Effect of An-pressing manipulation on the serum levels of T-AOC and CK-MM in volunteers with delayed onset muscle soreness in biceps brachii
2018-04-28JiangQuanrui蒋全睿LiWu李武LiuXiaowei刘小卫YuJun于隽AiKun艾坤LiJiangshan李江山
Jiang Quan-rui (蒋全睿), Li Wu (李武), Liu Xiao-wei (刘小卫), Yu Jun (于隽), Ai Kun (艾坤), Li Jiang-shan (李江山)
Hunan University of Chinese Medicine, Changsha 410208, China
Delayed onset muscle soreness (DOMS) refers to pain and stiffness felt in muscles after unaccustomed or strenuous exercise[1]. Symptoms include muscle soreness, stiffness, swelling, decreased muscle strength and restricted movement. At present, the pathogenesis of DOMS is not yet clear. In modern medicine, DOMS belongs to the scope of sports fatigue. In traditional Chinese medicine (TCM), muscle soreness after exercise is due to qi stagnation and blood stasis[2-3]. Studies have shown that tuina and acupuncture can prevent DOMS[4-5]. An-pressing and Gun-rolling manipulations are commonly used for DOMS. An-pressing manipulation can circulate blood to dissipate stasis and relieve pain by promoting qi activity[6], but the studies on its mechanisms of prevention and treatment of DOMS are rarely reported. This experiment aimed to explore the mechanism of An-pressing manipulation in improving DOMS from the aspects of anti-muscle cell damage and anti-oxygen free radical metabolism.
1 Subjects
Thirty male volunteers were recruited, with the height of 170-175 cm, body weight of 60-70 kg and age of 18-24 years. The volunteers must be right handed,physically healthy, and energetic before the test without obvious feeling of fatigue, not engaged in physical training or special athletic training. They were designated as 1-30 based in the order of registration,and then were randomly divided into a blank group, a model group and a treatment group by the random number table, 10 cases in each group. Volunteers in the blank group did not receive modeling or treatment;volunteers in the model group only received modeling but without An-pressing manipulation treatment;volunteers in the treatment group underwent modeling and received An-pressing manipulation treatment.
2 Methods
2.1 Modeling method
2.1.1 Maximum voluntary contraction (MVC) assay
According to the study of Wang JJ[7], the subjects were comfortably seated when the test began; the upper body remained upright; the hip, knee and ankle joints maintained at 90° by the adjustment of the seat height and the body posture; kept the feet flat on the ground. The left forearm was maintained vertical to the upper arm; the upper arm maintained vertical to the horizontal plane; the arm on the other side naturally drooped to the side of the body. Before the formal modeling exercise, each subject was tested for MVC in the flexor of elbow joint of the left side, using dynamic muscle test system (model PM1/MK2a, b), under the above mentioned posture conditions. Each subject was measured 3 times at an interval of 5 min. The maximum value was used as the individual MVC by unit of kilogram.
2.1.2 Modeling exercise
The 60% of the measured MVC was used as load weight. The left upper arm flexion elbow muscle eccentric exercise training was performed by the adjustment of the dumbbell, using a self-designed imitation PM Clarkson eccentric exercise training device[8]. The flexion elbow eccentric contraction mode was used in this test which easily led to DOMS on the less used non-favored hand (such as selection of the left upper limb for the right handed subject).
Subjects kept the posture of sitting, bending hips and knees with the left upper limb abducted by 30°. The left upper limb of the subjects naturally returned to the starting position by elbow flexion without weightbearing with the exercise aids pulling the dumbbells,after slowly extending to 170° from the initial 90°(Figure 1). Twenty-five times of eccentric contraction training were a set, and each subject received 2 sets of training at a 5-minute interval.

Figure 1. Modeling training
2.1.3 Model evaluation method
Based on human DOMS model evaluation method proposed by Xie W,et al[9], soreness grade was selected as an indicator to evaluate the modeling. DOMS often appeared 8-24 h after modeling exercise, climbed to the peak at 24-48 h, and recovered to normal at 168 h[10].The model was successfully established if the test results were in line with the expectations.
