooms.
Many biological assays have been carried out to estimate the toxicity of several jelly fish venoms.Hemolytic proteins in nematocyst venoms are key markers of the toxic effects of jelly fish stings,including erythema and edema (Li et al., 2013);therefore, hemolytic and cytotoxic activities of jelly fish venoms have been the most widely reported(Nagai et al., 2000a, b; Brinkman and Burnell, 2007;Helmholz et al., 2007; Yu et al., 2007; Marino et al.,2008, 2009). The hemolytic and cytotoxic activities of venoms from C. nozakii and N. nomurai have also been evaluated. Kang et al. (2009) assessed the hemolytic activity of N. nomurai venom on cat, dog,human, rabbit, and rat erythrocytes, and evaluated its cytotoxicity using C2C12 (skeletal myoblast) and H9C2 (heart myoblast) cell lines. Feng et al. (2010)partially characterized the hemolytic activity of nematocyst venom from C. nozakii on chicken erythrocytes. Lee et al. (2011) compared the cytotoxicity of venoms extracted from N. nomurai,Rhopilema esculenta, C. nozakii, and Aurelia aurita on NIH 3T3 mouse fibroblast cells. Li et al. (2012)investigated the cytotoxicity of C. nozakii on Bel-7402 and SMMC-7721 human hepatoma cells and on H630 human colon cancer cells. However, as far as we are aware, there is no published report comparing the activity intensity of venoms from C. nozakii and N. nomurai. Therefore, in the current study, we compared the toxic activities of crude venoms from C. nozakii and N. nomurai nematocysts using hemolytic and cytotoxic assays. The results provide a novel viewpoint for understanding the threats to human health posed by these two jelly fish species.
2 MATERIAL AND METHOD
2.1 Preparation of nematocysts
Specimens of C. nozakii and N. nomurai were collected from the near-shore area in Qingdao, China,in August 2012, and transferred to the laboratory immediately. All tentacles were removed from living jelly fish bodies, no less than ten tentacles were selected at random, and the remaining tentacles were stored at 80° C before use. The selected tentacles were unbended on aluminum foils separately and left stretching naturally at 4℃ for 5 min. Then three 5 mm-long sections were cut randomly from each tentacle and transferred to one drop of sterilized and filtered seawater on the glass slide under microscope to count the numbers of nematocysts. All sections cut from selected tentacles of jelly fish specimens were observed, and the distribution and density of nematocysts in clusters on the hollow tubular tentacles were estimated totally.
Nematocysts were isolated from frozen tentacles following the methods described previously (Bloom et al., 1998) with a slight modi fication. Brie fly, frozen tentacles were thawed at 4° C and immersed for 3 days in a volume of sterilized and filtered (0.45 μm) natural seawater that was twice that of the tissue volume to enable autolysis. The mixture was stirred gently twice daily. The resulting suspension was filtered using different-sized mesh (following the order 250 μm,160 μm, 90 μm, and 40 μm), and washed three times with sterilized and filtered natural seawater. After each round of washing, the filtrate was centrifuged at 3 412× g at 4° C for 20 min and the debris was removed with a pipette. All The final undischarged nematocysts were collected, examined microscopically, and then stored at -80°C until further use.
