蓝宝石衬底上PECVD生长石墨烯及其气敏传感器
2017-11-22蔚翠何泽召刘庆彬李娴谢丹蔡树军冯志红
蔚翠,何泽召,刘庆彬,李娴,谢丹,蔡树军,冯志红
(1河北半导体研究所专用集成电路重点实验室,河北 石家庄 050051;2清华大学微电子所,北京 100084)
蓝宝石衬底上PECVD生长石墨烯及其气敏传感器
蔚翠1,何泽召1,刘庆彬1,李娴2,谢丹2,蔡树军1,冯志红1
(1河北半导体研究所专用集成电路重点实验室,河北 石家庄 050051;2清华大学微电子所,北京 100084)
在蓝宝石衬底上,利用 PECVD在相对较低的温度和相对短的时间制备石墨烯。实验发现,在 950℃,生长15 min,可制备纳米晶石墨烯。所制备的石墨烯为双层结构,存在较多的缺陷,使得其适合用于制作气敏传感器。制作的纳米晶石墨烯气敏传感器对甲醛气体显示出良好的响应和恢复特性。分析发现纳米晶石墨烯中大量的晶界和褶皱使气体的吸附和解吸附能垒降低是其表现出良好气敏特性的主要原因。
石墨烯;吸附;膜;测量;甲醛;曝气
引 言
气敏传感器在环境监测,工业生产和安全,医疗诊断,军事和宇航等领域发挥着重要作用[1]。固态气敏传感器具有尺寸小、功率低、灵敏度高、成本低等优势。它们可以探测多种气体,探测浓度可以达到百万分之一(mg·L-1)量级[2]。甲醛气体在环境中非常常见,对人类的身体健康产生严重的影响,甲醛浓度高于几个mg·L-1时,会对人类的呼吸系统产生非常大的伤害[3-4]。此外,甲醛还被列为致癌物[5-6]。因此及时准确地在位探测甲醛气体对控制环境污染、保护人类健康极其重要。石墨烯具有高载流子迁移率,大的比表面积,低噪声等特性[7],是制作固态气敏传感器的理想选择。研究人员对石墨烯气敏传感器已开展大量的研究,取得了很大进展[8-11]。石墨烯气敏传感器可以探测 NO2、N2O、NO、CO2、O2、NH3、H2S、甲苯、乙醇等[12]。Li等[13]前期利用还原氧化石墨烯探测甲醛气体,10 mg·L-1下对甲醛气体的响应度约为1.8%,响应时间约16 s。Mu等[14]利用化学气相沉积生长石墨烯并转移至 Si/SiO2衬底,对 9 mg·L-1甲醛气体响应度2%,响应时间130 s。
