瑞巴派特通过抑制miR-877-5p的表达减轻阿司匹林对人胃黏膜上皮细胞损伤的研究
2018-06-27,,,,,
, ,, , ,
南京医科大学附属南京医院(南京市第一医院) 1.消化科; 2.中心实验室,江苏 南京 210006; 3.解放军南京总医院急诊科
【Abstract】ObjectiveTo investigate whether Rebamipide plays a protective role on Aspirin-induced injury through inhibiting the expression of miR-877-5p in human gastric epithelial cells (GES-1).MethodsCultured GES-1 cells were divided into control group, Aspirin injured group and different concentrations (0.25, 0.5, 1.0 mmol/L) Rebamipide plus Aspirin groups. The expression of miR-877-5p was detected by qRT-PCR. The Aspirin treated cells were transfected with miR-877-5p inhibitors. The combination of Rebamipide and Aspirin treated cells were transfected with miR-8877-5p mimics. Cell proliferation and apoptosis were detected by CCK-8 and flow cytometry. The targeted genes of miR-877-5p were predicted by miRNA target databases, the GO and KEGG pathway were analyzed by DAVID database.ResultsqRT-PCR showed that the expression of miR-877-5p in Aspirin group was the highest than others. The expressions of miR-877-5p in different concentrations (0.25, 0.5, 1.0 mmol/L) of Rebamipide plus Aspirin groups were 4.28±0.25, 2.45±0.28 and 1.47±0.17. The expression of miR-877-5p was gradually decreased with the increase of concentration of Rebamipide. The CCK-8 assay and flow cytometry showed that miR-877-5p mimics transfection blocked proliferations and promoted apoptosis in combination of Rebamipide and Aspirin treated cells, while miR-877-5p inhibitors promoted proliferation and inhibited apoptosis in Aspirin treated