2.2 An-pressing manipulation treatment
A homemade An-pressing manipulation stimulator was used to perform the An-pressing manipulation in this experiment[11]to replace the traditional manual operation. An-pressing manipulation stimulator was constituted by pushrod, control system and auxiliary system. The pushrod can be freely adjusted to select the massage head size and the angle of the An-pressing position according to the An-pressing position;temperature control system can control the massage head to be heated to 35-37 ℃, and to simulate the temperature of finger massage; the power control system can regulate massage head pressure power,dwell time and interval time by adjusting the pushrod.All the control parameters can be set through the man-machine exchange platform. The stimulator can simulate the manpower to achieve the constant temperature, fixed-point, quantitative and regular An-pressing manipulation on the specific area of the volunteer (Figure 2). The parameters in this test were set at the frequency of 2 Hz, the intensity of 1 kg, the time of 20 min, and the massage head area of 2.0 cm2.The An-pressing manipulation was performed at the junction of the biceps muscle belly with the tendon. The treatment period was from 3 d before modeling to 4 d after modeling, 20 min each time, once daily for 7 d.

Figure 2. An-pressing manipulation stimulator
2.3 Observed items and methods
Subjective rating of perceived exertion (RPE),subjective soreness sensation threshold and soreness grade were evaluated before modeling, immediately after modeling, 24, 48, 72, 96 and 120 h after modeling.The total antioxidant capacity (T-AOC) was determined before modeling, immediately after modeling, at 24, 48 and 72 h after modeling. Blood samples were collected before modeling, 24, 48 and 72 h after modeling, and creatine kinase MM isoenzyme (CK-MM) was measured by CK-MM kit. All operations were performed and recorded by the same person.
According to the modified measurement methods proposed by Wei JS,et al[12], the subjective soreness sensation threshold was measured with a simple home-made tenderness device. Subjects were supine with lower extremities relaxed naturally and straightened, upper extremities relaxed and straightened and putting on the sides of the body.Mercurial manometer was tied to the subject biceps brachii and gradually gave pressure in the same way of measuring blood pressure. The scale of mercurial manometer was recorded when the biceps brachii appeared soreness, and was used as the subjective soreness sensation threshold of the subject.
The six-grade pain assessment method, which is commonly used in the sport physiology field, was used for the soreness grade evaluation[13]. Grade 0: no pain or discomfort; grade 1: mild muscle soreness, evident with finger pinch; grade 2: muscle soreness which is significant with large amplitude movement, showing limited or un-limited movement, without affecting night sleep; grade 3: muscle soreness is obvious and activities are limited, but tolerable and not affecting sleep at night; grade 4: muscle soreness is serious than that of grade 3 with disturbed sleep; grade 5: severe persistent muscle soreness interfering with normal activities and movements and impeding sleep.
Exercise fatigue was tested using RPE scale[14]. The scale is divided into 15 grades from low to high of the exercise load (or fatigue). 6 points: not at all dificult; 7-8 points: very easy; 9-10 points: quite easy; 11-12 points:easy; 13-14 points: a little difficult; 15-16 points: difficult;17-18 points: quite difficult; 19 points: very difficult; 20 points: to the limit.
2.4 Statistical analysis
All data were input in the computer and processed by the first author using the SPSS 21.0 version software for Windows. The measurement data fitting the normal distribution and between-group homogeneous variance were all expressed as mean ± standard deviation (x±s)and analyzed by using one-way ANOVA; Kruskal-Wallis H test was used if the data did not meet the above criteria.Multiple samples were compared with each other by Student-Newman-Keuls (SNK)-q or Dunnett-T method.Repeated measurement data were processed by repeated measures data variance analysis. Ranked data were analyzed using rank-sum test.P<0.05 indicated a significant difference.
3 Results
3.1 Comparison of each item before modeling
There were no significant differences among groups in muscle soreness grade, RPE score, subjective soreness sensation threshold, T-AOC and CK-MM before modeling (allP>0.05), (Table 1).
3.2 Comparison of soreness grade
Intra-group comparisons: during the experiment,there was no significant change in the soreness grade of the blank group; the differences in the model group and the treatment group were statistically significant (bothP<0.05), and the grade showed an immediate increase after modeling and reached the peak at 24 h after modeling. There was no significant difference in the soreness grade of the treatment group between 120 h after modeling and before modeling (P>0.05); the difference in the soreness grade was statistically significant in the model group between 120 h after modeling and before modeling (P<0.05). Inter-group comparisons: the soreness grade of the treatment group was better than that of the model group at 24, 48,72 and 120 h after modeling (allP<0.05), (Table 2).