还原氧化石墨烯可控性较差,化学气相沉积在金属催化剂上生长的石墨烯则需要转移至其他绝缘衬底。在介质衬底上,不使用金属催化剂生长石墨烯可以直接用于制备石墨烯器件,具有很大的研究价值。蓝宝石是低成本制备石墨烯的理想候选衬底。一些课题组报告了在蓝宝石上直接生长石墨烯。Hwang等[15]在高温下(1350~1600℃)生长少层外延石墨烯。Miyasaka等[16]尝试通过乙醇CVD在相对较低的温度下(800~1000℃)生长石墨烯。Fanton等[17]在高温下(1425~1600℃)在蓝宝石上生长了石墨烯,其质量与SiC上外延石墨烯可以比拟。
同时,等离子体化学气相沉积(PECVD)被用来合成大尺寸石墨烯[18]。该方法与常规合成技术比,可以在较低温度下生长。本工作在蓝宝石衬底上生长了纳米晶石墨烯,并用于制备石墨烯气敏传感器。石墨烯气敏传感器对甲醛气体表现出良好的响应。
1 实验材料和方法
1.1 石墨烯材料制备
单面抛光的直径50.8 mm的单晶a-Al2O3(0001)衬底使用乙醇和丙酮清洗后放入冷壁低压 CVD系统中。生长温度为950℃,腔体压力1 kPa。甲烷作为碳源,氢气作为载气。衬底温度达到目标温度后,石墨烯生长前,蓝宝石衬底在H2中刻蚀7 min,气流 1000 ml·min-1。然后 H2和甲烷流速分别为 300 ml·min-1和 6 ml·min-1,通入 CVD 腔体。沉积过程中射频等离子体功率保持在100 W。沉积15 min后,H2和甲烷气流和射频电源关断,衬底在Ar气气氛下冷却至室温。
1.2 石墨烯气敏传感器制备
石墨烯气敏传感器通过标准光刻制备。沟道外的石墨烯通过氧等离子体刻蚀去除。金属Au作为欧姆接触金属,欧姆接触通过光刻、电子束蒸发沉积、剥离后获得。
图1为纳米晶石墨烯气敏传感器的光学显微(OM)照片。纳米晶石墨烯气敏传感器采用叉指电极结构。图1(a)为某一个石墨烯气敏传感器。图1(b)和(c)为局域放大图。
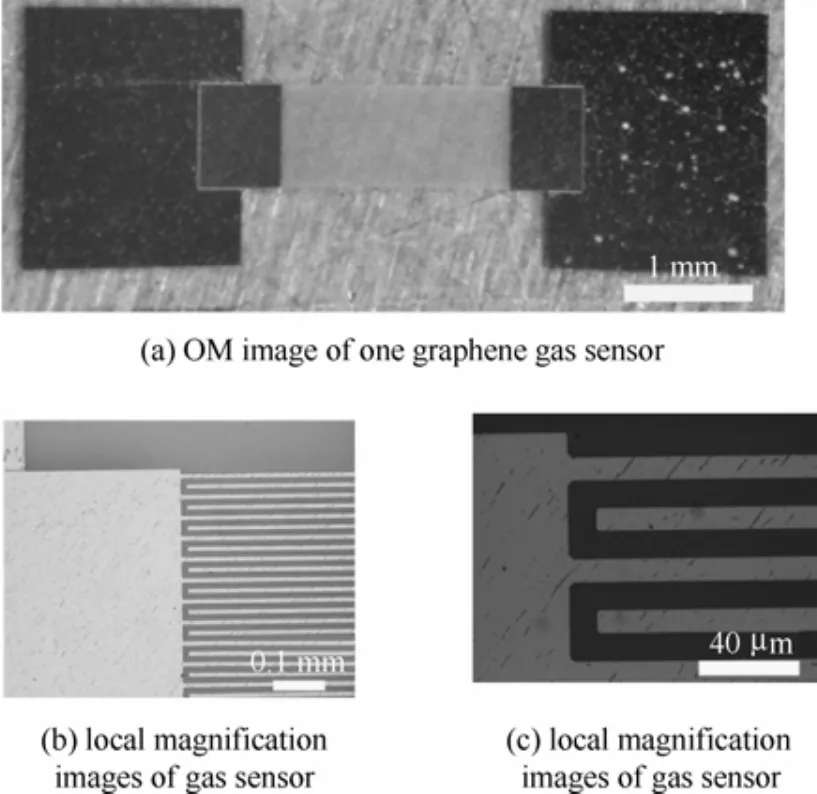
图1 纳米晶石墨烯气敏传感器光学OM照片Fig.1 OM images of nano-grain graphene gas sensor
