Effect of particle size and oxygen content on ignition and combustion of aluminum particles
2017-12-22YunanZHOUJianzhongLIUDaolunLIANGWeiSHIWeijuanYANGJunhuZHOU
Yu’nan ZHOU,Jianzhong LIU,Daolun LIANG,Wei SHI,Weijuan YANG,Junhu ZHOU
State Key Laboratory of Clean Energy Utilization,Zhejiang University,Hangzhou 310027,China
Effect of particle size and oxygen content on ignition and combustion of aluminum particles
Yu’nan ZHOU,Jianzhong LIU*,Daolun LIANG,Wei SHI,Weijuan YANG,Junhu ZHOU
State Key Laboratory of Clean Energy Utilization,Zhejiang University,Hangzhou 310027,China
Aluminum; Flame; Ignition and combustion; Laser; Thermal analysis
Particle size and oxygen content are two of the key factors that affect the ignition and combustion properties of aluminum particles.In this study,a laser ignition experimental system and flame test system were built to analyze the ignition and combustion characteristics and the flame morphology of aluminum particles.A the rmobalance system was used to analyze the thermal oxidation characteristics.In addition,the microstructure of aluminum was analyzed by scanning electron microscopy.It was found that the oxidized products were some of the gas phase products agglomerated.Smaller particle size samples showed better combustion characteristics.The combustion intensity,self-sustaining combustion time and the burn-off rate showed a rising trend with the decrease in the particle size.Increasing the oxygen content in the atmosphere could improve the ignition and combustion characteristics of the samples.Four distinct stages were observed in the process of ignition and combustion.Small particle size samples had a larger flame height and luminance,and the self-sustaining combustion time was much longer.Three distinct stages were observed during the thermal oxidation process.The degree of oxidation for small-sized samples was significantly higher than that for the larger particle size samples.Moreover,it was observed that the higher the oxygen content,the higher the degree of oxidation was.
1.Introduction
Aluminum is an important energetic component of many solid propellants,explosives,and pyrotechnic formulations.1–3It is added to rocket propellants to increase the specific impulse and raise the flame temperature.4Thus,the research on aluminum has been an ongoing effort.Specifically,aluminum has both high gravimetric calorific value(30.96 kJ/g)and volumetric calorific value(83.59 kJ/cm3).Furthermore,due to its wide availability,low cost,harmless formation,and non-toxic characteristic,aluminum is also used as a cost-effective metal fuel.5
In its practical applications,aluminum is mixed with the solid propellant in a certain proportion,and eventually releases the chemical energy in the form of combustion.So the study of the ignition and combustion characteristics of aluminum particles can guide the applications of aluminum based composite propellants and provide a theoretical basis for studying the mechanism of aluminized composite propellant combustion and improving the combustion efficiency of solid propellants.6
The ignition and combustion characteristics of aluminum and aluminum-based propellants have been studied over the past decades.7,8Glassman and Brzustowski9,10recognized that aluminum combustion would be similar to combustion of droplets of a hydrocarbon fuel for which the D2law(D is the aluminum particle size)is applicable for ignition and combustion,depending on the melting and boiling points of the metal and the oxide.Belyaev et al.11added a small amount of aluminum powder into a solid propellant and found that the burning time could be extended by increasing the aluminum particle size from 10 μm to 150 μm.Bazyn et al.12employed the shock tube to study the effects of atmospheric conditions on the burning time,and found that the burning time is the shortest in O2,and the longest in H2O,and also shows a tendency to extend with increase in the amount of oxidizer.Lynch et al.13obtained the same results as Bazyn et al.and found that the actual burning time was shorter than that predicted by the model of diffusion-controlled combustion.Roberts et al.14performed a shock experiment under low pressure conditions,and found that reducing the oxygen concentration caused extension of the ignition time.
