土壤pH值和含水量对土壤硝化抑制剂效果的影响
2018-05-13GuYanWuLianghuanHuZhaoping
Gu Yan, Wu Lianghuan,※, Hu Zhaoping
(1. Zhejiang Provincial Key Laboratory of Agricultural Resources and Environment, College of Environmental and Resource Sciences,Zhejiang University, Hangzhou 310058, China; 2. Ministry of Education Key Laboratory of Environmental Remediation and Ecosystem Health, College of Environmental and Resource Sciences, Zhejiang University, Hangzhou 310058, China; 3. State Key Laboratory of Nutrition Resources Integrated Utilization, Kingenta Ecological Engineering Group Co., Ltd., Linyi 276000, China)
0 Introduction
Nitrogen (N) is an essential nutrient for crop growing but amount of applied fertilizer N used by crops rarely exceeds 40%[1]. Most of the N fertilizers not taken up by target system tend to nitrify. Nitrification, the microbial oxidation of ammonia (NH3), via nitrite (NO2-), to nitrate(NO3-), is a critical component in the N cycle, which causes N losses in the form of NO3-leaching and nitrous oxide(N2O) emissions from agriculture system[2]. NO3-leaching could trigger waterway eutrophication and drinking water contamination[3-4]. N2O, the greenhouse gas, with about 300-fold greater warming potential than CO2on a per molecule basis, also relates to destruction of the stratospheric ozone layer[5-6]. When N fertilizers are applied such as urea or anhydrous ammonia, the microbial process of nitrification converts a large fraction of NH4+into NO3-within 2-3 weeks[7]. Hence, great efforts were devoted to decreasing nitrification rates to retain N in the soil for plant uptake, thus improving nitrogen use efficiency (NUE) and reduce environmental pollution[8-9]. One strategy that decreases the potential for N loss is the use of nitrification inhibitors (NIs). NIs slow the first step of NH3oxidation in soil, reducing its conversion to NO3-and the possibility of leaching while also having great potential for decreasing N2O emissions, thus increasing the chance for plant acquisition, immobilization, fixation and adsorption of NH4+[10-12].
Nitrapyrin [2-chloro-6- (tricholoromethyl)-pyridine, CP]is one of the most frequently used commercial nitrification inhibitors in agriculture. It was discovered in a fumigant that had been noted to occasionally stimulate plant growth and then manufactured by Dow Chemical Company under the trade name N-Serve[13-14]. So far, nitrapyrin has been widely applied to cropland in the United States[15-16]. It inhibits the first step of nitrification by chelating copper, the component of ammonia monooxygenase (AMO) that catalyzes the conversion of NH3to NH2OH and thus to inactivate AMO[17].Numerous laboratory and field trials have shown that nitrapyrin has been successfully used to decrease N losses and improve NUE for many years and contributed to decreases in N2O emissions from fertilized agricultural soil[18-20]. Nitrapyrin was recently reformulated by Zhejiang Aofutuo Chemical Co. Ltd.. Unlike the previous form of nitrapyrin, the purity of the new product has the potential to reach 98%. A reduction in impurities improves the efficacy of the compound and reduces dosage requirements to as low as 0.2%-0.3% of the pure N amount. Now it is made into commodity named as “N fertilizer synergist” and have access to markets. Adding nitrapyrin into N fertilizer as stabilized fertilizer is becoming widely used gradually.
China represents an enormous agricultural landscape with diverse soil types and pH value. The efficacy of NIs varied considerably with individual experiments and may be attributed to several physical, environmental and chemical factors that interact in a complex manner[21]. Among the various factors, pH value and soil moisture, both have significant effects on nitrification and the efficacy of nitrification inhibitors[22-24]. Soil pH value and moisture can influence its fate and transport processes such as volatilization, photolysis, sorption, degradation and leaching.As a result, it is important to determine the effects of those factors on the new nitrapyrin[25]. Soils in southern China are between acidic and weak alkaline and soil water in actual field condition vary with different land uses, as local field water content for upland is around 30%-45% of WHC(water holding capacity), while that for non-flooding paddy field with mulching film is around 80% and 60% of WHC for the earlier and late growth period, respectively. In this study we set five pH values and three soil moisture gradients in order to evaluate the effects of soil pH value and soil moisture on the nitrification inhibition efficacy of reformulated nitrapyrin. A better understanding on how these factors influence the nitrification inhibition of nitrapyrin is instructive for regulating N transformation via reformulated nitrapyrin application.
