The post-larval and juvenile fi sh assemblage in the Sukhothai Floodplain, Thailand*
2018-07-11SIRIWANSuksriBOONSATIENBoonsoong
SIRIWAN Suksri, BOONSATIEN Boonsoong
Animal Systematics and Ecology Speciality Research Unit(ASESRU),Department of Zoology,Faculty of Science,Kasetsart University,Bangkok,10900,Thailand
Received Nov. 1, 2016; accepted in principle Dec. 22, 2016; accepted for publication Mar. 7, 2017
© Chinese Society for Oceanology and Limnology, Science Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
AbstractThis study investigated abundance, species composition and spatial and temporal distributions of fi sh larvae and their relationship with some environmental variables in the Sukhothai floodplain in northern Thailand. Fish larvae were collected from 33 sampling stations on 8 occasions between August 2010 and October 2013. The study collected and identified 149 296 individuals, representing 32 families and 165 taxa. The species composition of larval fi sh was dominated by the Cyprinidae (47.27%), Cobitidae(7.88%), Siluridae (6.67%), Bagridae (6.06%) and Mastacembelidae (3.33%) families. The mostabundant larval species were the Striped fl ying barbEsomus metallicus(16.90%), the Siamese mud carpHenicorhynchus siamensis(8.48%) and the Sumatran river spratClupeichthys goniognathus(8.31%). The greatest abundance and species diversity of larvae were found when the river flow runs onto the floodplain.PCA and nMDS analysis revealed that the samples plot is associated with temporal distribution among years. The discharge was a major factor determining fi sh larvae assemblage and environmental variables in the Sukhothai floodplain. Four fi sh larval species were positively correlated with the samples for 2013. The result of the CCA ordination plot showed that only the discharge variable was strongly correlated with fi sh larvae abundance, especially two cyprinidRasboraspecies.
Keyword:fi sh larvae; distribution; diversity
1 INTRODUCTION
A floodplains is an ecotone that forms a transition between aquatic and terrestrial environments.Floodplains are very important because they provide a variety of habitats and areas of high biodiversity. All this depends on the natural water flow and the predictable, seasonal pattern of rising and falling floodwaters (Moss, 2010). Floodplains are one of the most important fishery resources, providing spawning and rearing grounds. Large river floodplains are considered key nursery habitats for many species of riverine fi sh (Górski et al., 2011), which allows aquatic organisms to migrate into inundated floodplains. One of the particular characteristics of river floodplains is that they provide dependable sources of food. Fish returning to the main channels as the floodwater recedes are fat from this source. In addition, floodplains provide some of the most productive and diverse freshwater fi sheries, especially in tropical regions (Moss, 2010).
Asia is the continent most frequently affected by floods, especially tropical parts of the continent.During the past 30 year, more flood disasters have occurred in Asia (40% of the total) than on any other continent, including the Americas (25%), Africa(17%), Europe (14%), and Oceania (4%) (Dutta and Herath, 2004). Asia contains many floodplains, the largest three of which are in China, India and Bangladesh, respectively (Hussain, 2010). The largest floodplain in Southeast Asia is the Great Lake, located in the Mekong River basin of Cambodia. The water level of this area increases standing water bodies by up to 9 m in flood season. Most floodplains are covered by several meters of floodwater for 3-4 months annually. In flood season, land and water together in the major flood zones account for 58 000 km2, much of which is covered with rice fi elds. During floods, adult fi sh move onto the floodplain to feed and spawn (Hortle, 2009). The Sukhothai floodplain is the second large floodplain of Thailand, and covers area about 320 km2. This floodplain is an importance area for fi sh productivity,spawning and rearing ground.

Fig.1 Map showing the location of sampling stations in the Sukhothai floodplain
Unfortunately, the basic taxonomic and ecological status of fi sh larvae assemblages on the floodplains in Thailand has received little study, and most previous studies have focused on adult fi sh communities in various parts of Thailand (Choi et al., 2005; Kottelat,2013; Phomikong et al., 2015). Tanaka et al. (2015)surveyed fi sh assemblages and investigated environmental and landscape parameters in a total of 135 floodplain waterbodies in the Chao Phraya River Basin. The study showed the population of juvenile fishes increasing in temporarily connected floodplain waterbodies to main rivers compared with isolated waterbodies (Tanaka et al., 2015). The hypotheses of this study were 1) fi sh larval assemblage are variable by the annual and seasonal patterns and 2) fi sh larvae and environmental factors are relate to those patterns.The objective of the present study was to evaluate the spatiotemporal variation in the abundance and diversity of fi sh larvae in the Sukhothai floodplain.