3.3 RPE comparison at each time point
Intra-group comparisons: there was no significant difference in RPE of the blank group among different time points (allP>0.05). The RPE in the model group and treatment group reached the peak immediately after modeling and then immediately decreased. RPE was recovered to normal at 96 h after modeling in the model group, while at 48 h after modeling in the treatment group. The values at 72, 96 and 120 h after modeling were lower than that before modeling (allP<0.05) in the model and treatment groups. Inter-group comparisons: after modeling, the RPE scores of the treatment group at each time point were better than those of the model group (allP<0.05), (Table 3).
Table 1. Comparison of various indicators among groups before the test
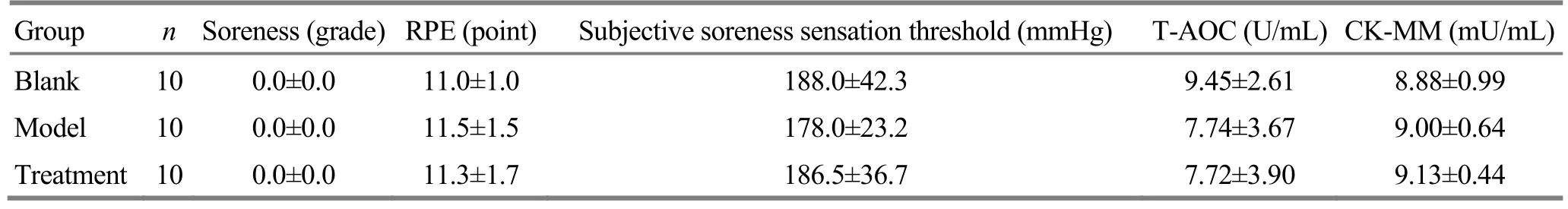
Table 1. Comparison of various indicators among groups before the test
Group n Soreness (grade) RPE (point) Subjective soreness sensation threshold (mmHg) T-AOC (U/mL) CK-MM (mU/mL)Blank 10 0.0±0.0 11.0±1.0 188.0±42.3 9.45±2.61 8.88±0.99 Model 10 0.0±0.0 11.5±1.5 178.0±23.2 7.74±3.67 9.00±0.64 Treatment 10 0.0±0.0 11.3±1.7 186.5±36.7 7.72±3.90 9.13±0.44
Table 2. Comparison of the soreness grades among groups before and after the modeling
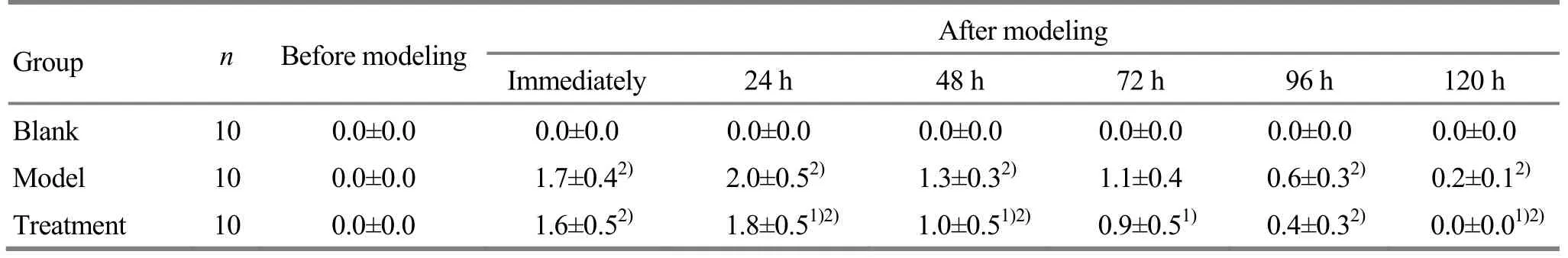
Table 2. Comparison of the soreness grades among groups before and after the modeling
Note: Compared with the model group at the same time point, 1) P<0.05; compared with the value at the previous time point in same group,2) P<0.05
?
Table 3. Comparison of RPE scores before and after modeling

Table 3. Comparison of RPE scores before and after modeling
Note: Compared with the model group at the same time point, 1) P<0.05; compared with the value at the previous time point in same group,2) P<0.05
?