cells. The pathways of miR-877-5p targeted genes were almost focused on cAMP signaling pathway, MAPK signaling pathway, PI3K-Akt signaling pathway, and so on.ConclusionRebamipide alleviates Aspirin-induced injury through inhibiting the expression of miR-877-5p in human gastric epithelial cells.
【Keywords】 Rebamipide; Aspirin; Gastric mucosa damage; miR-877-5p
近年来,随着阿司匹林在心脑血管疾病中的广泛应用,其相关不良反应尤其是胃黏膜损伤的发生率逐年上升。阿司匹林相关胃黏膜损伤的临床表现可以无症状或有消化不良、出血、穿孔等。瑞巴派特是临床上常用的胃黏膜保护剂,广泛应用于胃黏膜损伤和溃疡的防治中,尤其在阿司匹林相关胃黏膜损伤的防治中效果突出[2-4]。然而,瑞巴派特对胃黏膜保护作用的机制尚未完全明确,近年来研究[5-6]发现,miRNAs参与了细胞发育、增殖、分化和凋亡等重要过程,这为研究瑞巴派特对胃黏膜保护作用机制提供了新的研究方向。
有研究[7]发现,NSAIDs类药物引起的细胞损伤中miR-877-5p明显上调,而我们经生物信息学初步分析发现,其可能参与细胞的增殖和凋亡调控,因此推测NSAIDs药物阿司匹林对胃黏膜的损伤作用可能与miR-877-5p有关,而胃黏膜保护剂瑞巴派特也可能是通过调控该miRNA表达发挥保护性作用。因此,本研究将从阿司匹林和瑞巴派特对miR-877-5p表达的影响和miR-877-5p对人胃黏膜上皮细胞GES-1增殖、凋亡的影响两方面入手,阐明瑞巴派特防治阿司匹林相关胃黏膜损伤的作用机制,为瑞巴派特的临床应用提供更多的理论基础。
1 材料与方法
1.1材料人胃黏膜上皮细胞株GES-1由南京市第一医院中心实验室保存。瑞巴派特(上海阿拉丁生物技术有限公司),阿司匹林(美国Sigma公司),脂质体转染试剂 Lipofectamine 2000(美国 Invitrogen),miR-877-5p mimics和miRNA negative control(NC)、miR-877-5p inhibitor和miRNA inhibitor negative control(INC)、实时荧光定量试剂盒(上海吉玛),细胞总RNA提取试剂Trizol、CCK-8试剂盒、Annexin V-FITC/PI双染细胞凋亡检测试剂盒(南京翼飞雪生物科技有限公司)。
1.2 方法
1.2.1 细胞培养:GES-1细胞用含质量浓度为100 g/L胎牛血清的DMEM高糖培养基,在37 ℃、体积分数为5%的CO2条件下常规培养传代。
1.2.2 实验分组:研究瑞巴派特对细胞中miR-877-5p表达的影响,实验分为5组:不加药物干预的空白对照组(NC组);加入2.21 mmol/L浓度的阿司匹林培养液(即IC10浓度)处理24 h设为阿司匹林损伤组[8];加入不同浓度(0.25、0.5、1.0 mmol/L)瑞巴派特预处理2 h,再加入IC10浓度的阿司匹林培养液处理24 h,设为瑞巴派特保护组。实验重复3次。
研究miR-877-5p对GES-1细胞增殖和凋亡的影响,实验分为4组:1、2组均用终浓度为0.5 mmol/L瑞巴派特联合阿司匹林处理24 h后分别加入miR-877-5p mimics、NC进行转染;3、4组均用阿司匹林处理24 h后分别加入miR-877-5p inhibitor、INC进行转染。转染后进行细胞凋亡实验和细胞增殖实验。