2 MATERIAL AND METHOD
2.1 Study area
The Sukhothai floodplain is part of the Yom River Basin in northern Thailand. The floodplain is located in the Yom River Valley in southernmost northern Thailand (16°76′-17°04′N, 99°82′-100°13′E), at an altitude of 40-52 m above mean sea level. The Yom River Basin has a total catchment area of 23 616 km2,and its main channel runs down a steep valley at about 1:700. There are narrow plains along the river itself,which flows southwest through a large plain (Thailand Water Partnership, 2014).
2.2 Sample collection and processing
Sampling was undertaken at 33 stations in the Sukhothai floodplain. The stations were divided into 4 habitat types: (1) river (R), 5 stations; (2) main canal(M), 8 stations; (3) branch canal (B), 3 stations; and(4) flood areas (F), 16 stations (Fig.1). To sample flow within the floodplain, 3 distinct types of flood periods were chosen, each defi ned by flow orientation: (1)river flow runs into the floodplain (P1, August); (2)constant water level in floodplain (P2, September);and (3) river flow runs off of the floodplain (P3,October). Samples were obtained during all 3 flooding periods in all 3 collection years: 2010, 2011 and 2013.Samples were taken at the water’s surface. The pH,water temperature and electrical conductivity were measured in the fi eld using the pH meter (YSI,pH100A), thermometer and conductivity meter (YSI,EC 300), respectively. Using titration methods, the water samples were analyzed in the fi eld for dissolved oxygen (mg/L), alkalinity (mg/L), hardness (mg/L)and free CO2(mg/L). The transparency and depth were measured (m) for physical parameters. The daily periodicity of the discharge (m3) and rainfall (mm)data were obtained from Hydrology Irrigation for the Lower Northern Region, the Royal IrrigationDepartment of Thailand (RID) (Royal Irrigation Department, 2014).
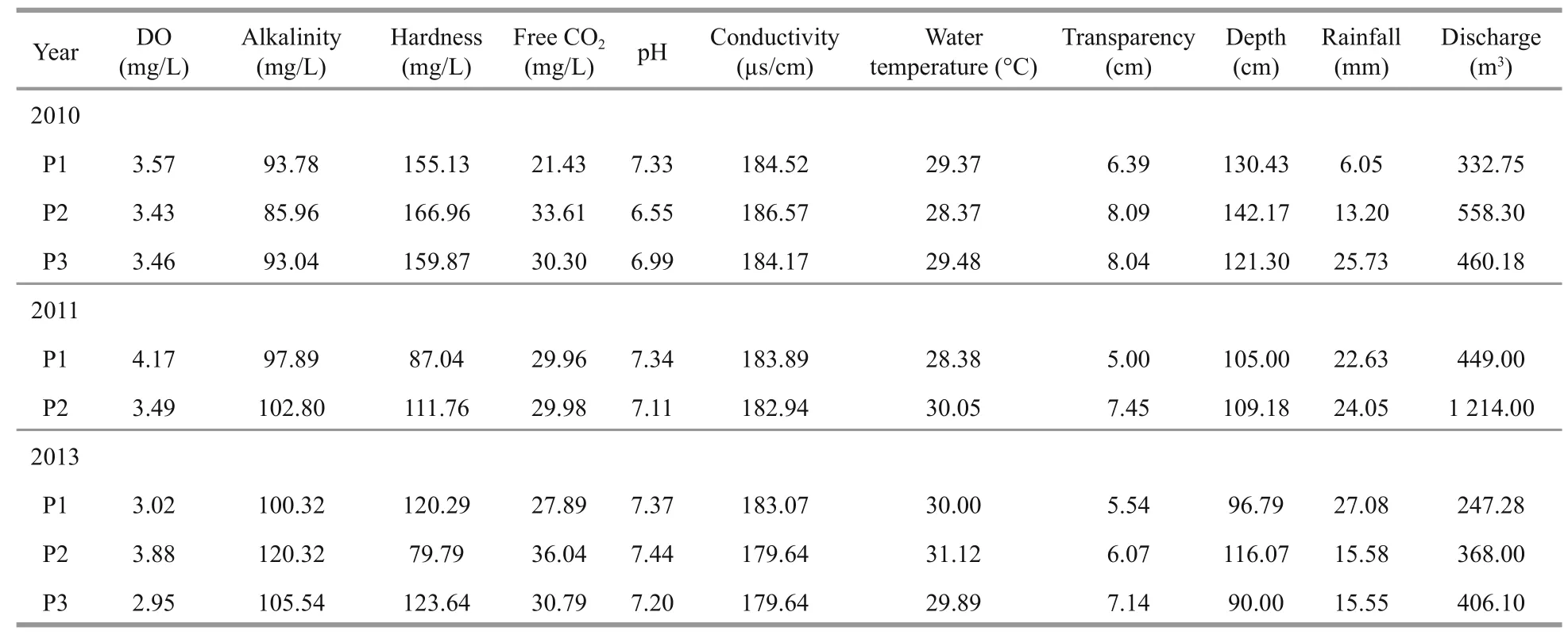
Table 1 Mean value of some physico-chemical and hydrological parameters of the Sukhothai floodplain in the period ofinvestigations from 2010 to 2013
In the margins, fi sh larvae were sampled using a micromesh seine net 30 m wide and 4 m deep, with 1-mm sized mesh. At each sample site, 3 sweeps of the net were made in different locations.