1.3 分析测试仪器
原子力显微镜(AFM,Asylum infinity),拉曼光谱仪(Raman,Horiba Jobin Yvon Lab RAM HR800),气敏特性测试系统(自制,其中器件的电阻采用Keithley 2700 数据采集系统测试)。
2 实验结果与讨论
2.1 石墨烯材料制备与表征
图2给出了PECVD在950℃生长石墨烯的拉曼光谱。如图2(a)所示,图中标出了石墨烯的D、G、D'和2D峰。G峰在1596 cm-1附近,2D峰在2696 cm-1附近。2D峰峰位表明石墨烯薄膜内的应力较小,说明石墨烯和蓝宝石衬底相互作用小[19]。2D峰半高宽(FWHM)为55 cm-1,且2D峰可以拟合为4个洛伦兹峰,2D峰与G峰面积比I2D/IG≈1.1,表明石墨烯为双层结构[20-23]。1335 cm-1附近为 D峰,来自碳原子空位或堆积,造成石墨烯材料的六方对称性被破坏。由于D峰强度很大,在1626 cm-1处出现了D'峰[19]。PECVD制备石墨烯的缺陷峰D峰特别强,强度比 ID/IG≈2.4。非常强的 D 峰表明PECVD法制备的石墨烯样品中存在较多缺陷。
图2(b)和(c)为20 μm×20 μm区域 2D峰 FWHM和峰位的面扫描结果。从图中可以看到,所制备的石墨烯样品2D峰FWHM和峰位在测试区域内变化很小,表明石墨烯层数和应力分布很均匀。
图3为PECVD在蓝宝石衬底上生长石墨烯的2 μm×2 μm区域范围的AFM图。从图中可以看到,样品为晶粒结构,晶畴尺寸约300 nm,并且在晶界处可观察到明显的石墨烯褶皱。Raman和AFM分析表明PECVD在蓝宝石衬底上生长的为缺陷密度很高的纳米晶石墨烯。
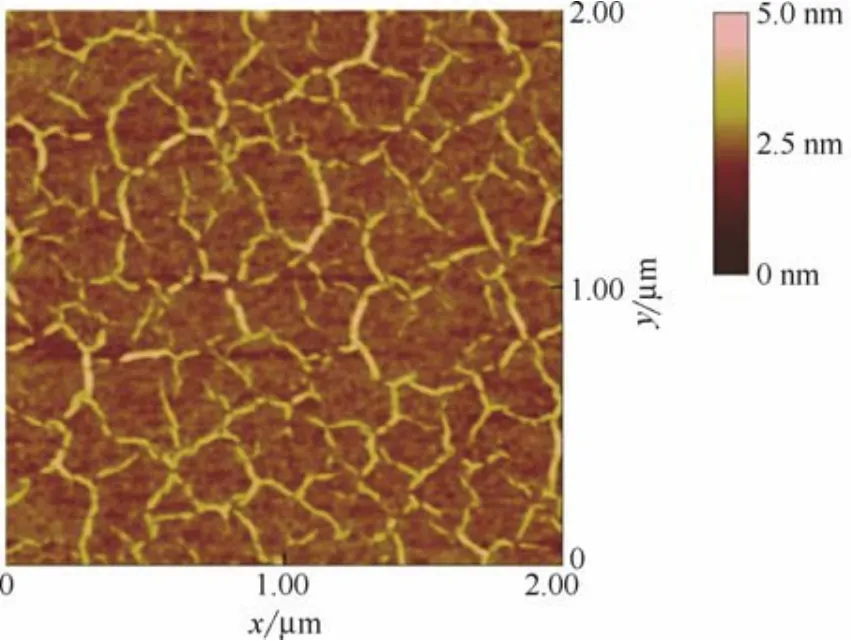
图3 PECVD在蓝宝石衬底上生长石墨烯的AFM图Fig.3 AFM image of graphene sample grown on sapphire by PECVD
2.2 石墨烯气敏传感器性能测试和分析