2.2 Venom extraction and preparation
Venom was extracted from nematocysts isolated from C. nozakii (VEC) or N. nomurai (VEN) using a modi fied version of the technique described by Carrette and Seymour (2004). In brief, nematocysts were resuspended using Hanks’ balance salt solution(HBSS, NaCl 137 mmol/L, KCl 5.6 mmol/L, CaCl21.26 mmol/L, MgSO40.81 mmol/L, Na2HPO40.38 mmol/L, KH2PO40.44 mmol/L and NaHCO34.2 mmol/L, the pH of HBSS was adjusted to 7.4 with 0.1 mol/L HCl and NaOH) containing 1 mmol/L of phenylmethylsulfonyl fluoride (PMSF). Before extraction, 100 μL of each nematocyst resuspension was examined using microscope respectively to check the discharge condition of the nematocysts,and the number of the nematocysts prepared for venom extraction was obtained by microscope count method. One milliliter of the nematocyst resuspension solution was placed into screw-top vials with 2.5 g of glass beads (0.5 mm in diameter),and the mixture was shaken five times in a mini bead beater (Mini-Beadbeater-16, Biospec Products,Bartlesville, USA) at 3 450 r/min for 20-s intervals with intermittent cooling on ice. After ensuring the absolute discharge of the nematocysts in resuspension microscopically, the venom was then centrifuged at 6 824× g at 4°C for 20 min and the supernatant used for the present study. The protein concentration of the venom extracts was determined following the method of Bradford (1976) and compared with bovine serum albumin (BSA) protein concentration standards, and the venoms were then used in the hemolytic and cytotoxicity assays based on their protein concentrations.
2.3 Hemolytic activity assay
The hemolytic activity of the venoms was tested using erythrocytes from chicken, pigeon, and sheep,which could easily be obtained from the local market.In brief, fresh blood samples were mixed with 1 mg/mL of heparin sodium as an anticoagulant at a 1:10 volume immediately after blood collection.Whole blood (60 mL) was centrifuged at 684× g for 10 min at 4°C, the supernatant was removed gently,and the erythrocytes were washed three times with HBSS. The erythrocytes were then resuspended in the same buffer to make a 1% (v/v) solution for the following hemolysis assay.
Various volumes of jelly fish venoms (0.1, 0.3, 0.5,0.7 and 0.9 mL) were added to 0.5 mL of the erythrocyte suspension obtained from the three species (chicken, pigeon, and sheep). The venomerythrocyte mixtures were adjusted using HBSS to a final volume of 1.5 mL and final protein concentrations of venoms (16, 49, 82, 115, and 147 μg/mL,respectively) were checked as mentioned above. All the mixtures were incubated at 37°C for 30 min. Then,1 mL of each mixture was diluted with 4 mL of HBSS and centrifuged at 1 000× g for 5 min at 4°C. The supernatants were transferred to 96-well microplates and the absorbance at 410 nm was determined using a visible spectrophotometer (7230G, Shanghai, China)to measure the extent of erythrocyte lysis. The half maximal effective concentration (EC50) values of each concentration of jelly fish venom protein were calculated to evaluate the hemolytic activity of the jelly fish venoms.
2.4 Cell culture and treatment
The A431 human epidermal carcinoma cell line was purchased from the Cell Bank of the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM Medium (Gibco, USA) with 4 mmol/L of L-glutamine, 100 μg/mL of streptomycin and 100 U/mL of penicillin, plus 15% fetal calf serum(FCS, TBD, China), and incubated at 37°C in a humidi fied 5% CO2atmosphere.
2.5 Cytotoxicity assay
Cytotoxicity was assessed by measuring the mitochondrial dehydrogenase activity, using a 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Brie fly, cells were plated on 96-well plates at a density of 4×103cells/well and cultured under normal conditions for 24 h. Venoms were diluted with HBSS to the same concentrations described above (16, 49, 82, 115, and 147 μg/mL),and added to pre-cultured cells, respectively. All incubations with various protein concentrations were then stopped at 24, 48, 72 and 96 h. The control group was treated simultaneously with HBSS only.
After incubation, the treated cells were observed and photographed using an inverted phase-contrast microscope (Nikon, Japan). Two hundred microliters of MTT solution (2.5 mg/mL) was added to each well and the plates were incubated for another 4 h at 37°C.The medium was then removed and replaced by 200 μL of dimethyl sulfoxide (DMSO) to solubilize the formazan crystal generated in the culture plates.The absorbance at 490 nm was read using a spectrophotometric microplate reader (MultiSkan FC,Thermo Scienti fic, USA) and the cell survival ratio(%) was calculated using Eq.1:

The half maximal inhibitory concentration (IC50)values were calculated to evaluate the cytotoxic activity of jelly fish venoms.