1.2.3 细胞转染:取对数生长期接种于细胞培养板中培养24 h,待细胞达70%~80%融合时进行转染。按照Lipofectamine 2000说明书进行转染,将miR-877-5p mimics、NC、inhibitor和INC分别转染至细胞中。6 h后更换新鲜培养基,48 h后收集细胞或进行相关实验,通过实时荧光定量聚合酶链式反应(qRT-PCR)验证转染是否成功。
1.2.4 qRT-PCR:收集细胞,提取总RNA,测定RNA浓度并按照说明书进行逆转录,将逆转录产物cDNA加入反应体系中进行qPCR反应,检测各组细胞间miRNA表达的差异。基因相对表达量用2-ΔΔCt表示。实验重复3次,取均值。
1.2.5 细胞增殖检测:细胞铺于96孔板中,分别继续培养24 h、48 h和72 h,每孔加入10 μl CCK-8,37 ℃孵育2 h,以450 nm为测定波长,在酶标仪上检测各孔密度值(OD值)。OD值可反映细胞增殖情况,每组设4个复孔,实验重复3次,取均值。
1.2.6 细胞凋亡检测:各组细胞转染48 h后,收集细胞,PBS洗涤2遍,依次加入500 μl结合缓冲液及Annexin V FITC和PI各5 μl,混匀后室温避光孵育15 min,上流式细胞仪检测。实验重复3次,取均值。
1.2.7 miR-877-5p靶蛋白预测:通过miRNA靶基因在线预测软件(Targetscan、miRWalk、miRanda),取3个数据库预测的交集靶基因进行GO功能富集分析和KEGG信号通路富集分析。
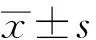
2 结果
2.1miR-877-5p在阿司匹林损伤组和瑞巴派特保护组中的表达情况qRT-PCR检测miR-877-5p表达量,设空白对照组表达量为1,阿司匹林损伤组为6.43±0.48,0.25 mmol/L瑞巴派特保护组为4.28±0.25,0.5 mmol/L瑞巴派特保护组为2.45±0.28,1.0 mmol/L瑞巴派特保护组为1.47±0.17,阿司匹林损伤组中miR-877-5p表达高于其他保护组(P<0.001)。不同浓度的瑞巴派特保护组中,miR-877-5p表达量依次降低,呈浓度依赖性(F=71.87,P<0.01)。以上结果说明,瑞巴派特能够抑制GES-1细胞中miR-877-5p的表达。
2.2转染miR-877-5pmimics和inhibitor的效果分析qRT-PCR结果显示,miR-877-5p mimics组miR-877-5p的表达是NC组的(92.77±8.00)倍,差异有统计学意义(P<0.001);miR-877-5p inhibitor组miR-877-5p的表达是INC组的(0.55±0.02)倍,差异有统计学意义(P<0.01)。以上结果说明转染成功。
2.3miR-877-5p对人胃黏膜上皮细胞GES-1增殖的影响细胞增殖结果显示,转染miR-877-5p mimics组的细胞增殖能力较NC组显著降低(P<0.01),第3天抑制增殖效果最明显[(0.683±0.035)vs(1.112±0.039),P<0.001]。转染miR-877-5p inhibitor组的细胞增殖能力较INC组明显增强(P<0.05)(见表1)。以上结果提示,miR-877-5p对人胃黏膜上皮细胞的增殖具有抑制作用。