The samples were fi xed in 10% neutral buffered formalin. Then, specimens were transferred to glass vials and preserved in 70% ethyl alcohol for identifi cation. Post-larval and juvenile stages of fishes were identified to the lowest possible taxon, usually the species level, according to Termvidchakorn (2003,2005), Termvidchakorn et al. (2005, 2007) and Termvidchakorn and Hortle (2013).
2.3 Data analysis
From the sample data for each site, abundance of larvae was standardized using population density methods (Wootton, 1992). Fish larvae assemblage change in response to habitats and years were visualized by performing a non-metric,multidimensional scaling algorithm (nMDS) on abundance data matrices (habitats) and presence/absence data (years). Temporal change of environmental variables among years was analyzed by a Principal Component Analysis (PCA). The vectors of all environmental variables to evidence the relationship between variables, axes and samples were shown in PCA ordination. It required an initial standardization of abundance and environmental data,which was performed by the general relativizations. A Sorensen distance matrix, using presence/absence data of taxa among years, was analysed by nMDS.The distance was computed, random staring confi gurations were used and 100 iterations were completed,by sample, with the sample and abundance of species collected in each sampling site, sampling period and year. The relationship between species distribution(abundance) and environmental factors was investigated using canonical correspondence analysis (CCA). All statistical analyses were performed using PC-ORD software version 6.08 (McCune and Mefford, 2011).
3 RESULT
3.1 Environmental variables
Table 1 contains the study’s physico-chemical parameters and hydrological data. Both physicochemical and hydrological parameters varied by period and year. Slight variations in environmental variables were found, including dissolved oxygen(DO) (range, 2.95-4.17 mg/L), alkalinity (range,85.96-120.32 mg/L), free CO2(range, 21.43-36.04 mg/L), conductivity (range, 179.64-186.57 μs/cm), water temperature (range, 28.37-31.12°C) and transparency (range, 5.00-8.09 cm). Water pH varied from 6.55-7.44. Hardness (range, 79.79-166.96 mg/L)and depth (range, 90-142.17 cm) increased during 2010. During the study period, peak discharge occurred in 2011, and the lowest discharge in 2013(Fig.2). The rainfall (range, 6.05-27.08 mm) and discharge (range, 247.28-1 214.00 m3) increased during periods of a constant water level in the floodplain (P2) during 2010 and 2011. In addition, the PCA analysis revealed that the environmental parameters in 2010 were clearly different from those in 2011 and 2013. Among the environmental variables,discharge strongly correlated with axis 2 and increased in periods of a constant water level in the floodplain(P2) in 2011 (Fig.3).

Fig.2 Hydrograph showing the annual flow events (discharge, m3) in the Sukhothai during the study period (2010-2013)
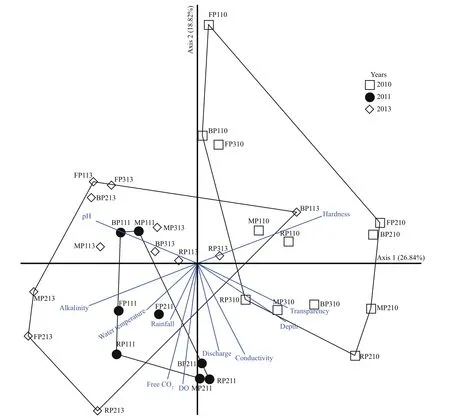
Fig.3 Biplot of sample coordinates on the fi rst two axis of PCA with distance-based biplot scores, including all 32 samples for those an environmental variables

Fig.4 nMDS ordination plot of samples by taxa dissimilarities (abundance data), with habitat identified (stress=16.82%),including all 32 samples for those a faunal list was established (solid dots=fish species)
3.2 Larval fi sh composition, abundance and habitats
Table 2 contains the species number and abundance of fi sh larvae in each habitat. The main canal (M) and flood areas (F) habitats accounted for 147 species and 140 species, respectively, of the total catch, while the river (R) and branch canal (B) habitats accounted for 131 and 108 species, respectively. In terms of larval density, the catch was dominated by taxa associated with the flood areas and branch canal habitats, which accounted for 4 733 and 4 006 ind./100 m2, respectively.The remaining portion of the catch consisted of taxa associated with the main canal (3 376 ind./100 m2)and river (2 529 ind./100 m2) habitats. Figure 4 presents the results of ordination using nMDS analysis on transformed larval fi sh-abundance data. It is clear that the river habitat samples on the left side of the plot (axis 3) were separated from the other habitats on the right side of the plot. Four species were positively correlated with the river habitat, includingPapuligobiusocellatus,Mystusbocourti,HypophthalmichthysnobilisandHeteropneustes kemratensis, indicating that they were the most-abundant species in the river habitat. This, in turn,indicates that the spatial pattern of the larval fi sh abundance was better represented by the river habitat type.