2.6 Statistical analysis
All data were expressed as means±SD of three parallel measurements. The SPSS16.0 software was used for statistical analysis, and statistically signi ficant differences between groups were determined by oneway ANOVA. For further comparison between two groups, the Student-Newman-Keuls (SNK) method was used for equal variance, whereas the Games-Howell test was used for unequal variances.Differences were considered signi ficant at P <0.05.EC50and IC50values were calculated using a probit regression model.
3 RESULT
3.1 Observations of nematocysts
As shown in Table 1, the density of nematocysts isolated from C. nozakii or N. nomurai was calculated after observations under an inverted microscope.There were fewer nematocysts per unit area from tentacles of N. nomurai than from tentacles of C. nozakii. Nematocysts isolated from tentacles of N. nomurai were predominant spheroidal, whereas those from C. nozakii were oval (Fig.1).

Fig.1 The microscopic structure of nematocysts isolated from N. nomurai (left) and C. nozakii (right)
3.2 The total protein content of nematocyst venoms
The protein concentration per nematocyst was calculated based on the protein concentration of the venoms and nematocyst suspension density. As shown in Fig.2, the protein concentration of VEN was higher than VEC at the same nematocyst density. Therefore,the protein concentration per nematocyst from N. nomurai was higher than that of C. nozakii.Interestingly, Fig.2 (left side) shows lower concentrations of protein per nematocyst with the increasing of nematocyst densities. That was probably due to the partly discharged nematocysts in resuspension during the process of venom extraction, and protein in supernatant might induce an overestimate of protein concentration per nematocyst at lower density.
3.3 Hemolysis
The hemolytic activities of venoms were assessed using erythrocyte suspensions from chicken, pigeon,and sheep. Both VEC and VEN showed strong hemolytic activities. After incubation with either VEN or VEC, all chicken, pigeon, and sheep erythrocytes showed obvious hemolytic reaction including morphological changes and obvious lysis (Fig.3)

Fig.2 Comparison of the protein concentration of nematocysts (left) and venoms (right) from C. nozakii (white bars) and N.nomurai (black bars)
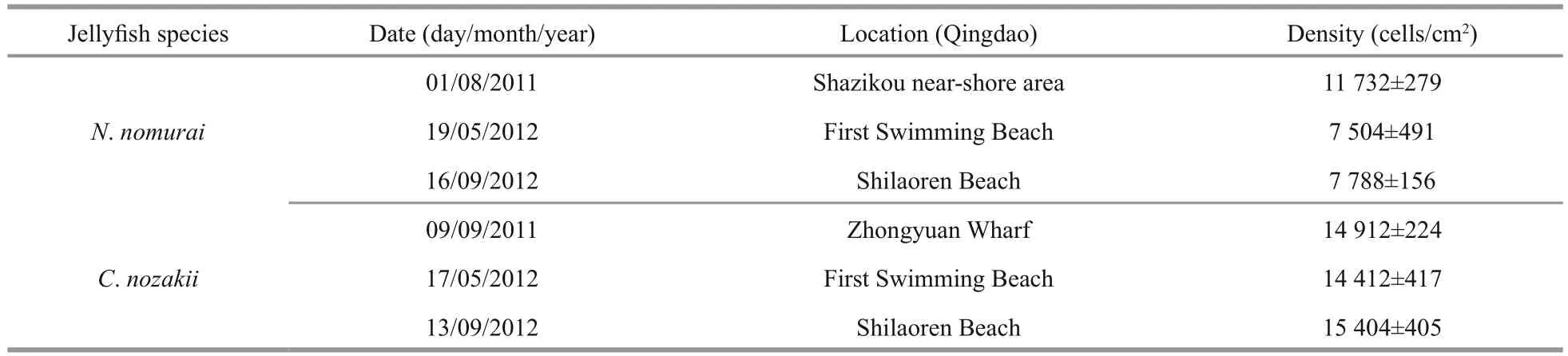
Table 1 The density of nematocysts in tentacles of N. nomurai and C. nozakii

Fig.3 Morphological changes of chicken, pigeon, and sheep erythrocytes before (upper) and after (lower) incubation with 147 μg/mL protein concentration of jelly fish venoms for 30 min

Fig.4 Hemolytic effects of VEN (white bars) and VEC (black bars) on erythrocytes from chickens after 30- (a) or 60- (b) min incubation ( n=3, P <0.05)