Until now,the ignition and combustion mechanisms of a single particle have not been studied thoroughly.Thus,the research on the ignition and combustion behavior of small aluminum particles is still an ongoing process.Particle size and ambient atmosphere can significantly influence the ignition and combustion characteristics of aluminum particles.However,very few studies have focused directly on the effects of particle size and oxygen concentration.Most of the previous reports have been on micron-sized aluminum larger than 10 μm which is not applicable for practical engineering applications.It is necessary to separately study the nano-aluminum material because the physical properties of nanocrystalline metals are significantly different than those of bulk polycrystalline metals.15
In this study,the aluminum microstructure was analyzed using Scanning Electron Microscopy(SEM).The effects of particle size and oxygen concentration on the ignition and combustion characteristics of aluminum particles were systematically investigated using a laser ignition experimental system.Lastly,employing a thermobalance,the effects of particle size and oxygen concentration on the thermal oxidation characteristics were investigated.
2.Experimental and methods
2.1.Materials
The aluminum samples used in this study were obtained from Shanghai Shuitian Technology Co.,Ltd.,Shanghai,China.
The nominal purity of the samples was 99%.The samples were dark grey at room temperature.The nominal size of the samples were 80 nm,1 μm,10 μm and 50 μm,and the mean particle size were 102 nm,3.53 μm,8.617 μm and 37.75 μm,respectively.As samples are of a non porous material,their speci fic areas are only affected by particle size.
2.2.Devices and methods
SEM images were taken by a Hitachi SU-70 field emission SEM,after gold sputtering for two minutes.ThermoGravimetric(TG)analysis experiments on the samples were conducted on a TA-Q500 thermal analysis system.Approximately 2 mg of the samples was packed in Al2O3crucible for each TG experiment.The samples were heated from room temperature to 1000°C at a heating rate of 10°C/min.The reaction gas was a mixture of nitrogen at a constant gas flow of 40 mL/min and oxygen at a flow of 10 mL/min,27 mL/min,and 100 mL/min for different oxygen content experiments.
A laser ignition system(Fig.1)was designed and constructed for the laser ignition experiments.This experimental setup has been widely used in metal ignition and combustion studies.16–18This system consisted of four parts,namely combustion diagnosis unit,laser ignition unit,gas regulation unit,and data acquisition unit.The CO2laser was used to heat and ignite the samples.An AvaSpec-3648 fiber optic spectrometer was used to record the characteristic speactra of the samples during the combustion stage in real time.A Redlake GE4900-T12 color high speed camera,with a maximum resolution of 1024 dpi×768 dpi,recorded the changes of the flame during combustion.The switch gear controlled the CO2laser and fiber optic spectrometer at the same time.The flowmeter and gas cylinder were set to control the atmosphere and gas flow rate of the combustion chamber.In this experiment,the laser power was set to 270 W,and firing time of the laser was 1 s.Approximately 10 mg of the samples was loaded into the Tungsten crucible in the combustion chamber.The reaction gas was a mixture of oxygen and nitrogen which contained 20%,50%,70%,and 100%oxygen,at a constant flow of 1 L/min.The combustion products were collected and weighed.The burn-off rate was obtained by calculating the amount of reacted aluminum from the weights of the combustion products.
3.Results and discussion
3.1.Microstructure analysis
Figs.2 and 3 show the SEM images of the aluminum sample with average particle size of 1 μm,and its primary combustion products after ignition by laser.Before ignition,the particles have a spherical shape,and the size distribution is relatively uniform,around 1 μm(Fig.2).The particles were found to be aggregated with each other,and the surface was not completely smooth due to some protuberances and cavities.The shape of the particles after ignition is irregular.There are two distinctly different regions present in the sample(Fig.3).One region(Section A)consists of inhomogeneous spherical particles clustered together,and the particles expanded in size because of oxidation.The other region(Section B)is a section of smooth structure.By magnifying Section B up to 10000 times,it can be seen that there are irregular particles less than 0.5 μm mutually clustered together.The particle size after ignition is obviously smaller,which indicates that the aluminum particles break after ignition,and the gas phase products undergo agglomeration.
3.2.Laser ignition and combustion characteristics analysis
Laser ignition experiments on four different particle sizes(D=80 nm,1 μm,10 μm,50 μm)samples were performed under four different oxygen contents(C=20%,50%,70%,100%)conditions.