Table 2 Species number and abundance of fi sh larvae assemblages of the Sukhothai floodplain in each habitat during 2010 to 2013
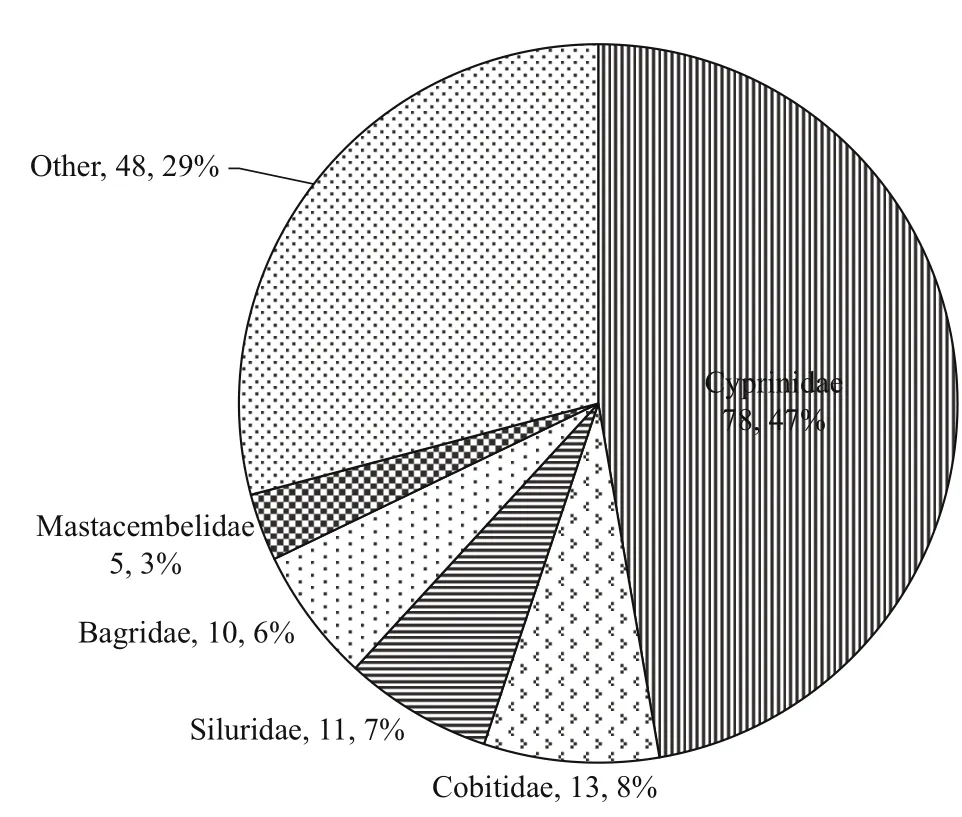
Fig.5 Percentage of species composition of the dominant families of fi sh larvae sampled in the Sukhothai floodplain from 2010-2013
The study collected 149 296 fi sh larvae representing 11 orders, 32 families, 84 genera and 165 species from 33 sampling stations along the Sukhothai floodplain. Figure 5 shows each of the dominant families of fi sh larvae as a percentage of total catch,including the number of species within each family.The catch was dominated by five families: Cyprinidae(78 species, 47.27%), Cobitidae (13 species, 7.88%),Siluridae (11 species, 6.67%), Bagridae (10 species,6.06%) and Mastacembelidae (5 species, 3.33%).Table 3 lists the 10 most-dominant larval fi sh species,each of which contributed more than 2% of the total catch. Of these 10, the top 4 were the cyprinidEsomus metallicus, the cyprinidHenicorhynchussiamensis,the clupeidClupeichthysgonionathusand the cyprinidHenicorhynchuscaudimaculatus, which accounted for 16.90%, 8.48%, 8.31% and 6.66% of the total catch, respectively. The next six species each accounted for less than 5% of the total catch: cyprinidsParachelaoxygastroides(4.83%),Barbonymus gonionotus(3.41%),Amblypharyngodon chulabhornae(2.88%) andParambassissiamensis(2.45%), the gobiidGobiopteruschuno(3.01%) and the clupeidClupeichthysaesarnensis(2.29%).