3.4 Comparison of subjective soreness sensation threshold at different time points
Intra-group comparisons: there was no significant difference in subjective soreness sensation threshold of the blank group between each time point (P>0.05). The subjective soreness sensation threshold in the model group began to decline after modeling, reached the lowest value at 48 h after modeling, then began to recover, and back to the normal value at 96 h after modeling. The subjective soreness sensation threshold in the treatment group decreased after modeling and immediately reached the lowest value, and was recovered to normal at 48 h after modeling. However,
the subjective soreness sensation threshold in the model and treatment groups at 72 h and 120 h after modeling were higher than those before modeling (all
P<0.05). Inter-group comparisons: there was no significant difference among groups before modeling(P>0.05). The sensory threshold of subjective soreness in the treatment group was better than that in the model group immediately after modeling, 24, 48, 72 and 96 h after modeling (allP<0.05), (Table 4).
3.5 The change of serum T-AOC at different time points
Intra-group comparisons: there were no significant differences in T-AOC values of the blank group immediately, at 24 h and 48 h after modeling compared with the value of before modeling (P>0.05). The T-AOC values in the model group and the treatment group increased rapidly to the peak after modeling, and rapidly dropped to the normal value at 24 h after modeling. Inter-group comparisons: the serum T-AOC in the treatment group was higher than that in the model group immediately after modeling (P<0.05); there was no significant difference in serum T-AOC between the treatment group and the model group at 24, 48 and 72 h after modeling (allP> 0.05), (Table 5).
3.6 The change of serum CK-MM at different time points
Intra-group comparisons: there were no significant differences in serum CK-MM of blank group among all time points (allP>0.05). In the model group, the CK-MM value was significantly different at 24 h after modeling versus before modeling (P<0.05); there was no statistically significant difference in CK-MM values between 48 h and 72 h after modeling versus 24 h after modeling (P>0.05). It indicated that the CK-MM value of the model group reached to the peak at 24 h after modeling and maintained to 72 h after modeling. The CK-MM value of the treatment group reached to the peak at 24 h after modeling, then decreased rapidly and returned to normal at 72 h after modeling. Inter-group comparisons: there was no significant difference in CK-MM value between the treatment group and the model group both before modeling and at 24 h after modeling (allP>0.05). However, CK-MM value in the treatment group was lower than that in the model group at 48 h and 72 h after modeling (allP<0.05),(Table 6).
Table 4. Comparison of subjective soreness sensation threshold before and after modeling

Table 4. Comparison of subjective soreness sensation threshold before and after modeling
Note: Compared with the model group at the same time point, 1) P<0.05; compared with the value at the previous time point in same group,2) P<0.05
Group n Before modeling After modelingⅠmmediately 24 h 48 h 72 h 96 h 120 h Blank 10 188.0±42.3 182.3±28.1 180.0±61.8 169.7±63.0 180.7±71.6 179.7±66.4 185.5±64.0 Model 10 178.0±23.2 146.9±52.32) 146.7±29.5 135.9±25.12) 153.3±43.82) 175.6±37.52) 194.1±52.02)Treatment 10 186.5±36.7 159.0±45.41)2) 179.0±60.91)2)183.5±59.81)2)193.7±60.91)2) 198.2±47.71) 203.1±54.62)
Table 5. Change of T-AOC at different time points before and after modeling
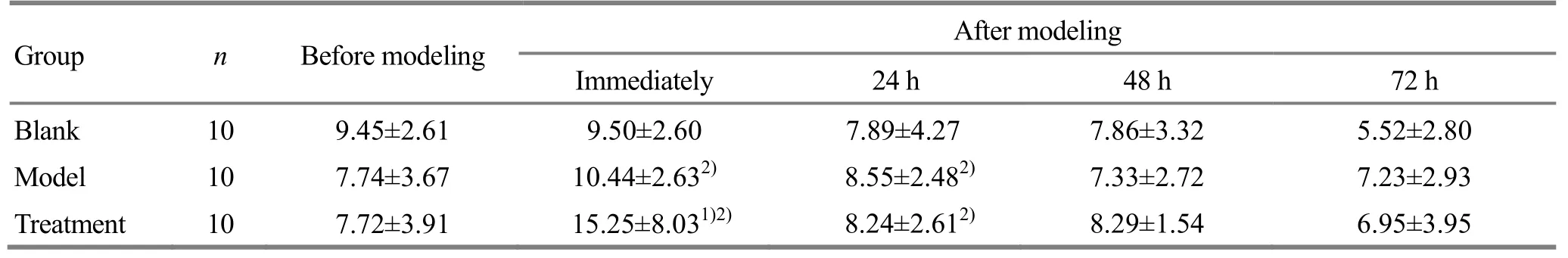
Table 5. Change of T-AOC at different time points before and after modeling
Note: Compared with the model group at the same time point, 1) P<0.05; compared with the value at previous time point in same group, 2)P<0.05
?