Venom extracts were applied at concentrations of 16, 49, 82, 115 and 147 μg/mL. Absorbance analysis with a one-way ANOVA showed that the time- and concentration-dependent hemolytic effects were in fluenced signi ficantly by the protein concentration and incubation time ( P <0.01). As shown in Figs.4–6,treatment with 16 μg/mL of VEC for 30 min resulted in hemolysis ratios in chicken, pigeon, and sheep erythrocytes of 22.4%, 19.8% and 24.7%, respectively,whereas the hemolysis ratios of the erythrocytes treated with the same concentration of VEN were 13.8%, 12.5% and 23.8%, respectively. With an extended treatment time of 60 min, the hemolysis ratios of chicken, pigeon, and sheep erythrocytes treated with VEC increased to 26.2%, 27.6% and 33.5%, respectively, whereas the hemolysis ratios of the erythrocytes treated with VEN increased to 21.6%,24.5% and 27.1%, respectively. The hemolytic effects of both jelly fish venoms at other protein concentrations showed similar differences, and all erythrocytes showed complete lysis after incubation with 147 μg/mL of venom (Figs.4–6).

Fig.5 Hemolytic effects of VEN (white bars) and VEC (black bars) on erythrocytes from pigeons after 30- (a) or 60- (b) min incubation ( n=3, P <0.05)

Fig.6 Hemolytic effects of VEN (white bars) and VEC (black bars) on erythrocytes from sheep after 30- (a) or 60- (b) min incubation ( n=3, P <0.05)

Fig.7 EC 50 of VEC (black bars) and VEN (white bars) on erythrocytes from chicken, pigeon, and sheep at different exposure times

Fig.8 Number of nematocysts from N. nomurai (white bars)and C. nozakii (black bars) required to meet the EC 50 level on chicken, pigeon, and sheep erythrocytes over 30- and 60-min exposure times
The EC50of both VEC and VEN on erythrocytes from the three species reduced over time ( P <0.05).Comparison of the EC50results indicated that sheep nematocysts were the most sensitive to the hemolytic effects of both jelly fish venoms, and VEC had a stronger hemolytic activity than VEN at the same protein concentrations (Fig.7). In addition, to enable the hemolytic activity to reach the EC50level, the number of nematocysts and tentacle area required from C. nozakii and N. nomurai were calculated according to the nematocyst densities listed above. Comparisons showed that similar numbers of nematocysts from the two species were needed to reach the EC50level in chicken, pigeon, and sheep erythrocytes for either the 30- or 60-min exposure time (Fig.8). Given that the number of nematocysts per unit area in tentacles of N. nomurai was lower than in C. nozakii, the tentacle area of N. nomurai corresponding to the EC50levels on erythrocytes from the three species was larger compared with C. nozakii (Fig.9).