1 Material and Methods
1.1 Experimental materials
The soil (0-20 cm) used in this study was a purple sand silt soil, collected in April 2013 from Yuyao City, in Zhejiang Province, China (30°24′N, 119°51′E). Upon collection, soil samples were hand-sorted to remove live plant material and other woody debris, immediately transported to the laboratory. Soils were air-dried,homogenized by sieving (2 mm mesh size), pooled and stored at room temperature. Physicochemical properties of the soil samples including pH value,total N (TN),alkali-hydrolyzable N, organic matter (OM), mechanical composition, NO3--N, NH4+-N, cation exchange capacity(CEC) were determined. Soil pH value was measured with a pH meter in a solution with a soil/distilled water ratio of 1:2.5 (w/v). TN was determined by Kjeldahl digestion.Alkali-hydrolyzable N determination used the alkaline hydrolysis diffusion method. OM was determined using the K2Cr2O7wet oxidation and titration by FeSO4. Particle size analysis was measured by pipette settling method. NO3--N and NH4+-N were extracted with 2 mol/L KCl in a ratio of 1:5 (w/v) and analyzed by indophenol blue colorimetric method[26]and dual- wavelength ultraviolet spectrophotometry[27]. CEC was determined by 1 mol/L neutral ammonium acetate elution method. Basic properties of the soil were as follows: pH value 7.24, TN 0.46 g/kg, alkalihydrolyzable N 37.01 mg/kg, OM 12.06 g/kg, sand content 63.54%, silt content 25.19%, clay content 11.27%, NO3--N 3.57 mg/kg, NH4+-N 79.13 mg/kg, CEC 10.38 cmol/kg. In this experiment, nitrapyrin was offered by Zhejiang Aofutuo Chemical Co. Ltd. and used in emulsifiable concentrate formulation, which has 24% active ingredient.
1.2 Experimental design
1.2.1 Effect of soil pH value
200 g air-dried soils were placed in sealed plastic bags under aerobic condition. Samples were pre-wetted and incubated for 1 week under 25 ℃ to equilibrate the soil before the application of treatments. After pre-incubation,(NH4)2SO4at a rate of 500 mg/kg soil (N content) and nitrapyrin at a rate of 1.5 mg/kg soil were added to each of the soil samples. The pH value gradient was controlled via the addition of finely powdered CaCO3[28]and H2SO4[29].The quantities of H2SO4(1 mol/L) applied were 7.5, 6, 2.4 and 0 mL, while 1% (w/w, relative to the weight of the air-dried soil) CaCO3was applied. Soil was homogenized and pH value was measured on the first day. The soil pH value was adjusted to 4.66, 5.60, 6.25, 7.24 and 7.70,respectively. Control treatments without nitrapyrin were included and the entire set was triplicate. The soils were mixed and then placed in a Plant Growth Chamber at 25 ℃and 80% humidity. A needle was used to prick a few small holes in each of the bags near its seal, in order to create an aerobic environment. During incubation, soil moisture was adjusted to and maintained at 60% of WHC. Water loss was monitored by reweighing and replenished by adding distilled water as required.
1.2.2 Effect of soil moisture
To investigate the effect of soil moisture on the nitrification inhibition rate of nitrapyrin, soil moisture levels were set to 40%, 60% and 80% of WHC to simulate low,medium and high soil moisture[30-31]. Soil pH value was its natural pH value. Other incubation procedures were identical to those described in section “1.2.1”.