Table 6. Comparison of serum CK-MM among groups before and after modeling

Table 6. Comparison of serum CK-MM among groups before and after modeling
Note: Compared with the model group at the same time point, 1) P<0.05; compared with the value at the previous time point in same group,2) P<0.05
Group n Before modeling After modeling 24 h 48 h 72 h Blank 10 8.88±0.99 8.86±1.42 8.85±1.10 9.41±0.96 Model 10 9.13±0.44 11.08±5.462) 11.15±2.85 11.61±2.07 Treatment 10 9.00±0.64 10.22±1.992) 9.42±1.541)2) 9.18±0.581)2)
4 Discussion
So far, the tuina manipulation was performed by professional staffs in most teaching, research and clinical practice. Moreover, terms such as ‘appropriate’and ‘suitable’ were used to describe the specific manipulations, which lacks of quantified presentation.In order to promote tuina to the world faster and better,it is necessary to improve the standardization of tuina manipulations, especially in the rigorous basic scientific experiments about tuina. Tuina manipulation is urgently needed to be standardized. The experimental procedure conducted with the tuina instrument is characterized by uniform manipulation, quantitative stimulation and simulated temperature. Uniformity of manipulation is a basic requirement of operation, and also a requirement of teaching, scientific research and curative effect[15].However, it is difficult to be controlled by manual operation, which requires long training[16]. The temperature of hand is crucial to the efficacy. The local thermal effects of An-pressing manipulation come from heat transfer, stamping work and body thermogenesis[17]. If the temperature of the operating site is too low, the warming caused by the tuina manipulation will be partially offset. In this experiment,homemade An-pressing stimulator was used, which could not only simulate the temperature of the fingers,but also quantitate the intensity, dwell time, force and withdrawal speed of An-pressing manipulations, and also guarantee the homogeneity of each An-pressing manipulation compared with that of the manual operation, thus to make the manipulation more objective and standardized.
Su WenofHuang Di Nei Jing(Essential QuestionsofYellow Emperor’s Classic of Internal Medicine) records that An-pressing manipulation can warm the local area,and show effects of promoting qi to activate blood circulation and analgesic. Modern studies have shown that the peripheral mechanism of pain relief by An-pressing manipulation includes improving local microcirculation, supplying blood and oxygen, reducing the accumulation of algesic substances in plasma and accelerating their clearance[18]; correcting muscle spasm and other pathological changes, and promoting absorption of congestion and edema[19]; improving the body's antioxidant capacity, effectively controlling lipid oxidation damage, and protecting normal muscle cells[20]. Clinical manifestations of DOMS are local muscle soreness, stiffness, swelling and distension,affecting exercise capacity. The main mechanism is the mechanical damage to the muscle caused by exercise,resulting in insufficiency of muscular oxygen and muscle spasms, inactivation of enzyme system, abnormalities of Na+, K+, Ca2+, inhibition of mitochondrial energy supply,and increased free radicals and cell necrosisin vivo[21].Thus, An-pressing manipulation can improve the corresponding symptoms of DOMS by aiming directly at the pathogenesis.
In this study, we performed DOMS modeling with the biceps brachii and did not give treatments to the model group. Compared with the blank group, it can clearly reveal the occurrence and development of DOMS. The results of this study showed no significant changes in the soreness grade of the blank group at different time points, while the soreness grade of the model group reached its peak at 24 h after the modeling and then slowly dropped to the normal level, indicating that human DOMS model was successfully replicated in this experiment. The soreness grade of the treatment group significantly improved compared with that of the model group at 24, 48 and 72 h after modeling (allP<0.05),and recovered to the normal level at 120 h after modeling, while the soreness grade of the model group was higher than the normal level at the same time point.Although the subjective soreness sensation threshold decreased immediately after modeling in both the model and treatment groups, significantly better in the treatment group than in the model group, indicating that An-pressing manipulation can improve the local subjective soreness sensation threshold and thus produce some analgesic effect on DOMS. The RPE level in the treatment group was significantly lower than that in the model group at each time point after modeling(P<0.05) indicating that An-pressing manipulation can alleviate the fatigue caused by DOMS to a certain extent.