3.3 Larval fi sh distribution

Fig.6 nMDS ordination plot of samples by taxa dissimilarities (presence/absence data), with year identified (stress=14.33%),including all 32 samples for those a faunal list was established (solid dots=fish species)
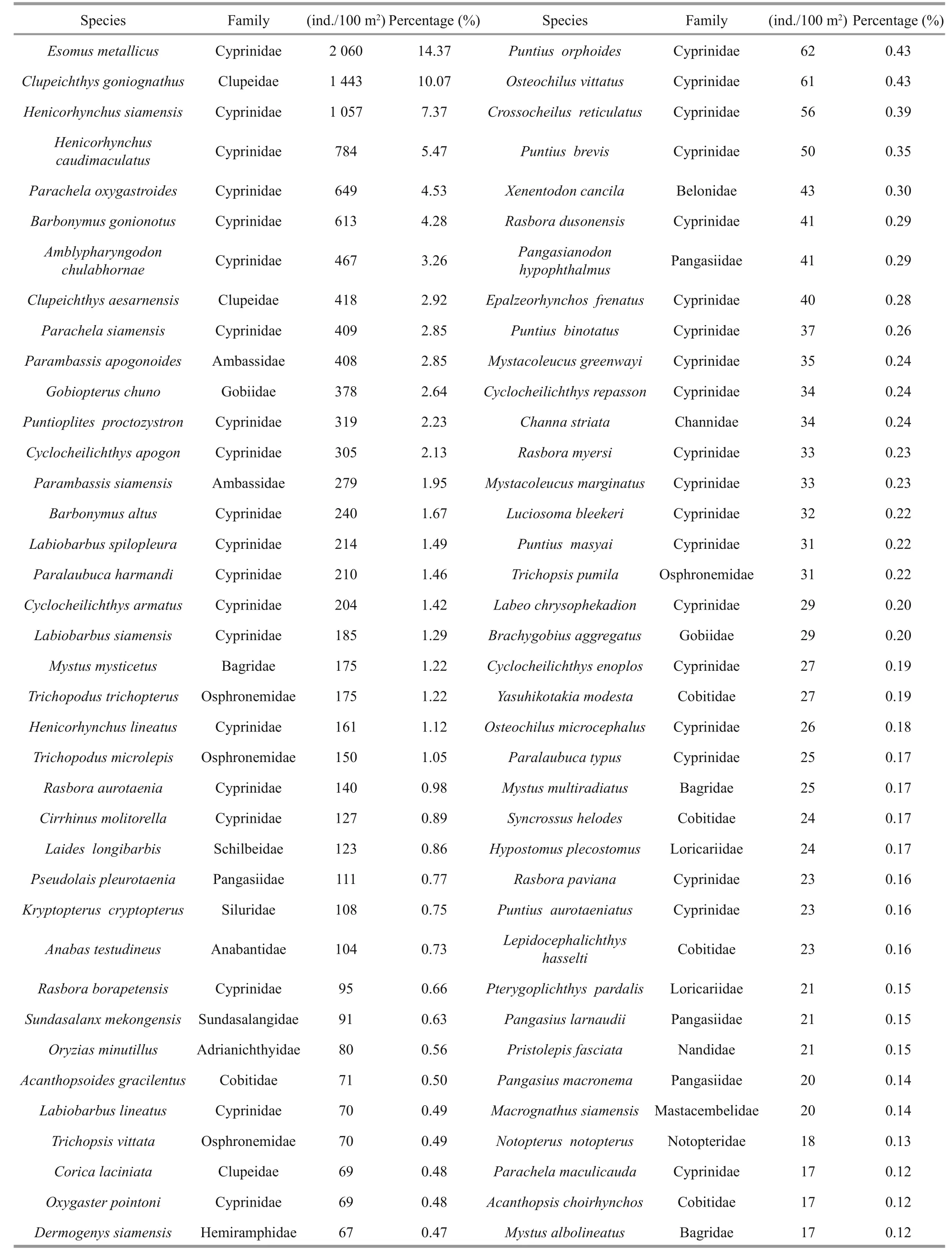
Table 3 Species list of fi sh larvae collected during the period (ordering by dominant taxon)
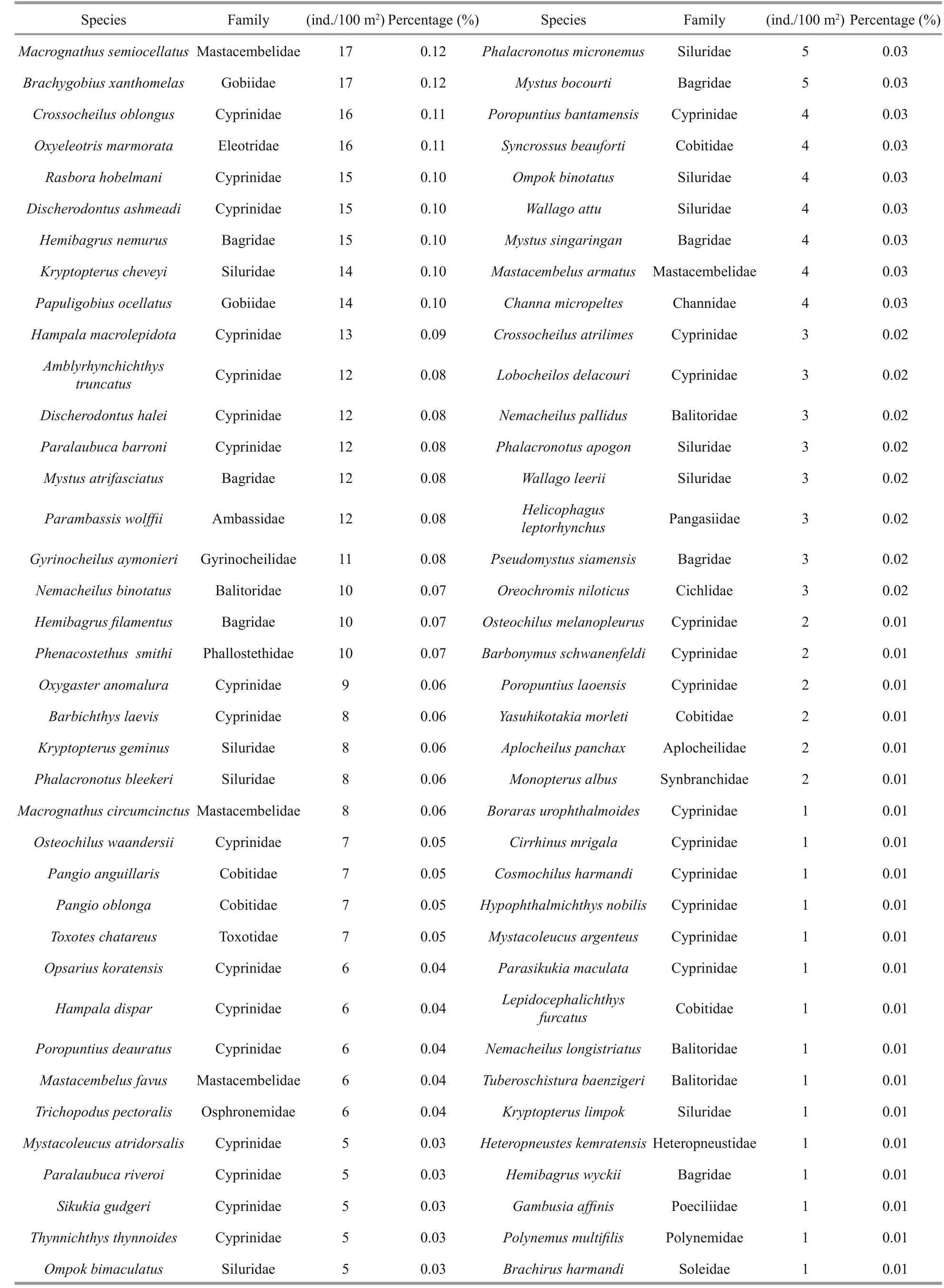
Table 3 Continued

Fig.7 CCA ordination plot of samples by environmental variable vector (discharge) and abundance of Rasbora aurotenia (a)( R2=0.389) and Rasbora dusonensis (b) ( R2=0.402), with taxa abundance superimposedIn the plot, the larger symbols denote greater values (higher abundance).
Figure 6 summarises nMDS analysis of the composition and structure of the fi sh larvae assemblages at each station during each period in each year. After 100 iterations, the stability criterion was met with a fi nal stress of 14.33 (Monte Carlo test:P=0.009 91) for a three-dimensional solution. The location of the points along axis 1 (Fig.6) identified years (temporal scale) as the main pattern in the composition and structure of the fi sh larvae assemblages. Samples for 2010 were positively correlated with axis 1. In contrast, samples for 2013 were negatively correlated with axis 1. It is clear that the samples plot is associated with temporal distribution among years. Figure 6 shows the species plot: cyprinidDischerodontushalei, gyrinocheilidGyrinocheilusaymonieri, cyprinidOpsarius koratensisand balitoridNemacheilusbinotatustypically populate the samples for 2013, located on the left side of the plot. This ordination indicates that they were positively correlated with the samples for 2013. No spatial or temporal patterns were observed in axis 3.