表1 CCK-8法检测miR-877-5p对人胃黏膜上皮细胞GES-1增殖的影响Tab 1 Analysis of proliferation in miR-877-5p transfected GES-1 cells by CCK-8
注:与NC组相比,*P<0.05,**P<0.001;与INC组相比,#P<0.05。
2.4miR-877-5p对人胃黏膜上皮细胞GES-1凋亡的影响流式细胞凋亡实验结果显示,NC组、miR-877-5p mimics组、INC组和miR-877-5p inhibitor组细胞凋亡率分别为(8.93±0.74)%、(24.87±3.35)%、(13.4±0.96)%、(5.87±0.53)%。与NC组比较,miR-877-5p mimics处理的细胞凋亡率明显增加,差异有统计学意义(P<0.01)。与INC组比较,miR-877-5p inhibitor处理的细胞凋亡率降低,差异有统计学意义(P<0.05)(见图1)。以上结果表明,上调细胞内miR-877-5p的表达可促进人胃黏膜上皮细胞的凋亡,而下调其表达,可抑制细胞的凋亡。
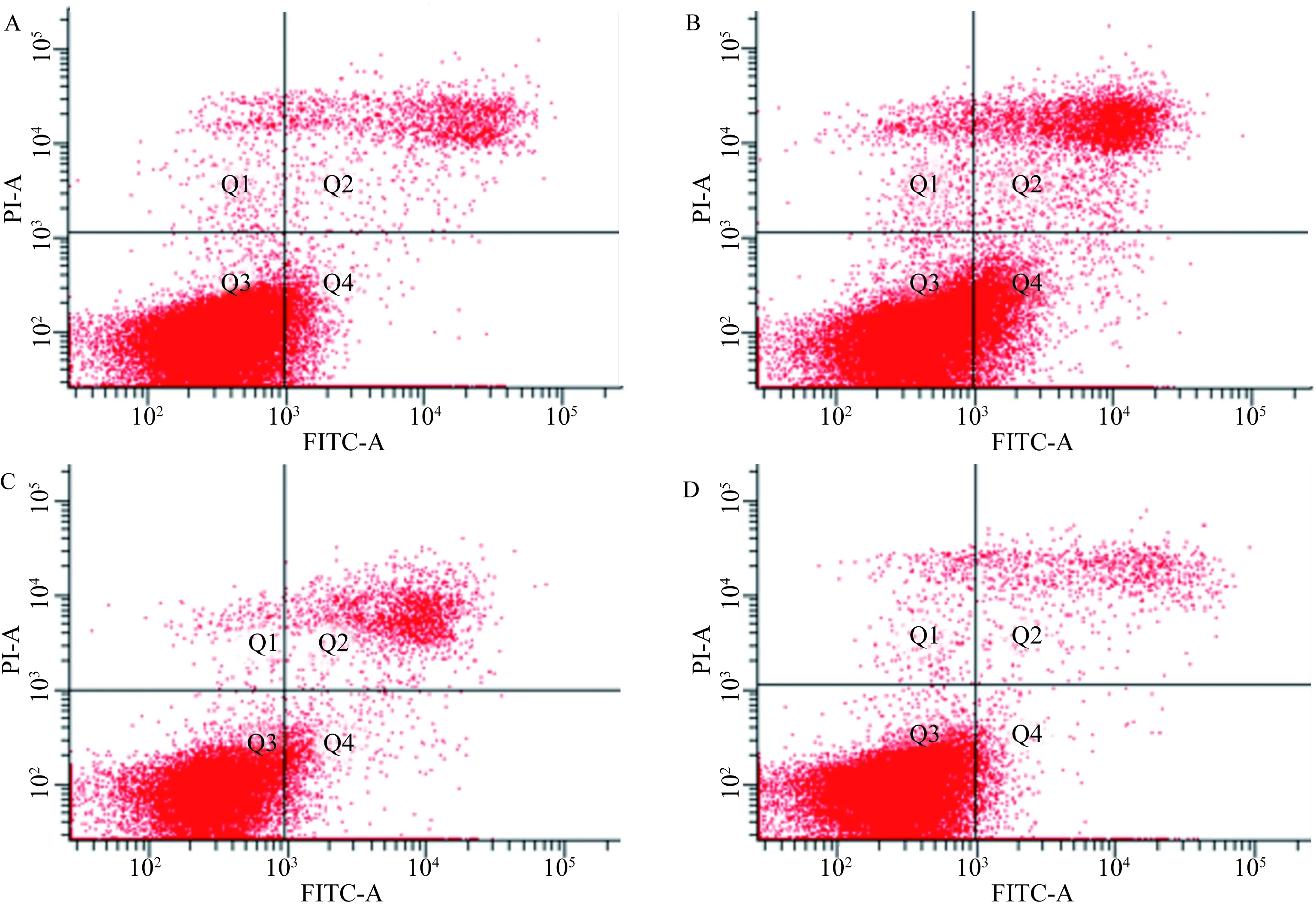
图1 流式细胞术检测miR-877-5p对人胃黏膜上皮细胞凋亡的影响
A:NC组;B:miR-877-5p mimic组;C:INC组;D:miR-877-5p inhibitor组
Fig1AnalysisofapoptosisinmiR-877-5ptransfectedGES-1cellsbyflowcytometry
A: NC group; B: miR-877-5p mimic group; C: INC group; D: miR-877-5p inhibitor group
2.5miR-877-5p靶基因预测和功能分析应用Targetscan、miRWalk和miRanda 3个数据库进行靶基因预测并取交集基因,共发现交集基因有945个。对这些靶基因富集进行GO功能富集分析发现,miR-877-5p靶基因主要参与了金属离子结合、转录调节、细胞内蛋白修饰、蛋白定位等生物功能(P<0.05)。对这些靶基因进行KEGG通路分析发现,共有65个明显富集的通路(P<0.05),其中富集指数较高的通路有cAMP信号通路、钙离子信号通路、PI3K-Akt信号通路、MAPK信号通路等(见图2)。
3 讨论
miRNA是近年来生命科学领域研究的一个热点,它是一类广泛存在于真核生物体内,长度在19~25个核苷酸的内源性非编码RNA。它能通过干扰mRNA翻译过程即碱基互补配对方式来调节靶基因的表达[9],对体内细胞的增殖、分化、损伤、凋亡等方面发挥重要作用。随着近几年研究的不断深入,细胞损伤及凋亡与miRNA的联系越来越清晰。多项研究[10-14]发现,阿司匹林可通过调节多种特异性miRNA的表达,在多种疾病如先兆子痫、胃肠道肿瘤等中发挥作用。既往研究发现,miR-877-5p参与多种细胞凋亡的过程[15-18],在多种药物引起的肝损伤中[19-22],miR-877-5p表达上调且参与肝损伤过程。
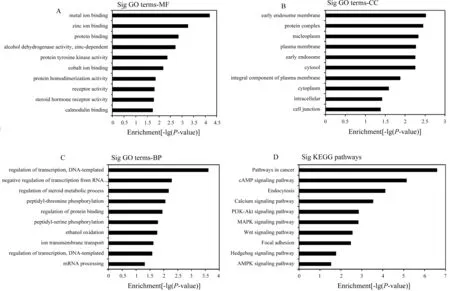
注:-lg(P-value):条形图长度,表示富集指数。
图2miR-877-5p靶基因富集的GO分析和KEGG通路分析
A:GO分类中分子功能领域(MF);B:GO分类中细胞成分领域(CC);C:GO分类中生物过程领域(BP);D:KEGG通路分析结果
Fig2Top10significantfunctionalGOtermsandKEGGpathwaysofmiR-877-5ptargetgenes