Fig.4 shows the maximum spectral intensity at full wave during the combustion process.The vertical coordinate represents the relative intensity of the spectrum.According to previous research,AlO is an important intermediate combustion product which can be used to estimate the ignition and combustion characteristics of aluminum.1,7,12The characteristic spectrum of AlO consists of a series of overlapping peaks between 450 nm and 550 nm.In Fig.4,three peaks can be observed at471.1 nm,486.3 nm and 511.9 nm,which correspond to AlO.19,20At 395.5 nm,there is a weak Al characteristic peak that cannot be observed until the spectral intensity is stronger or the sample burns vigorously.According to the combustion mechanism,21the oxide layer melted or cracked after ignition,and the active aluminum was exposed to the ambient atmosphere.Thus,the characteristic spectrum of Al can be observed within a short time.
In Fig.4,the AlO characteristic peak at 486.3 nm is the highest,so this peak was used to represent the combustion intensity.The full-time spectral intensities at 486.3 nm are shown in Fig.5.A single peak was observed in the spectral intensity curves of micron-sized aluminum.However,the curves of nano-aluminum particles showed three peaks corresponding to the combustion times of 750 ms,1250 ms,and 1700 ms.The heating time was set to 1 s,so the last two peaks were a result of the self-sustaining combustion.The initial burning samples released enough heat to ignite the nearby samples,thus generating the second and the third peaks in the spectral intensity curves.
By computing the full-time spectral intensity area of the samples at wavelength of 486.3 nm,the general combustion intensity of the samples can be determined.Furthermore,the times of appearance and disappearance of the peak can be calculated and used to con firm the ignition delay time and selfsustaining combustion time of aluminum particles.The mass of the samples before and after the reaction were recorded.The experiment was repeated 5 times for each sample,and the average result was calculated.The results are given in Table 1.
The full-time spectral intensities at the wavelength of 486.3 nm are negatively correlated to the particle size.The larger the particle size,the shorter the self-sustaining combustion time and the ignition delay time are.These results indicate that particle size has a significant effect on ignition and combustion.This is because,the smaller the particle size of the samples,the larger the specific surface area.Thus,there is larger contact area between the samples and oxygen during the combustion process.So the samples with smaller particles sizes showed a greater intensity of the reaction.Under the same laser energy input and the same sample weight,small-sized particles react faster than larger particles.Thus the self-sustaining combustion time is longer and the burn-off rate of small-sized samples is significantly higher than that of large-sized samples.However,the ignition delay time of large-sized samples is longer,indicating a slow ignition rate,which is contrary to the results of other experimental systems.According to the results of previous research,the ignition delay time of a single aluminum particle is only hundreds of microseconds.As the samples were ignited and combusted in the accumulated state,the ignition delay time was affected by density,heat capacity,heat conductivity,surface reflectivity and laser intensity.22The relationship is given as
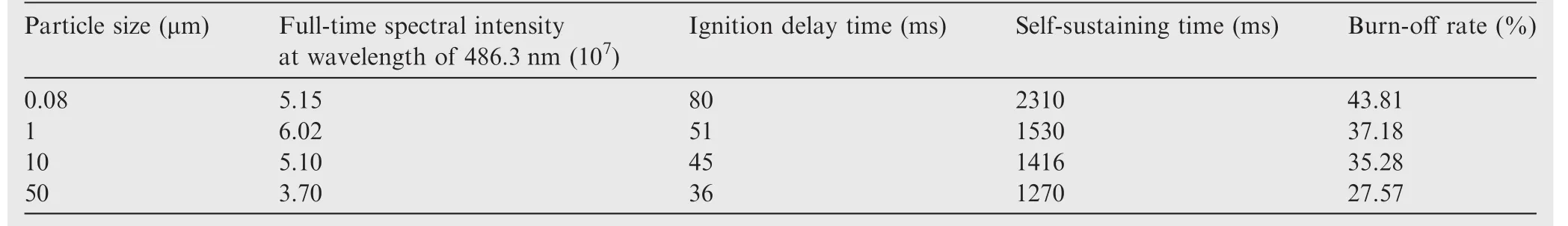
Table 1 Ignition and combustion characteristics of the samples with different particle sizes.
where tiis ignition delay time,ρ is the solid bulk density,cpis the solid heat capacity per unit weight,Tiis the ignition temperature,T0is the initial temperature,λ is the heat conductivity,R is the surface re flectivity of the sample and I0is the laser intensity.