Fig.9 Tentacle areas of N. nomurai (white bars) and C. nozakii (black bars) required to meet the EC 50 level on chicken, pigeon, and sheep erythrocytes over 30- and 60-min exposure times
3.4 Cytotoxicty
Morphological changes in A431 cells following their exposure to the jelly fish venoms were observed under a microscope. The cells of the untreated control group grew well and their nuclei were round and clear. Cells treated with either VEC or VEN were misshapen and some were broken. Nuclei were marginalized in some cells (Fig.10). The cytotoxic activities of the venoms were evaluated using the MTT assay, and the results showed a dose-dependent inhibition of either VEN or VEC on cell viability with increasing protein concentrations (Fig.10). After incubation with VEC and VEN for 24 h at a concentration of 16 μg/mL, the survival rates of A431 cells were 64% and 80.2%, respectively. The survival rates at 24 h reduced to 8.1% and 9.5% when the protein concentrations of venoms increased to 147 μg/mL. Meanwhile, the cytotoxic activities of both venoms were signi ficantly related to incubation time ( P <0.05). After incubation for 96 h with VEC and VEN, the survival rates of A431 cells at a concentration of 16 μg/mL reduced to 49.7% and 52.8%, respectively. The survival rates of cells incubated with 147 μg/mL of venom reduced to 5.5%and 5.6%, respectively. Lower values of IC50for VEC at different incubation times (Fig.11) compared with VEN were also recorded (Figs.12, 13).
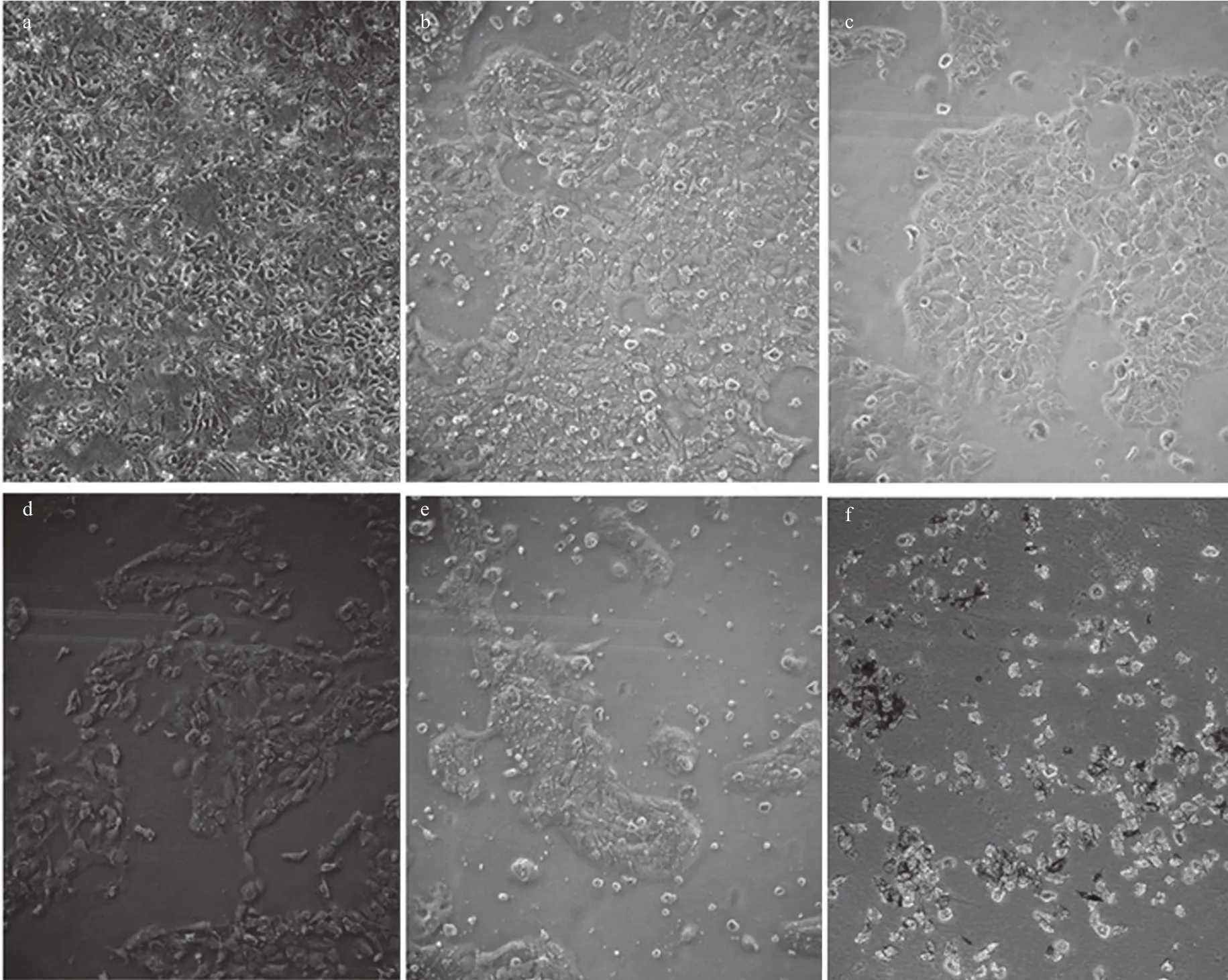
Fig.10 Morphological changes in A431 cells following 24-h incubation with different concentrations of jelly fish venom

Fig.11 Comparison of the cytotoxic effects of VEC (black bars) and VEN (white bars) on the viability of A431 cells following their incubation with venom for 24, 48, 72, and 96 h
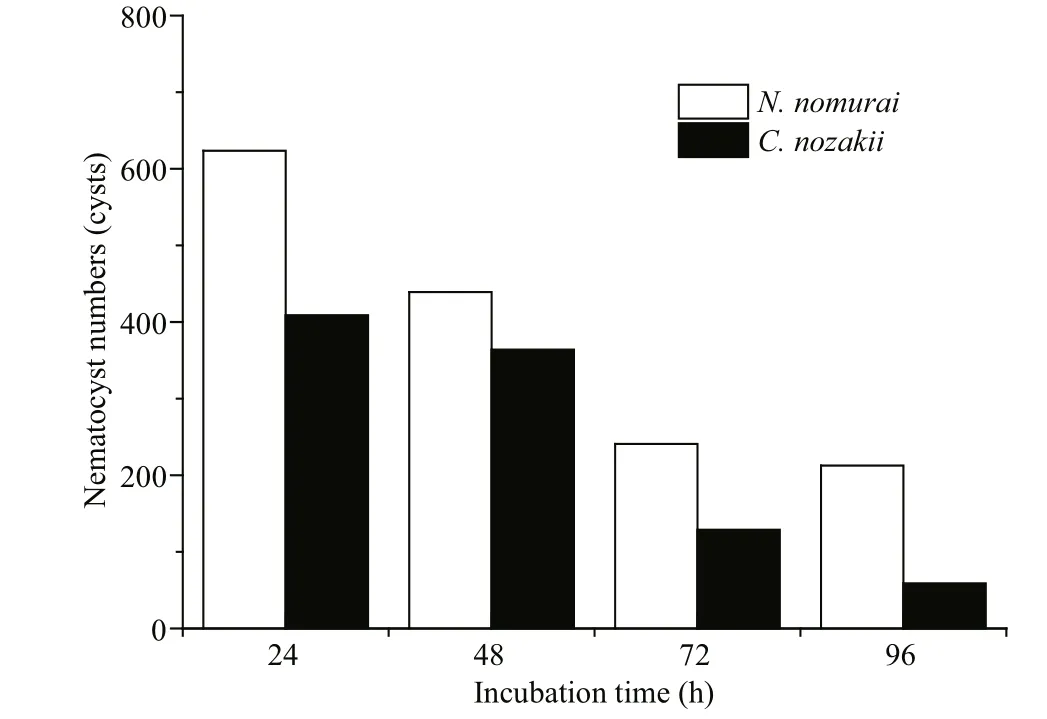
Fig.12 Number of nematocysts of N. nomurai (white bars)and C. nozakii (black bars) needed to meet the IC 50 cytotoxic level on A431 human epidermal carcinoma cells over different incubation times