A: the field of molecular function (MF) in GO classification; B: the field of cellular components (CC) in GO classification; C: the biological processes (BP) of GO classification; D: the analysis results of KEGG pathway
既往研究[23-29]发现,瑞巴派特主要通过促进胃黏液的分泌,促进前列腺素E(postaglandin E2, PGE2)的产生,抑制炎症细胞和炎症因子的产生,拮抗氧化应激等发挥胃黏膜保护作用。有研究[30-31]报道,瑞巴派特可通过改善胃肠道黏膜屏障来预防阿司匹林引起的胃黏膜损伤。但其是否通过改变miRNA发挥胃黏膜保护作用,目前尚无报道。
本研究发现,阿司匹林损伤的胃黏膜上皮细胞株GES-1中miR-877-5p表达明显升高,而经瑞巴派特预处理后miR-877-5p的表达降低,说明miR-877-5p参与瑞巴派特保护阿司匹林所致胃黏膜损伤的过程。为进一步探讨miR-877-5p的作用,我们向阿司匹林损伤组的GES-1细胞中转染miR-877-5p inhibitor,发现细胞增殖能力增强,细胞凋亡减少;用miR-877-5p mimics转染瑞巴派特保护组的GES-1细胞,细胞增殖受到抑制,凋亡增加,这些结果提示,miR-877-5p对GES-1细胞具有抑制增殖促进凋亡的作用。通过生物信息学分析发现,miR-877-5p可能参与细胞生长、增殖和凋亡等生物行为的调控过程。以上实验说明,瑞巴派特通过抑制GES-1细胞中阿司匹林所诱导的miR-877-5p的表达,发挥胃黏膜保护作用。目前已知miRNA对细胞功能活动的影响往往是通过在转录后水平下调某些特定的功能基因,即靶基因的表达实现的[9]。本课题组下一步将在靶基因水平和蛋白水平上验证miR-877-5p的靶基因在GES-1细胞中的功能,进一步证实miR-877-5p发挥的具体作用机制。
本研究证实了瑞巴派特发挥胃黏膜保护作用与miR-877-5p相关,通过抑制GES-1细胞中miR-877-5p的表达,减轻阿司匹林引起的胃黏膜上皮细胞的损伤。
[1] RAHME E, BERNATSKY S. NSAIDs and risk of lower gastrointestinal bleeding [J]. Lancet, 2010, 376(9736): 146-148. DOI: 10.1016/S0140-6736(10)60839-2.
[2] ZHANG S, QING Q, BAI Y, et al. Rebamipide helps defend against nonsteroidal anti-inflammatory drugs induced gastroenteropathy: a systematic review and meta-analysis [J]. Dig Dis Sci, 2013, 58(7): 1991-2000. DOI: 10.1007/s10620-013-2606-0.
[3] KIM H K, KIM J I, KIM J K, et al. Preventive effects of rebamipide on NSAID-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers [J]. Dig Dis Sci, 2007, 52(8): 1776-1782. DOI: 10.1007/s10620-006-9367-y.