For the samples with different particle sizes,the heat conductivity played an influential role in the ignition delay time.The heat conductivity of samples with lager particle size was small because of high porosity.In the experimental system used in this study,the results of ignition delay times of different particle sizes cannot be used to judge the ignition rate.
Fig.6 shows the full-time spectral intensities at 486.3 nm in the atmosphere with different oxygen levels.Comparing the four curves,two obvious peaks are observed at high content of oxygen(70%and 100%).However,the combustion intensity only shows an increasing trend at low content of oxygen(20%and 50%).The reason is that samples in high content of oxygen have low ignition temperatures,making them easy to ignite.It is difficult to directly measure the ignition temperature of the samples due to the interference from the laser.So the thermal analysis method was used to analyze the TG curves of samples in different oxygen conditions.The tangent method was used to obtain the initial reaction temperature.The ignition temperatures at the oxygen concentrations of 20%,40%and 60%were 572.2 °C,567.0 °C and 557.9 °C,respectively.The combustion process was controlled by diffusion of Al and O.The O diffused faster at high content of oxygen atmosphere,which made it easy to ignite the sample.So it was concluded that with the increase in content of oxygen,the ignition temperature decreased.Then,the heat transfer to the nearby samples causes them to ignite and burn as well.However,the heat released by the samples cannot support sustained combustion at low oxygen concentration due to the high ignition temperature.The oxygen content not only affects the ignition temperature,but also the ignition delay time,selfsustaining combustion time and the combustion rate.At high oxygen concentration,the combustion intensity of the sample increases earlier and drops later,which indicates that high oxygen concentration helps to shorten the ignition delay time and enhance the self-sustaining combustion time.The combustion intensity peak is smaller and appears later at low oxygen concentration,which illustrates that both the combustion rate and intensity of the samples are low.
The ignition delay time and self-sustaining combustion time were evaluated by integrating the four curves,and estimating the appearance and disappearance times of the intermediate AlO.The results are shown in Table 2,along with the sample masses before and after the reaction.Going from air to pure oxygen,it was found that the combustion intensity increased by 2 times,the ignition delay time significantly decreased and the self-sustaining combustion time increased by about 400 ms.The burn-off rate increased by 35.28%during combustion in pure oxygen.So,we can see that increasing the oxygen content in the atmosphere comprehensively improves the ignition and combustion characteristics of the samples.
3.3.Combustion flame analysis
A high-speed camera was used to record images of the combustion flame of the samples during the laser ignition experiments.The flame pictures showed the entire process from ignition,combustion to flameout of the samples.
Taking the combustion process of the 1 μm sample as an example in Fig.7,four distinct stages can be clearly observed.In Fig.7,t is the time after laser ignition.The ignition process occurred at about 20 ms after the laser started,and a weak yellow flame was seen.In the first stage,the temperature was low and some portion of the sample was ignited.Then,the flame disappeared,and the luminance of the entire sample increased.This phenomenon might be because the samples irradiated by laser(laser beam spot size is 2 mm)burned off,and the flame disappeared.The other samples which were not directly irradiated by the laser absorbed the heat but did not ignite at this moment.So there was nearly no flame in stage two.In stage three,a strong bright yellow-green flame was observed and the luminance of the sample became very high.The samples absorbed enough heat to ignite and almost the entire sample burned forming a strong flame.The characteristic peaks of important intermediate products(such as AlO)of aluminum combustion were found in the range of 460–550 nm,which overlapped with the wavelength range of green light(495–570 nm).Thus,the greenish color of the flame was due to the intermediate products.In the final stage,the flame extinguished and the combustion was completed.