1.3 Sampl ing and analysis
10 g soil samples were collected at 9intervals,immediately extracted with 2 mol/L KCl solution, and analyzed for NH4+-N and NO3--N content. NH4+-N was measured using the indophenol blue colorimetry, NO3--N was measured via dual-wavelength ultraviolet spectrophotometry.
The nitrification inhibition rate is a characteristic of the inhibition intensity, which was calculated using the following formula
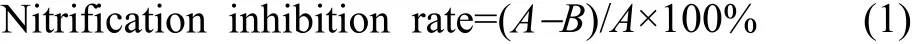
Where A represents the difference in NO3--N content after incubation compared to initial levels in soil not treated with the inhibitor, mg/kg; B represents the difference in NO3--N content after incubation compared to initial levels in soil treated with inhibitors, mg/kg
The apparent nitrification rate of the soil corresponds to its nitrification intensity, which was calculated as follows
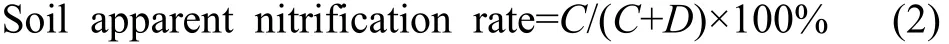
Where C is the NO3--N content, mg/kg; D is the NH4+-N content, mg/kg.

Fig. 1 Dynamics of NH4+-N and NO3--N concentration as affected by applications of nitrapyrin under different soil pH value
1.4 Stati stical analysis
The results of each treatment were reported as the means of the three replicates and expressed on the basis of soil air-dried weight. All data was analyzed using one-way ANOVA in Statistics, Version 5.5 (Windows) and origin 8.0.The significance of the differences among mean values was tested using Duncan’s multiple range method (P<0.05).
2 Results
2.1 E ffects of s oil pH val ue on the ni trification inhibition of nitrapyrin
The NH4+-N concentrations constantly decreased over time during the incubation period (Fig.1a) and displayed dissimilarly in different treatments. The NH4+-N concentration was lower and the rate of descent was faster at higher pH value. At the 9th day, NH4+-N concentration was 447.27 mg/kg at pH value of 4.66 while 210.31 mg/kg at pH value of 7.24, and dropped to 371.82 and 65.97 mg/kg at the 18th day, respectively. NH4+-N concentration of the treatment with nitrapyrin was significantly greater than that without nitrapyrin at the same time point (P<0.05). NH4+-N at pH value of 7.70 without nitrapyrin dramatically declined to 39.48 mg/kg at the 9th day while that with nitrapyrin remained 59.79 mg/kg at the 18th day. NH4+-N concentrations at pH value of 4.66 and 5.60 without nitrapyrin at the 36th day were 259.78 and 217.26 mg/kg, which amount to those with nitrapyrin at the 45th day.
The NO3--N concentration changed in an inverse pattern to that of NH4+-N concentration in all treatments and gradually increased with incubation time (Fig.1b). NO3--N concentration of the treatment with nitrapyrin was significantly lower than that without nitrapyrin at the same time point (P<0.05). Moreover, the higher the pH value, the higher the NO3--N concentration. At the 54th day, NO3--N concentration in the treatment of pH value of 4.66 adding nitrapyrin was 310.10 mg/kg, significantly lower than other treatments.
The apparent nitrification rates varied significantly with soil pH value and incubation time (P<0.05) (Table 1).Inductively, apparent nitrification rates for all soils attained the lowest value initially, increased subsequently and reached the highest towards the end of the incubation(P<0.05). Excessive acidification significantly inhibited soil nitrification, as demonstrated by the fact that apparent nitrification rates had a decline tendency as pH value decreased. At pH value of 4.66, apparent nitrification rate of treatment without nitrapyrin was just 8.51% at the 9th day and did not achieve 91.21% until the 54th day, while that reached up to 93.14% only at the 9th day at pH value of 7.70.Apparent nitrification rate at pH value of 4.66 and 5.60 without nitrapyrin were significantly higher than those in treatments including nitrapyrin during all the incubation time (P<0.05). At the 54th day, nitrapyrin at pH value of 4.66 still reduced apparent nitrification rate by 32.20%.However, nitrapyrin showed great inhibition on nitrification at pH value of 7.70 only for 18 d. Especially at the 9th day,apparent nitrification rate without nitrapyrin was just 7.87%,remarkably lower than that with nitrapyrin (P<0.05).