Fig.13 Tentacle area of N. nomurai (white bars) and C.nozakii (black bars) needed to meet the IC 50 cytotoxic level on A431 human epidermal carcinoma cells over different incubation times
4 DISCUSSION
The jelly fish C. nozakii is widely distributed along the coast of China and blooms have been reported in the northern East China Sea, Yellow Sea, and Bohai Sea (Dong et al., 2010). It can ruin fishing nets, prey on juvenile fish, crabs and mollusks, and produce toxins that are poisonous to humans and marine animals (Zhou and Huang, 1956; Peng and Zhang,1999; Zhong et al., 2004; Dong et al., 2006). In humans, the venom of C. nozakii can produce a burning feeling that develops into severe pain,although no deaths have been attributed to this species(Tibballs, 2006; Helmholz et al., 2007). N. nomurai is also a dominant species throughout seas along East China, including Bohai Sea, Yellow Sea, and northern East China Sea (Kawahara et al., 2006). Blooms of N.nomurai were reported in the northern part of the East China Sea, Yellow Sea, and Liaodong Bay in 2003,2005, and 2007, respectively (Cheng et al., 2004;Ding and Cheng, 2005). This species was responsible for most of the severe or fatal cases of jelly fish stings in Chinese seas (Zhang et al., 1993; Xu et al., 2007;Jiang et al., 2008). Given that hemolytic proteins in nematocyst venoms are key molecules determining the toxic effects of jelly fish stings, including erythema and edema (Li et al., 2013), the hemolytic activity of venoms extracted from jelly fish tentacle nematocysts has been widely reported against erythrocytes from different species (Bloom et al., 2001; Torres et al.,2001; Nagai et al., 2002; Tibballs, 2006). The sensitivity with which erythrocytes re flect these conditions varies from species to species (Table 2).Kang et al. (2009) assessed the hemolytic activity of N. nomurai on cat, dog, human, rabbit, and rat erythrocytes and showed a concentration-dependent activity of venom extracts starting at 10 μg/mL of protein equivalents; dog erythrocytes were the most sensitive (EC50=151 μg/mL, 30 min). The hemolytic activity of extracts from C. nozakii was also documented on human and chicken erythrocytes(Helmholz et al., 2007; Feng