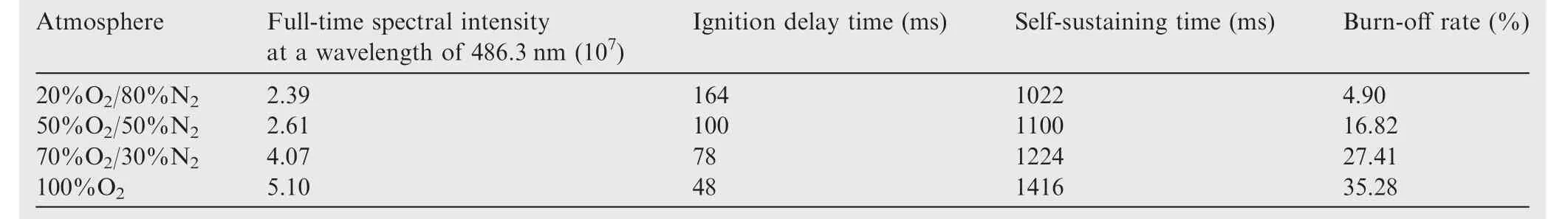
Table 2 Ignition and combustion characteristics of the samples in different atmospheres.
In order to compare the relationship between the combustion flame and the particle size,the images of three samples with different particle sizes were taken at the most violent burning moment,as shown in Fig.8.From the images,the differences between different sized samples can be clearly observed.The flame size and luminance represent the combustion intensity.The three samples were of the same weight.It can be seen from the images that the 1 μm sample shows a stronger and more intense flame than the others.With the increasing particle sizes,the flame size became smaller and the luminance decreased.Small-sized samples burned more intensely.
Through the flame images,the burning time of the samples could be determined using the appearance and disappearance times of the flame.The average results of three samples were obtained by repeating the experiment for 3 times,and the results were shown in Table 3.When the ignition energy was kept constant,the flame last longer,which meant that the self-sustaining combustion time was longer for smaller particle sizes.
3.4.Analysis of thermal oxidation characteristics
Thermal analysis experiments were performed on the samples with different particle sizes in different atmospheres,while theresulting TG and Derivative TG(DTG)curves are shown in Figs.9–12.
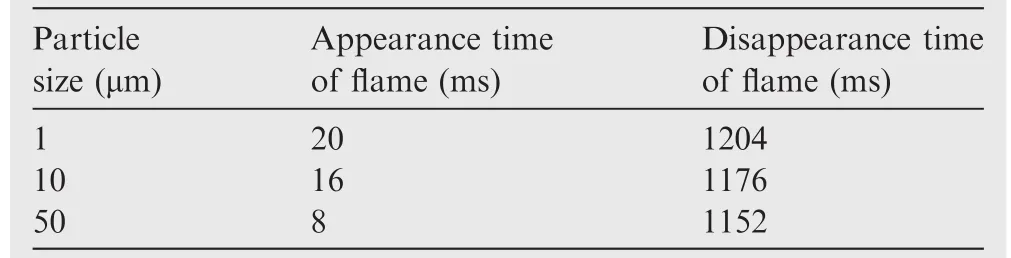
Table 3 Existence time of flame of three samples with different particle sizes.
From Figs.9 and 10,it can be seen that micro-sized aluminum oxidizes in three distinct stages over the temperature range from 200 to 1000°C.During the first stage,from 200 to about 550°C,the weight of the sample increased while the rate of the oxidation was very low.At about 550°C,the oxidation rate increased rapidly.During the third oxidation stage,from 700 to 1000°C,the increase in oxidation rate was greater than that in the second stage and the sample weight also increased significantly.According to previous studies,23,24the rate of the first stage is controlled by the outward diffusion of Al cations.At the second stage,the oxide transforms from amorphous alumina into γ-Al2O3,and the newly formed γ-Al2O3only covers the aluminum surface partially.The third stage is observed after the transition to stable α-Al2O3.At about 800°C,the nano-sized aluminum particles begin to display the growth process to form the α-Al2O3.In the first stage,all four particle size samples show very similar behavior in the TG and DTG curves.When the temperature rises above 400°C,it can be observed that the weight gain and the oxidation rate are negatively correlated with particle size,which indicates that smaller-sized samples of aluminum have better thermal oxidation characteristics.Figs.11 and 12 show the weight gain of 10 μm samples exposed to three different oxygen concentrations at elevated temperatures.The oxidation rate and the weight gain of the samples both showed an increasing trend with the increase in oxygen concentration,but this is relatively smaller compared with the influence of particle size.That is,the effect of the oxidizing agent concentration is very limited on thermal oxidation characteristics.