Anyone unfortunate enough to encounter a porcupine s quills knows that once they go in, they are extremely difficult to remove. Researchers at MIT and Brigham and Women s Hospital now hope to exploit the porcupine() quill s unique properties to develop new types of adhesives(), needles and other medical devices. In a new study, the researchers characterized, for the first time, the forces needed for quills to enter and exit the skin. They also created artificial devices with the same mechanical features as the quills, raising the possibility of designing less-painful needles, or adhesives that can bind internal tissues more securely.
The nitrification inhibition rate of nitrapyrin was significantly affected by soil pH value. We could conclude that in soils with pH value of 4.66-7.70, nitrapyrin was the most effective on the 9th day,and had a lower efficiency over sampling time (Fig.2). Initially, nitrapyrin was the most effective when the pH value was 7.70; however, its efficacy then dropped sharply with the nitrification inhibition rate was down to 12.05% on the 18th day, while the inhibition ceasing almost entirely after that point. Nitrification inhibition rates at pH value of 4.66 and 5.60 had a slower declining tendency and they showed nitrapyrin less effective at the beginning, but the trend in nitrapyrin activity reversed on the 27th day, when nitrapyrin began to perform better as the pH value decreased. Finally, at the end of the incubation,nitrapyrin was most effective at pH value of 4.66. At pH value of 7.24, the inhibition of nitrification continued for 45 days, while the nitrapyrin remained effective in other more acidic soil during the entire incubation period.
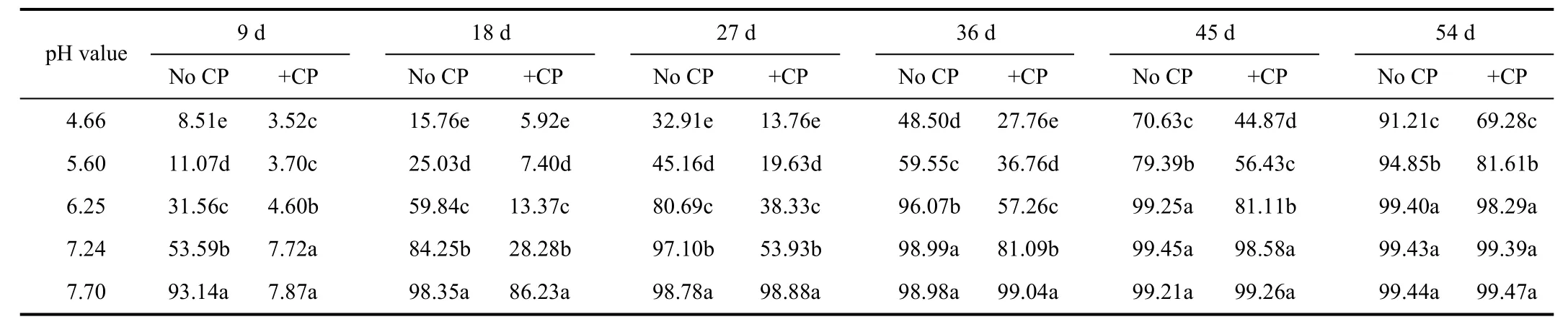
Table 1 Appar ent nitrification rates under different soil pH values %

Fig.2 Dynamics of nitrification inhibition rate of nitrapyrin under different soil pH values
2.2 Effects of soil moisture on nitrification inhibition of nitrapyrin
The NH4+-N concentration of all the treatments gradually decreased over time during all the incubation period (Fig.3a). NH4+-N concentration under different soil moisture levels was 80%WHC>40%WHC>60%WHC.NH4+-N concentration declined to 12.47 mg/kg at the 27th day as soil moisture was 60% of WHC, but it remained 66.94 and 254.90 mg/kg when 40% and 80% of WHC.Adding nitrapyrin to soil of different humidity all can delay the NH4+-N transformation. When soil moisture was 60% of WHC, NH4+-N concentration was 210.31 mg/kg at the 9th day while that was 453.88 mg/kg including nitrapyrin and decreased to 215.99 mg/kg until the 27th day. The NH4+-N concentration decreased with a delay of 18 days as well as in the treatments when soil moisture was 40% of WHC.