Oxygen free radicals can react with fatty acids on the cell membrane to produce peroxides, which invade the body's nucleic acids and proteins, thus cause a series of cell-damaging effects[22]. Muscle fatigue and injury can increase the body's endogenous free radicals, resulting in further muscle damage[23]. Antioxidants play an extremely important role in protecting against peroxide damage caused by exercise. Increasing the antioxidant capacity is beneficial to the body after exercise, by reducing the production of free radicals or accelerating free radical scavenging to combat the side effects of free radicals, which can delay the onset of exerciseinduced fatigue, and speed up the recovery of physical fitness for the human body after exercise[24]. In this experiment, there was no significant difference in T-AOC value of the model group at 24, 48 and 72 h after modeling versus before modeling (P>0.05). T-AOC value in the treatment group was higher immediately after modeling than that before modeling (P<0.05), and there was also a significant difference compared with the model group at the same time point (P<0.05). However,the T-AOC value recovered to the normal level at 24 h after modeling (P>0.05), indicating that An-pressing manipulation can increase the body's antioxidant capacity in a short term, reduce fatigue and protect the body.
CK-MM is mainly found in various muscle cells. Under normal conditions, CK-MM is released into the blood from the muscle cells through the cell membrane with a low concentration; a large number of CK-MM is released into the blood after excessive load exercise,due to changes in muscle fiber structure or muscle cell membrane structure[25].
Therefore, CK-MM can be used as a biochemical indicator to evaluate muscle damage and recovery[26]. In this experiment, the CK-MM value of the model group increased to the peak at 24 h after modeling and maintained till 72 h after modeling; the CK-MM value of the treatment group also reached to the peak at 24 h after modeling and rapidly decreased to the normal value. These results indicate that DOMS produced by heavy exercise produces certain muscle damage to cause excess release of CK-MM into the bloodstream.The CK-MM value rapidly increased, and the release of CK-MM can be reduced by An-pressing manipulation treatment.
In summary, An-pressing manipulation can reduce the degree of muscle soreness caused by exercise,relieve fatigue, increase the body's antioxidant capacity in a short term, reduce the damage in vivo caused by oxygen free radicals, protect the integrity of muscle cells and promote the repair of muscle damage and prevent exercise-induced DOMS.
Conflict of Interest
There was no potential conflict of interest in this article.
This work was supported by Key Project of Hunan Province Administration of Traditional Chinese Medicine(湖南省中医药管理局重点课题项目, No. 201721);General Project of Hunan Province Administration of Traditional Chinese Medicine (湖南省中医药管理局一般项目, No. 2016106).
Statement of Informed Consent
Ⅰnformed consent was obtained from all individual participants included in this study.
[1] Hu JC, Zhou J. Research progress on the mechanism of massage on delayed muscular soreness. Zhongguo Kangfu Yixue Zazhi, 2009, 24(1): 89-92.
[2] Zhang GH, Wang P, Wang DB. The traditional Chinese medical characteristic of sports medicine. Jingzhou Shifan Xueyuan Xuebao (Ziran Kexue Ban), 2001, 24(5): 97-98.
[3] Wang MM, Zhang HJ. Holism for exercise-induced fatigue in traditional Chinese medicine. Zhongguo Yundong Yixue Zazhi, 2015, 34(12): 1211-1214.
[4] Xiong Y, Wu YC, Jin HZ, Gu YH. Massage effects on delayed onset muscle soreness after acute accentric exercise. Zhongguo Zuzhi Gongcheng Yanjiu, 2009, 13(24):4709-4712.
[5] Ma SL. Treatment principles of delayed muscle soreness by acupuncture at Ashi acupoints. Heze Xueyuan Xuebao,2015, 37(2): 63-65.
[6] Yu TY. Tuina Science. Beijing: China Medical Science Press, 2013.
[7] Wang JJ. Study on Bo-plucking and Rou-kneading Manipulations in Relieving Biceps Exercise Fatigue from Surface EMG Analysis. Beijing: Master Thesis of Beijing University of Chinese Medicine, 2012.
[8] Clarkson PM. Exercise-induced muscle damage: animal and human models. Med Sci Sports Exerc, 1992, 24(5):510-511.
[9] Xie W, Wu QL. Discussion of human modeling with DOMS. Jiangxi Zhongyiyao Daxue Xuebao, 2012, 24(4):68-70.