et al., 2009, 2010). This study compared the hemolytic activities of venoms extracted from nematocysts of C. nozakii and N. nomurai on erythrocyte suspensions from chickens,pigeons, and sheep. Sheep erythrocytes were the most sensitive to the hemolytic effects of these two jelly fish venoms, with EC50values of VEN and VEC of 69.69 and 63.62 μg/mL over a 30-min exposure. In addition,the venom from C. nozakii showed a stronger hemolytic activity than that from N. nomurai on all three erythrocyte suspensions at the same protein concentration. Based on the same relative amount of each jelly fish nematocyst needed to reach the EC50level on erythrocytes, the hemolytic activities per nematocyst from N. nomurai and C. nozakii were similar. However, the tentacle area of N. nomurai corresponding to the EC50level was larger than that of C. nozakii. Thus, the hemolytic activity per unit area of tentacle from C. nozakii was stronger than that of N. nomurai.

Fig.14 The IC 50 of N. nomurai (white bars) and C. nozakii(black bars) nematocyst venoms on A9, A673, and A431 cells following 24–96-h exposure
There are two general mechanisms to explain this hemolytic activity. The first is an enzymatic mechanism,whereby cytolytic components bind preferentially to membrane glycolipids or glycoproteins (Burnett and Calton, 1987). The other is a stoichiometric mechanism,whereby toxin molecules bind to, and insert in, the plasma membrane followed by oligomerization to form transmembrane pores (Bhakdi and Tranum-Jensen, 1988). Many studies have indicated that jelly fish venoms form pore-like structures in target membranes, causing rapid cell lysis (Rottini et al.,1995; Edwards et al., 2002), and that the pore formation only occurs at the site of envenoming, possibly serving to aid the entry of other venom components into the host (Bailey et al., 2005). Thus, the hemolytic activity is likely to occur in combination with the envenomation of the jelly fish venom. However, it is not fully understood how the venoms of C. nozakii and N. nomurai elicit their hemolytic potencies in different species, and so requires further investigation.