Contrary to the NH4+-N concentration change tendency,NO3--N concentration increased with the incubation time(Fig.3b). When soil moisture was 60% of WHC, the NO3--N concentration was higher than others and remained relatively stable at (420±10) mg/kg since the 27th day. NO3--N concentration in the treatment of 40% of WHC was significantly higher than that of 80% during the whole incubation period (P<0.05). The addition of nitrapyrin effectively retarded the NO3--N formation in three different soil moisture treatments, especially in the initial stage. At the 9th day, NO3--N concentration in the treatment of 40% of WHC was 156.64 mg/kg, significantly decreased to 24.40 mg/kg with nitrapyrin at the same time (P<0.05).Compared with 40% of WHC, nitrapyrin made NO3--N concentration decrease by 65.48% when soil moisture was 80% of WHC at the 9th day.

Fig. 3 Dynamics of NH4+-N and NO3--N concentration as affected by nitrapyrin under different soil moisture
The apparent nitrification rates showed significant differences with soil moisture and incubation time (P<0.05)(Table 2). Apparent nitrification rates increased gradually with longer incubation time in all treatments. Apparent nitrification rates were the highest when the soil moisture was 60% of WHC and followed by those of 40% of WHC during the whole incubation period. Nitrapyrin could effectively retard the process of NH4+-N converted to NO3--N, since that apparent nitrification rates with nitrapyrin were significantly lower than those without nitrapyrin during 54 days at 40% and 80% of WHC, and 45 days at 60% of WHC (P<0.05). When soil moisture was 40% of WHC, apparent nitrification rate of treatments without nitrapyrin got to 40.60% at the 9th day, while that reached to 45.31% until the 27th day in treatments including nitrapyrin. Results indicated that nitrapyrin inhibited nitrification and had a retardant effect for about 18 days as well as in treatment of 60% of WHC.
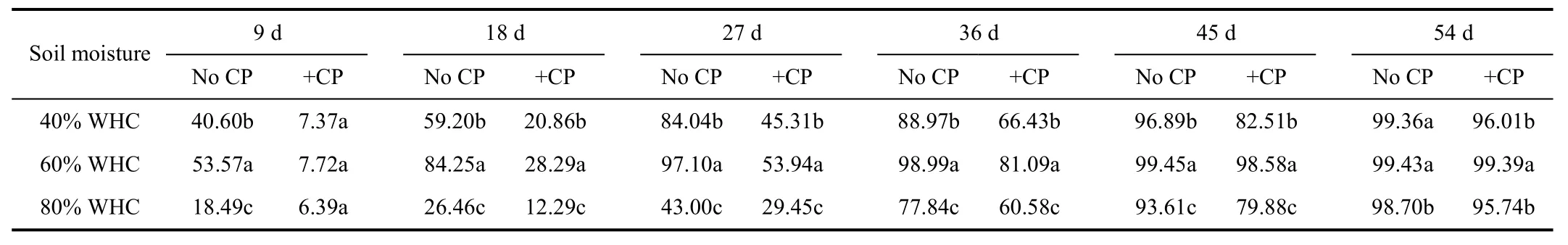
Table 2 Appar ent nitrification rates under different soil moisture %
The effects of soil moisture on the efficacy of nitrapyrin were presented in Fig. 4. Nitrification inhibition rates of nitrapyrin decreased as incubation time in all treatments.Nitrapyrin was significantly less effective in soils of 80% of WHC than that of 40% of WHC (P<0.05) during the first 27 days while there was no significant difference in nitrification inhibition rates between soils of 40% and 60% of WHC.From day 36 to 54, soil nitrification inhibition rates at 40%of WHC were significantly higher than those of 60% of WHC (P<0.05). Simultaneously, inhibition rates were similar under 60% and 80% of WHC. The duration of nitrification inhibition was prolonged as soil moisture decreased. When the soil moisture was 60% or 80% of WHC,the inhibition of nitrification persisted for 45 days, while the nitrification inhibition rate remained at 15.40% when the soil was 40% of WHC.