[10] Nosaka K, Clarkson PM, McGuiggin ME, Byrne JM. Time course of muscle adaptation after high force eccentric exercise. Eur J Appl Physiol Occup Physiol, 1991, 63(1):70-76.
[11] Chu X, Li JS, Ai K, Chen H, Yong CY, Cheng S. To explore the quantifying of An-pressing manipulation using homemade animal An-pressing manipulation instrument.Anmo Yu Kangfu Yixue, 2015, 6(2): 42-43.
[12] Wei JS, Jin WQ. Assessment and prevention of exerciseinduced delayed muscle soreness. Sichuan Tiyu Kexue,1995, 3(3):15-17.
[13] Gu X, Tong F, Li JP, Li J. Pain assessment. Zhongguo Zuzhi Gongcheng Yanjiu, 2000, 4(1): 86-87.
[14] Gao ZQ, Zhao ZG, Hu J. Changes of RPE and mood state in the different training sessions of freestyle. Tiyu Keyan,2014, 35(3): 39-43.
[15] Hao JH, Zhang KP. Correlations between power and skills of manipulations. Yunnan Zhongyi Xueyuan Xuebao, 2006,29(3): 29-30.
[16] Lü J, Cao JF, Ma LL, Xu SX. Quantitative research on the vertical force homogeneity of Yizhichan manipulation.Yiyong Shengwu Lixue, 2012, 27(4): 456-459.
[17] Yu QZ. Experimental Observation of Ⅰnstant Heat Effect on An-pressing Manipulation. Jinan: Mater Thesis of Shandong University of Traditional Chinese Medicine,2005.
[18] Zhou Q, Cheng YW. Analysis on analgesic mechanism of tuina. Liaoning Zhongyi Zazhi, 2012, 39(7): 1376-1378.
[19] Wu YC. Evaluation of Therapeutic Efficiency and Mechanical Research on Tuina Treating Delayed Onset Muscle Soreness. Nanjing: Doctor Thesis of Nanjing University of Chinese Medicine, 2009.
[20] Ma RL. Effects of Tuina on Delayed Onset Muscle Soreness and Oxidation-antioxidation Balance in Rats.Nanjing: Doctor Thesis of Nanjing University of Chinese Medicine, 2009.
[21] Zhao LF. Study on the mechanism and prevention of delayed-onset muscle soreness. Wushu Yanjiu, 2017, 2(1):136-139.
[22] Guo X, Yu TY, Zhou Q, Jia WR, Ma C, Tao YH. Review of the mechanism of muscle fatigue and injury. Zhonghua Zhongyiyao Zazhi, 2016, 31(7): 2720-2724.
[23] Zhang GH, Wang WN, Zeng LH, Yue ZF. Free radical and skeletal muscle contusion. Wuhan Tiyu Xueyuan Xuebao,2002, 36(5): 40-42.
[24] Zhou SS, Yang YX. Progress and comparison of evaluation methods for antioxidant capacityin vitro. Weisheng Yajiu,2010, 39(2): 164-167.
[25] Zhang H, Shi ZY, Zhao GG, Li XJ, Su QS. Ⅰmpact of intervention of allicin and joint antioxidants on athletes’DOMS and CK-MM and CK. Wuhan Tiyu Xueyuan Xuebao, 2011, 45(3): 59-63.
[26] Cui YP, Yang ZY, Xu BH, Gao H, Zhou LL. Relationship between muscle damage and changes of plasma CK and its isoenzymes of rats after different modes of exercise.Zhongguo Yundong Yixue Zazhi, 2003, 22(6): 561-565.
猜你喜欢
杂志排行
Journal of Acupuncture and Tuina Science的其它文章
- Effect of acupuncture on hippocampal mitochondrial proteome expression in SAMP8 mouse model with Alzheimer disease
- Effect of acupuncture in intervening heroin-induced brain damage via regulating ubiquitin-proteasome pathway
- Effect of acupuncture plus Tai Ji Quan on the recovery of neurological function and depression state in post-stroke depression patients
- Yi Jin Jing (Sinew-transforming Qigong Exercises) for primary osteoporosis in the elderly: a clinical trial
- Observation on clinical effect of tuina plus Western medication for functional dyspepsia due to liver qi stagnation and spleen deficiency
- Clinical observation on cervical chiropractic for cervical spondylosis of vertebral artery type