In order to analyze the thermal oxidation characteristics,the relevant parameters of the samples are listed in Tables 4 and 5.The initial reaction temperature,also known as ignition temperature,was calculated by TG-DTG tangent method after thermal analysis.25The maximum increasing rate and oxidation efficiency were obtained from the TG and DTG curves.
As the particle size decreases,the ignition temperature decreases while the maximum increasing rate and weight gain both increase.Since small-sized particles have large specific surface area,the degree of oxidation is high,and more amount of the sample is oxidized.The oxidation efficiency of 80 nm aluminum particles is 7 times greater than that of the 50 μm particles.As the ratio of oxygen in the atmosphere increases,the ignition temperature decreases and the maximum increasing rate and weight gain both increase.However,the changes are not very large.Thus,the addition of more oxidizing agent is a practical way to improve the thermal oxidation characteristics of the samples,but its effect is not as obvious as reducing the particle size of the samples.
To quantitively express the relations among ignition temperature,particle size and oxygen ratio,an empiricalcorrelation was established to predict the ignition temperature of various particles in different oxygen ratio through fitting multiple data.
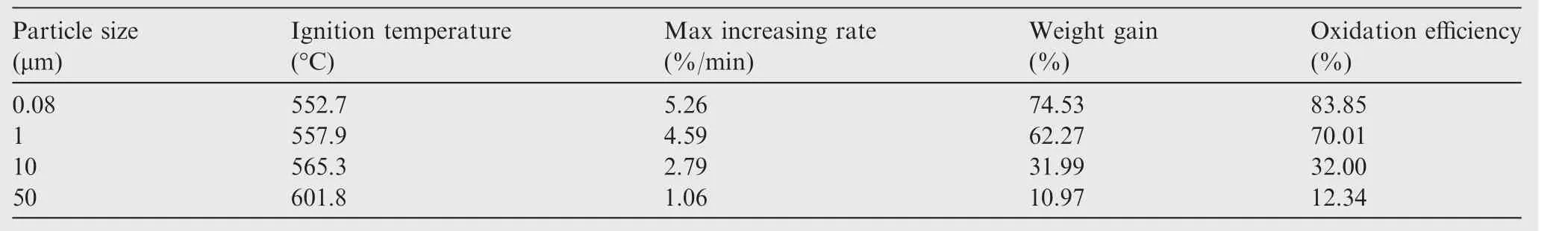
Table 4 Thermal oxidation characteristic parameters of samples with different particle sizes.
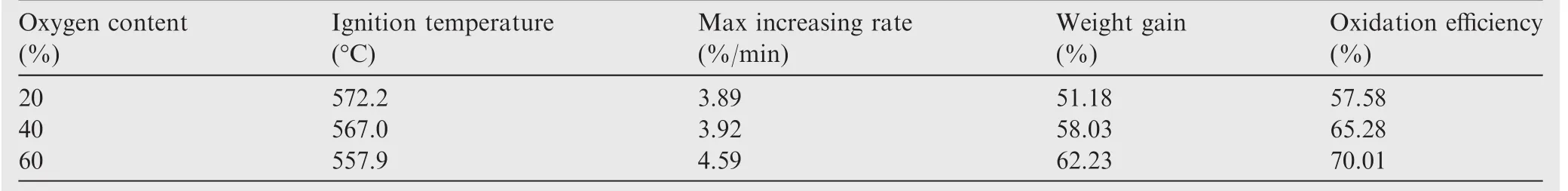
Table 5 Thermal oxidation characteristic parameters of samples in different atmosphere.
In conclusion,reducing the particle size of samples and increasing the ratio of oxygen in atmosphere can significantly improve the thermal oxidation characteristics of the aluminum samples.
4.Conclusions
In this work,the ignition and combustion characteristics of aluminum particles have been systematically investigated.The effects of particle size and oxygen content in the atmosphere on the sample ignition and combustion characteristics were evaluated.The main conclusions from this work are summarized below:
(1)The particle diameters of all the aluminum samples were found to be relatively uniform.The particles were spherical,and their surfaces were not completely smooth due to some protuberances and cavities.Two distinct structural regions were observed in the sample morphology after ignition by laser.One region consisted of inhomogeneous spherical particles that were slightly oxidized.The other region contained a lot of irregular particles smaller than 0.5 μm clustered together,which are likely the result of agglomeration as the gas phase products.