3.4 Larval fi sh abundance and environmental linkages
Relationships between fi sh larvae abundance and abiotic variables were analysed using CCA analysis.The result of the ordination plot showed that only the discharge variable was strongly correlated with fi sh larvae abundance (Fig.7). The discharge vector strongly correlated with axis 3, increasing in periods of a constant water level in the floodplain (P2) in 2011. Two cyprinid species,RasboraauroteniaandRasboradusonensis, were strongly correlated with axis 3, providing values ofR2=0.389 and 0.402,respectively (Fig.7). These species were observed on the lower part of the plot, associated with the P2 samples and with high discharge values. The larvae ofR.auroteniaandR.dusonensisdominated and accounted for mean values (±SD) of 32.48±11.04 and 70.19±29.70 ind./100 m3, respectively.
4 DISCUSSION
4.1 Environmental variables
Generally, both physico-chemical and hydrological parameters varied by year and period. Most parameters did not exceed the surface-water quality standard of Thailand and the appropriate water-quality criteria for aquatic living. The water temperature and pH varied from 23-32°C and from 5.0-9.0, respectively. Most dissolved oxygen concentrations were greater than 3 mg/L, which is appropriate for freshwater aquatic resources (Pollution Control Department, 1992). In periods of a constant water level in the floodplain, the discharge profi le and the water level showed increasing trends in 2011. Changing in limnological characteristics during the flooding period may be affect to fi sh species (Baumgartner et al., 1997).Increasing floodplain area probably reflected a direct association with floodplain utilization by migratory fi sh species (Tanaka et al., 2015).
4.2 Larval fi sh assemblages
The dominant larval species were the cyprinids,represented mainly by the common speciesEsomus metallicus. This cyprinid specie is abundant in the Mekong and Chao Phraya River systems and is found in both freshwater and brackish habitats. It is the most-common and widely distributed species in Indochina and Sumatra. In all floodplains within its natural distributional range, this species is usually abundant and one of the commonest freshwater fi sh(Arbsuwan et al., 2012). The larvae ofE.metallicuswere approximately 17% of the total catch, which means it was relatively highly abundant. According to Tanaka et al. (2015) found thatE.metallicusis one of four common species increased in temporarily connected survey sites in waterbodies of the Chao Phraya river basin.
The second most-abundant species of freshwater cyprinid fi sh, the Siamese mud carpHenicorhynchus siamensis, is a variety of Asian carp native to the Mekong and Chao Phraya Rivers in Southeast Asia. It is very common in floodplains during the wet season and migrates upstream in the Mekong, the migration pathway begins in Cambodia (Suvarnaraksha et al.,2011).
The third most-abundant species,Clupeichthys goniognathus, is a pelagic clupeid fi sh inhabiting the lower reaches of rivers, although it has also been recorded in reservoirs. This species also occurs in floodplains and lakes during flooding season, which agrees with the results of the present study. It is widely distributed in Thailand, Malaysia, Indonesia,Cambodia, and the Lao PDR (Vidthayanon, 2012a).Jutagate et al. (2003) and Cowx et al. (2015) have identified differences in species composition in the ichthyoplankton drift in the Lower Mekong Basin in various seasons. They found that the flooding period(maximum water level in the Mekong is from August-October) was dominated by Cyprinidae, which agrees with the results of this study. In the study by Cowx et al. (2015), the most abundant species wereLaides longibarbis(11.2%),Cyclocheilichthysenoplos(10.3%) andPuntioplitesproctozysron(8.0%), all which were found in the main channels of rivers. In the present study, all the commonly abundant fi sh larvae are associated with floodplain areas, and all are used locally both as food and commercially, in the aquarium trade, although pollution and large-scale overfishing may be threaten these species in the future(Mondal et al., 2010).
The spatial pattern of larval fi sh abundance is represented by the habitat type. The abundance of four larval fi sh species,Papuligobiusocellatus,Mystusbocourti,HypophthalmichthysnobilisandHeteropneusteskemratensis, correlated with the river(R) habitat. Two native fi sh species,P.ocellatusandM.bocourti, are riverine fi sh recorded in several areas of Thailand. In addition,M.bocourtiis a vulnerable fi sh species. Another native fi sh species,H.kemratensis, is distributed throughout the Irrawaddy, Sittoung and Salaween Basins in Myanmar and the Chao Phraya, Eastern and Tapi Basins in Thailand (Ratmuangkhwang, 2007). The present study also found an invasive species, a bighead carp,H.nobilis. Bighead carp are native to the large rivers and associated floodplain lakes of eastern Asia. This specie is one of the most important fi sh in aquaculture(Kara, 2012).