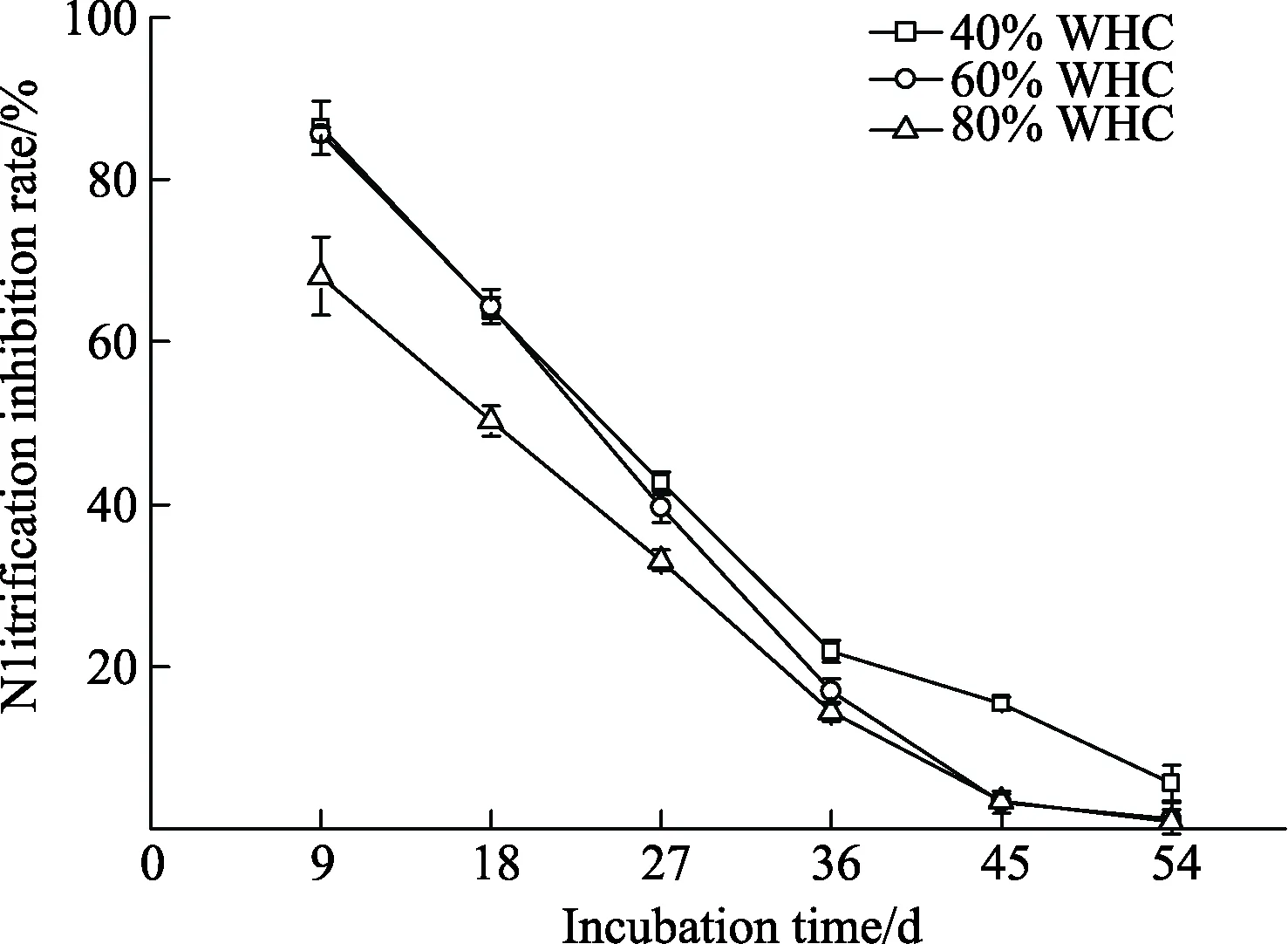
Fig. 4 Dynamics of nitrification inhibition rate of nitrapyrin under different soil moisture
3 Discussion
Temporal variations of nitrification inhibition rates were to investigate the effects of pH value and soil moisture on the efficacy of nitrapyrin. Results indicated that NO3--N concentration and apparent nitrification rate increased with soil pH value (Table 1). Under acidic conditions, inorganic nitrogen exists mainly in the protonated form, NH4+rather than NH3(pKa=9.25), yet NH3is a suitable substrate for ammonia monooxygenase[32]. There is a wide range in the reported optima for nitrification, but the general trend indicates that as the pH value decreases, the rate of nitrification decreases[33-34]. This is the reason why NO3--N concentration and apparent nitrification rate was lower at lower pH value. Liming of soil increased pH value and initiate nitrification, but meanwhile may probably cause increased nitrifier activity and general microbial activity,resulting in the rapid biodegradation of nitrapyrin[35].Additionally, the rate of nitrapyrin disappearance rose with pH value[36]. All these may explain our result. At the 9th day,nitrification was stronger and yet the effect of nitrapyrin was better at higher pH value. As nitrapyrin degraded gradually along with the incubation time and diminished faster at high pH value, consequently it performed better progressively at lower pH value during the latter incubation time. A similar result had been showed in the experiment carried out by Rovita and Killorn[37], which suggested that nitrapyrin in the soil of higher pH value and organic showed less efficacy on nitrification inhibition. Therefore, nitrapyrin will likely be more effective in limed soils at incipience of application, yet can persist longer in more acidic soils.