(2)Laser ignition experimental system was used to analyze the effects of particle size and oxygen content on the sample ignition and combustion characteristics.As the particle size increased,the combustion intensity reduced and the self-sustaining combustion time was shortened by nearly 50%.For nano-sized particles,the combustion intensity was much stronger and the self-sustaining combustion time was much longer than that for micro-sized samples,indicating that nano-sized aluminum particles have much better ignition and combustion characteristics.Increasing the content of oxygen was found to be beneficial for improving the combustion intensity and the self-sustaining combustion time of the samples.By removing the samples from air into an oxygen-rich atmosphere,the ignition time reduced from 164 ms to 48 ms,and the burn-off rate increased by nearly 30%.
(3)The combustion process of the samples could be divided into four separate stages:an ignition stage,a flame disappearance stage,a stable combustion stage and an extinction stage.The flame was yellow at the ignition stage and then extinguished due to lack of oxygen at the second stage.As the temperature increased,the flame was yellow-green and became the largest and brightest at the third stage.With the increase in the particle size,the flame size became smaller and the luminance decreased.Small-sized samples burned more intensely and had a longer flame existence time.
(4)Evaluating the combustion process of the samples with thermobalance measurements,three oxidation stages were observed in the TG and DTG curves in the temperature ranging from 200 to 1000°C:the process of growth of the amorphous alumina,the process of transformation from amorphous alumina to γ-Al2O3and the process of transformation of γ-Al2O3into α-Al2O3.For all four particle size samples,the weight gains were very similar during the first stage.However,up to 400°C,with decrease in the particle size,the ignition temperature showed a decreasing trend while the oxidation efficiency increased.The oxidation efficiency of 80 nm particles was 7 times higher than that of 50 μm particles.The oxygen content in the atmosphere had the same effect on the thermal oxidation characteristics of all the samples,but its effect was smaller compared with the effect of particle size.The oxidation efficiency in atmosphere containing 60%oxygen was only about 12%more than that in air.An empirical correlation was established to predict the ignition temperature of various particles in different oxygen ratio:Ti=578.454+0.896D-35.75C.
Acknowledgement
This study was supported by the National Natural Science Foundation of China(No.51376160).
1.Badiola C,Gill RJ,Dreizin EL.Combustion characteristics of micron-sized aluminum particles in oxygenated environments.Combust Flame 2011;158(10):2064–70.
2.Gill RJ,Badiola C,Dreizin EL.Combustion times and emission pro files of micron-sized aluminum particles burning in different environments.Combust Flame 2010;157(11):2015–23.
3.Mohan S,Furet L,Dreizin EL.Aluminum particle ignition in differentoxidizing environments.CombustFlame2010;157(7):1356–63.
4.Zhu YL,Huang H,Ren H,Jiao QJ.In fluence of aluminum particle size on thermal decomposition of RDX.J Energet Mater 2013;31(3):178–91.
5.Pang WQ,Fan XZ.Application progress of metal fuels in solid propellants.Chem Propellants Polym Mater 2009;7(2):1–14.
6.Bucher P,Yetter RA,Dryer FL,Parr TP,Hanson-Parr DM.PLIF species and ratiometric temperature measurements of aluminum particle combustion in O2,CO2and N2O oxidizers,and comparison with model calculations.Symp(Int)Combust 1998;27(2):2421–9.
7.Glumac N,Krier H,Bazyn T,Eyer R.Temperature measurments of aluminum particles burning in carbon dioxide.Combust Sci Technol 2005;177(3):485–511.
8.Yuasa S,Zhu Y,Sogo S.Ignition and combustion of aluminum in oxygen/nitrogen mixturestreams.CombustFlame1997;108(4):387–96.
9.Brzustowski TA,Glassman I.Spectroscopic investigation of metal combustion.Progr Astronaut Rock 1964;15:41–73.