In 2010, the main canal (M) habitat showed the highest values for fi sh larvae species richness,evenness and SDI, but slight differences were observed in the diversity and richness of species among the spatiotemporal scales, which may be related to the characteristics of these sites (Daga et al.,2009). The Sukhothai floodplain suffered flooding in 2011 and drought in 2013, causing changes in the pattern, quantity and intensity of flow in the area. If they continue, these condition may affect the diversity and richness of larval fi sh species in the Sukhothai floodplain.
In 2013, four distinctive species were observed in the larval fi sh assemblage. The fi rst was the cyprinidDischerodontuscolemani, which found in the upper Chao Phraya basin in northern (Vidthayanon, 2012b).The second was the cyprinidOpsariuskoratensis,which is also found in clear water with sandy-rocky bottoms (Rainboth, 1996). The third was the gyrinocheilidGyrinocheilusaymonieri, which inhabits flowing streams and tributaries with substrates of boulders, pebbles, gravel and sand(Rainboth, 1996) and occurs in medium- to largesized rivers and enters flooded fi elds. The fourth was the balitoridNemacheilusbinotatus, which inhabits streams and rivers with moderate current over sandy or pebbly substrate.
The study’s CCA analysis revealed that in addition to the temporal changes noted, the main environmental gradient was due to discharge. Discharge gradient is a common feature of tropical rivers and has pronounced temporal effects on fi sh composition and distribution.Shuai et al. (2016) found that mean water temperature,river discharge, atmospheric pressure, maximum temperature and precipitation play important roles in larval occurrence patterns. The cyprinid larval species,Megalobramaterminalis,Xenocyprisdavidi,andCirrhinusmolitorella, are associated with high precipitation, high river discharge, low atmospheric pressure and low DO concentrations which featured during the summer month (Shuai et al., 2016). While,no association was observed between environmental variables and spatial and temporal patterns of the ichthyoplankton assemblages of the floodplain of Upper Parana River, in Ilha Grande National Park,southern Brazil (Gogola et al., 2013).
River discharge and the timing of floods are increasingly being recognised as important causes ofinter-annual variability in the recruitment success of cyprinid fi sh (Nunn et al., 2007). In addition, discharge variables correlate with the abundance of larvalR.auroteniaandR.dusonensis. Both two species occur near the surfaces of ponds, canals and streams, are often found in turbid waters and migrate from rivers into flooded forests and swamps (Rainboth, 1996). In the present study, twoRasboraspecies seem not to be migratory species, however, two larval species(HemicorhynchussiamensisandHemicorhynchus caudimaculatus) of top 10 abundant fishes categorise as migratory fishes (Cowx et al., 2015). This presence of the larvae of migratory species indicated that the Sukhothai floodplain may be important to maintaining these species. In general, migratory species spawn in the open waters of the main channel or in tributaries,and the eggs and larvae are transported passively by currents to flooded areas and marginal lagoons, where they complete their development (Daga et al., 2009).Therefore, the larvae of migratory species in the Sukhothai floodplain possibly originated from spawning events in the Yom River.
5 CONCLUSION
The post-larval and juvenile fi sh assemblages and environmental parameters in the Sukhothai floodplain were investigated in August 2010 and October 2013.The dominant larval family was Cyprinidae,accounting for 78 taxa.Esomusmetallicuswas the most abundant species, followed byHenicorhynchus siamensis,Clupeichthysgonionathus(Clupeidae) andHenicorhynchuscaudimaculatus. The results of this study indicate that variations in larval fi sh assemblage are temporal in nature. The larval fi sh assemblage in 2013 was different from that in 2010 and 2011, and some trends were distinguishable. Four fi sh species,Discherodontuscolemani,Gyrinocheilusaymonieri,OpsariuskoratensisandNemacheilusbinotatustypically populate the samples for 2013. The water discharge was a major factor determining fi sh larvae composition and structure in floodplain. Specifically,there was evidence for the changes in larval fi sh assemblage in 2013 (the discharge decreasing, and the water level and rainfall also showed decreasing trends in 2013). Moreover,RasboraauroteniaandRasboradusonensiswere strongly correlated with the discharge parameter.
6 ACKNOWLEDGEMENT
We are most grateful to our colleagues for assistance during fi eld trips. We would like to thank the Department of Fisheries and Department of Zoology for facilities and assistance.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Response of the North Pacific Oscillation to global warming in the models of the Intergovernmental Panel on Climate Change Fourth Assessment Report*
- Effect of mesoscale wind stress-SST coupling on the Kuroshio extension jet*
- Surface diurnal warming in the East China Sea derived from satellite remote sensing*
- Cross-shelf transport induced by coastal trapped waves along the coast of East China Sea*
- Observations of near-inertial waves induced by parametric subharmonic instability*
- Seasonal variation and modal content ofinternal tides in the northern South China Sea*