In the soil system, soil aeration, was closely related to soil moisture, which significantly affects the activity of soil nitrifying bacteria. The nitrification rate in soil is near its maximum when the soil moisture is 60% of WHC. The nitrification of soils saturated with water occurs slowly or stops entirely because of the associated deficit of molecular oxygen[38-39]. Nitrification also ceases in dry soils, but it would appear that higher soil moisture has a greater negative impact on nitrification than a lower moisture[40]. Phenomena were reconfirmed in this experiment, which found that the ranking of the apparent nitrification rates observed at different soil moisture levels was 60% WHC>40%WHC>80% WHC. Nitrapyrin is insoluble in water and can be hydrolyzed to 6-chloropicolinic acid[41], which has no effect on the oxidation of ammonia in the soil, even when applied at rates as high as 79 μg/g soil[42]. Besides, the volatilization of nitrapyrin is more pronounced in wet soils than in dry soils[21]. As a result, the nitrapyrin retention time is shorter in the very moist soil. The efficacy of nitrapyrin positively correlated to its retention time in soil and this coincides with our conclusion that the efficacy of nitrapyrin in soils of different moisture levels was 40% WHC>60%WHC>80% WHC. Our results of this study suggested that nitrapyrin is more suitable for application in upland soils.
4 Conclusions
Nitrification is stronger in alkaline soil and at 60% of WHC soil moisture. The efficacy of nitrapyrin is greater at higher pH value initially, whereas it is better at lower pH value later and lasts longer. Additionally, nitrapyrin performs better when the soil is at lower moisture. As a result, nitrapyrin is suggested to apply in acidic soil and upland fields.
Besides the factors mentioned above, its own movement and the relative migration rate of ammonium may also influence the efficacy. Moreover, further research is required to obtain molecular-level information about the mechanism underlying nitrification inhibition by nitrapyrin.