[4] KIM J H, PARK S H, CHO C S, et al. Preventive efficacy and safety of rebamipide in nonsteroidal anti-inflammatory drug-induced mucosal toxicity [J]. Gut Liver, 2014, 8(4): 371-379. DOI: 10.5009/gnl.2014.8.4.371.
[5] YEKTA S, SHIH I H, BARTEL D P. MicroRNA-directed cleavage of HOXB8 mRNA [J]. Science, 2004, 304(5670): 594-596. DOI: 10.1126/science.1097434.
[6] BARTEL D P. MicroRNAs: genomics, biogenesis, mechanism, and function [J]. Cell, 2004, 116(2):281-297.
[7] YU D, WU L, GILL P, et al. Multiple microRNAs function as self-protective modules in acetaminophen-induced hepatotoxicity in humans [J]. Arch Toxicol, 2018, 92(2): 845-858. DOI: 10.1007/s00204-017-2090-y.
[8] 孙沂, 樊宏伟, 王书奎, 等. 阿司匹林、氯吡格雷联用对人胃黏膜上皮细胞株GES-1增殖的影响[J]. 胃肠病学和肝病学杂志, 2010, 19(6): 520-523.
SUN Y, FAN H W, WANG S K, et al. Effect of combined Aspirin and Clopidogrel on proliferation of human gastric epithelial cell line GES-1 [J]. Chin J Gastroenterol Hepatol, 2010, 19(6): 520-523.
[9] FRIEDMAN R C, FARH K K, BURGE C B, et al. Most mammalian mRNAs are conserved targets of microRNAs [J].Genome Res, 2009, 19(1): 92-105. DOI: 10.1101/gr.082701.
[10] KIM J, LEE K S, KIM J H, et al. Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia [J]. Free Radic Biol Med, 2017, 104: 185-198. DOI: 10.1016/j.freeradbiomed.2017.01.010.
[11] YIANNAKOPOULOU E. Targeting epigenetic mechanisms and microRNAs by aspirin and other non-steroidal anti-inflammatory agents--implications for cancer treatment and chemoprevention [J]. Cell Oncol (Dordr), 2014, 37(3): 167-178. DOI: 10.1007/s13402-014-0175-7.
[12] LAN F, YUE X, HAN L, et al. Genome-wide identification of TCF7L2/TCF4 target miRNAs reveals a role for miR-21 in Wnt-driven epithelial cancer [J]. Int J Oncol, 2012, 40(2): 519-526. DOI: 10.3892/ijo.2011.1215.
[13] MIKAMI J, KUROKAWA Y, TAKAHASHI T, et al. Antitumor effect of antiplatelet agents in gastric cancer cells: an in vivo and in vitro study [J]. Gastric Cancer, 2016, 19(3): 817-826. DOI: 10.1007/s10120-015-0556-2.
[14] GUO X, YU L, CHEN M, et al. miR-145 mediated the role of aspirin in resisting VSMCs proliferation and anti-in ammation through CD40 [J]. J Transl Med, 2016, 14(1): 211. DOI: 10.1186/s12967-016-0961-2.
[15] WANG Y, GU X, LI Z, et al. microRNA expression profiling in multidrug resistance of the 5-Fu-induced SGC-7901 human gastric cancer cell line [J]. Mol Med Rep, 2013, 7(5): 1506-1510. DOI: 10.3892/mmr.2013.1384.
[16] SHI Q, XU X, LIU Q, et al. MicroRNA-877 acts as a tumor suppressor by directly targeting eEF2K in renal cell carcinoma [J]. Oncol Lett, 2016, 11(2): 1474-1480. DOI: 10.3892/ol.2015.4072.
[17] HUANG X, QIN J, LU S. Up-regulation of miR-877 induced by paclitaxel inhibits hepatocellular carcinoma cell proliferation though targeting FOXM1 [J]. Int J Clin Exp Pathol, 2015, 8(2): 1515-1524.