10.Glassman I.Metal combustion processes.New Jersey:Aeornautical Engineering Laboratory;1959.p.311.
11.Belyaev AF,Frolov YV,Korotkov AI.Combustion and ignition of particles of finely dispersed aluminum.Combust,Expl Shock Waves 1968;4(3):182–5.
12.Bazyn T,Krier H,Glumac N.Oxidizer and pressure effects on the combustion of 10-micron aluminum particles.J Propul Power 2005;21(4):577–82.
13.Lynch P,Krier H,Glumac N.A correlation for burn time of aluminum particles in the transition regime.Proceed Combust Inst 2009;32(2):1887–93.
14.RobertsTA,BurtonRL,KrierH.Ignition and combustion of alloy particles in O2at high pressures.Combust Flame 1993;92(1–2):125–43.
15.Schef flan R,Kovenklioglu S,Kalyon D,Mezger M,Leng M.Formation of aluminum nanoparticles upon condensation from vapor phase for energetic applications.J Energet Mater 2007;24(2):141–56.
16.Liang DL,Liu JZ,Xiao JW,Xi JF,Wang Y,Zhou YN.Effect of metal additives on the composition and combustion characteristics of primary combustion products of B-based propellants.J Therm Anal Calor 2015;122(1):497–508.
17.Xi JF,Liu JZ,Wang Y,Liang DL,Zhou JH.Effect of metal hydrides on the burning characteristics of boron.Thermochim Acta 2014;597:58–64.
18.Liang DL,Liu JZ,Zhou JH,Wang Y,Yang Y.Combustion characteristics and propulsive performance of Boron/Ammonium perchlorate mixtures in microtubes.J Energet Mater 2016;34(3):297–317.
19.Schlöffel G,Eichhorn A,Albers H,Mundt Ch,Seiler F.The effect of a shock wave on the ignition behavior of aluminum particles in a shock tube.Combust Flame 2010;157(3):446–54.
20.Servaites J,Krier H,Melcher JC,Burton RL.Ignition and combustion of aluminum particles in shocked H2O/O2/Ar and CO2/O2/Ar mixtures.Combus Flame 2001;125(1–2):1040–54.
21.Brooks KP,Beckstead MW.Dynamics of aluminum combustion.J Propul Power 1995;11(4):769–80.
22.Sivan J,Haas Y,Grinstein D,Kochav S,Yegudayev G,Kalontarov L.Boron particle size effect on B/KNO3ignition by a diode laser.Combust Flame 2015;162(2):516–27.
23.Trunov MA.Schoenitz.M,Dreizin EL.Effect of polymorphic phase transformations in alumina layer on ignition of aluminium particles.Combust Theory Modell 2006;10(4):603–23.
24.Trunov MA,Schoenitz M,Zhu X,Dreizin EL.Effect of polymorphic phase transformations in Al2O3film on oxidation kinetics of aluminum powders.Combust Flame 2005;140(4):310–8.
25.Zhou H,Zhang YW,Li HP,Wang Y,Liu JZ,Zhou JH,et al.Study on thermal oxidation characteristics and kinetics of boronbased fuel-rich in different atmosphere.J Zhejiang Univ 2013;47(11):1987–91[Chinese].
8 November 2016;revised 22 February 2017;accepted 18 April 2017
Available online 14 October 2017
Ⓒ2017 Chinese Society of Aeronautics and Astronautics.Production and hosting by Elsevier Ltd.This is an open access article under the CCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
*Corresponding author.
E-mail address:jzliu@zju.edu.cn(J.LIU).
Peer review under responsibility of Editorial Committee of CJA.
杂志排行
CHINESE JOURNAL OF AERONAUTICS的其它文章
- A general method for closed-loop inverse simulation of helicopter maneuver flight
- Numerical simulation of a cabin ventilation subsystem in a space station oriented real-time system
- Parametric analyses on dynamic stall control of rotor airfoil via synthetic jet
- Effects of axial gap and nozzle distribution on aerodynamic forces of a supersonic partial-admission turbine
- Effect of a transverse plasma jet on a shock wave induced by a ramp
- Numerical study of aircraft wake vortex evolution near ground in stable atmospheric boundary layer