Cytotoxicity is also a common bioactivity of jelly fish venoms (Table 3). Li et al. (2012) assessed the cytotoxicity of C. nozakii venom on Bel-7402 and SMMC-7721 human hepatoma cells and H630 human colon cancer cells. H630 cells were most sensitive to the venom (IC50=15.9, 8.8, and 5.1 μg/mL after incubation for 12, 24, and 48 h, respectively) followed by Bel-7402 (17.9 μg/mL) and SMMC-7721(24.3 μg/mL). C2C12 (muscle myoblast) and H9C2(heart myoblast) cell lines were used to assess the cytotoxicity of crude venom from N. nomurai, which showed high cytotoxic effects against H9C2 heart myoblasts (IC50=2 μg/mL; Kang et al., 2009). Lee et al.(2011) assessed the cytotoxicity of venom from four scyphozoan jelly fish ( Nemopilema nomurai, Rhopilema esculenta, C. nozakii, and Aurelia aurita) on NIH 3T3 mouse fibroblast cells, and reported a cytotoxic potency scale of C. nozakii > N. nomurai > A. aurita >R. esculenta. We had previously compared the cytotoxicity of venoms from C. nozakii and N. nomurai on A9 (mouse subcutaneous connective tissue) and A673 (human rhabdomyosarcoma) (Xu et al., 2014).The results from both our studies demonstrated that venom from C. nozakii nematocysts was more cytotoxically potent than that from N. nomurai nematocysts at the same protein concentration. Further calculations also indicated the stronger cytotoxic activities per nematocyst and per unit area of tentacle from C. nozakii compared with N. nomurai. In addition,a comparison of IC50values of C. nozakii and N. nomurai nematocyst venoms on A9, A673, and A431 cells showed that A431 cells were most sensitive to the cytotoxic effects of both jelly fish venoms (Fig.14).
In conclusion, our results showed that both C. nozakii and N. nomurai jelly fish venoms have strong hemolytic and cytotoxic activities, which might be attributed to the protein content of the venoms, with venoms from C. nozakii showing stronger toxic activities than those from N. nomurai. Sheep erythrocytes were the most sensitive to the hemolytic effects of both jelly fish venoms, and the EC50values of venoms from N. nomurai and C. nozakii were 69.69 and 63.62 μg/mL following 30-min exposure, respectively. The IC50values of venoms from N. nomurai and C. nozakii were 68.6 and 40.9 μg/mL, respectively, following 24-h incubation with A431 human epidermal carcinoma cells. Given that jelly fish nematocysts contain at least one toxic component, further biochemical investigations are required to characterize the different components of jelly fish nematocyst extracts and to clarify their mechanism of action.
5 CONCLUSION
Comparative study on the toxic activities of crude venoms from C. nozakii and N. nomurai nematocysts shows that nematocyst venom from C. nozakii had stronger hemolytic and cytotoxic activities than that from N. nomurai. We concluded that C. nozakii might be more harmful to the health of humans than be N.nomurai, and, thus, people are advised to pay more attention on the risk of sting by C. nozakii.
猜你喜欢
杂志排行
Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Recent insights into physiological responses to nutrients by the cylindrospermopsin producing cyanobacterium,Cylindrospermopsis raciborskii*
- Response of Microcystis aeruginosa FACHB-905 to different nutrient ratios and changes in phosphorus chemistry*
- In fluence of light availability on the speci fic density, size and sinking loss of Anabaena flos- aquae and Scenedesmus obliquus*
- Application of first order rate kinetics to explain changes in bloom toxicity—the importance of understanding cell toxin quotas*
- Regime shift in Lake Dianchi (China) during the last 50 years*