[18] LIANG Y, ZHAO G, TANG L, et al. MiR-100-3p and miR-877-3p regulate overproduction of IL-8 and IL-1β in mesangial cells activated by secretory IgA from IgA nephropathy patients [J]. Exp Cell Res, 2016, 347(2): 312-321. DOI: 10.1016/j.yexcr.2016.08.011.
[19] MITSUGI R, ITOH T, FUJIWARA R. MicroRNA-877-5p is involved in the trovafloxacin-induced liver injury [J]. Toxicol Lett, 2016, 263: 34-43. DOI: 10.1016/j.toxlet.2016.10.002.
[20] YAMASHITA Y, ASAKURA M, MITSUGI R, et al. microRNA expression in the vildagliptin-treated two- and three-dimensional HepG2 cells [J]. Drug Metab Pharmacokinet, 2016, 31(3): 201-209. DOI: 10.1016/j.dmpk.2016.02.004.
[21] YANNG X, GREENHAW J, SHI Q, et al. Identification of urinary microRNA profiles in rats that may diagnose hepatotoxicity [J]. Toxicol Sci, 2012, 125(2): 335-344. DOI: 10.1093/toxsci/kfr321.
[22] WARD J, BALA S, PETRASEK J, et al. Plasma microRNA profiles distinguish lethal injury in acetaminophen toxicity: a research study [J]. World J Gastroenterol, 2012, 18(22): 2798-2804. DOI: 10.3748/wjg.v18.i22.2798.
[23] ISHIHARA K, KOMURO Y, NISHIYAMA N, et al. Effect of rebamipide on mucus secretion by endogenous prostaglandin-independent mechanism in rat gastric mucosa [J]. Arzneimittelforschung, 1992, 42(12): 1462-1466.
[24] QI Z, JIE L, HAIXIA C, et al. Effect of rebamipide on quality of peptic ulcer healing in rat [J]. Dig Dis Sci, 2009, 54(9):1876-1883. DOI: 10.1007/s10620-008-0577-3.
[25] KLEINE A, KLUGE S, PESKAR B M. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats [J]. Dig Dis Sci, 1993, 38(8): 1441-1449.
[26] MURAKAMI K, OKAJIMA K, UCHIBA M, et al. Rebamipide attenuates indomethacin-induced gastric mucosal lesion formation by inhibiting activation of leukocytes in rats [J]. Dig Dis Sci, 1997, 42(2): 319-325.
[27] AIHARA M, AZUMA A, TAKIZAWA H, et al. Molecular analysis of suppression of interleukin-8 production by rebamipide in Helicobacter pylori-stimulated gastric cancer cell lines [J]. Dig Dis Sci, 1998, 43(9 Suppl): 174S-180S.
[28] YOSHIKAWA T, NAITO Y, TANIGAWA T, et al. Free radical scavenging activity of the novel anti-ulcer agent rebamipide studied by electron spin resonance [J]. Arzneimittelforschung, 1993, 43(3): 363-366.
[29] NAITO Y, YOSHIKAWA T, TANIGAWA T, et al. Hydroxyl radical scavenging by rebamipide and related compounds: electron paramagnetic resonance study [J]. Free Radic Biol Med, 1995, 18(1): 117-123.
[30] SUZUKI T, YOSHIDA N, NAKABE N, et al. Prophylactic effect of rebamipide on aspirin-induced gastric lesions and disruption of tight junctional protein zonula occludens-1 distribution [J]. J Pharmacol Sci, 2008, 106(3): 469-477.
[31] DIAO L, MEI Q, XU J M, et al. Rebamipide suppresses diclofenac-induced intestinal permeability via mitochondrial protection in mice [J]. World J Gastroenterol, 2012, 18(10): 1059-1066. DOI: 10.3748/wjg.v18.i10.1059.
doi:10.3969/j.issn.1006-5709.2018.06.016