纳米晶石墨烯薄膜的气敏特性通过在室温下暴露于甲醛气氛下测试其电阻变化来表征。图4(a)给出了纳米晶石墨烯气敏传感器对室温下不同浓度甲醛气体的响应。纳米晶石墨烯气敏传感器对 20 mg·L-1甲醛气体室温响应度为 3%。响应时间 9.92 min,恢复时间17 min。较低的甲醛浓度下,响应度变低。10和 5 mg·L-1甲醛气氛下响应度分别为1.6%和0.8%。传感器对不同浓度甲醛气体表现出良好的响应和恢复特性。本文的PECVD蓝宝石基纳米晶石墨烯气敏传感器与文献中报道的氧化还原石墨烯气敏传感器和化学气相沉积石墨烯气敏传感器响应度基本相当[13-14]。作为对比,也制备了SiC基外延石墨烯气敏传感器,如图4(b)所示。在SiC (0001)衬底上生长了单层石墨烯,生长细节参照之前的工作[24-25]。SiC上外延石墨烯在甲醛气氛下方阻降低,这是因为外延石墨烯为n型,甲醛对石墨烯为n型掺杂,因此使其电阻降低。外延石墨烯对甲醛气体也有明显的响应,但是其恢复特性很差。气体关闭后,长时间恢复后,样品的电阻基本不变。
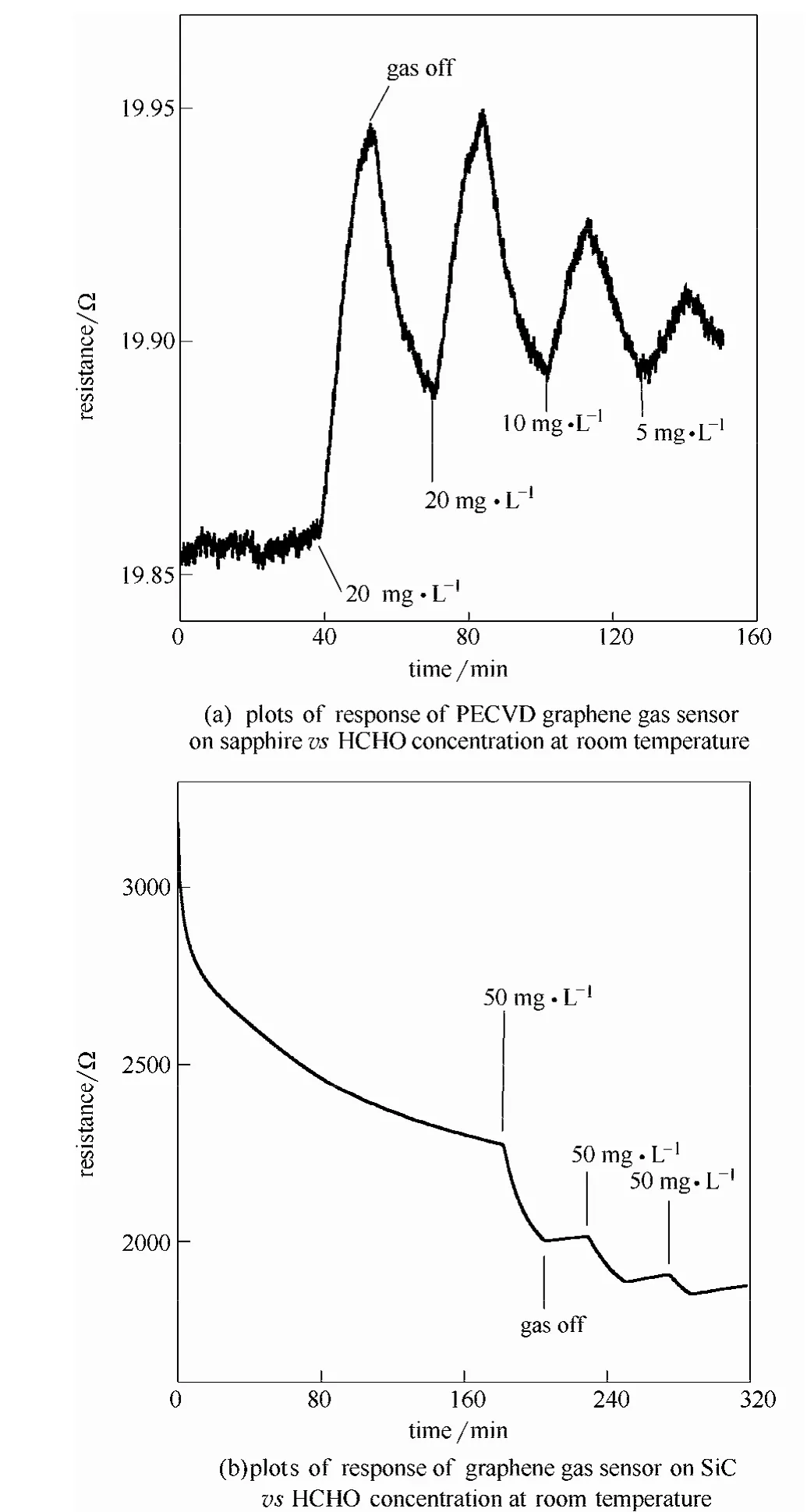
图4 室温下石墨烯气敏传感器对不同浓度甲醛气体的响应曲线Fig.4 Plots of response of graphene gas sensor vs HCHO concentration at room temperature
PECVD法在蓝宝石上生长的纳米晶石墨烯对甲醛气体表现出良好的响应,气体关闭后具有良好的恢复特性。外延石墨烯气敏传感器对甲醛气体有良好的响应,但是其恢复特性很差。这应该是由于两个样品中缺陷种类和数量不同造成的。PECVD法在蓝宝石上制备的纳米晶石墨烯中含有大量晶界和褶皱。在这些缺陷区域,气体吸附和脱附的能量势垒低[26-29]。外延石墨烯的晶体质量高,拉曼光谱ID/IG≈ 0.36,且没有D'峰[25]。D峰主要来源于过渡层,H插入去除过渡层后缺陷D峰消失[30]。外延石墨烯气敏传感器,甲醛气体主要吸附在石墨烯晶格上,而不是缺陷处。由于其大的能量势垒,气体分子难以从石墨烯薄膜中解吸附,因此,关闭气体后,电阻难以恢复。
3 结 论
用PECVD方法在蓝宝石衬底上制备了纳米晶石墨烯,研究了其气敏传感性能。蓝宝石上石墨烯气敏传感器对不同浓度的甲醛气体表现出良好的响应和恢复特性。PECVD在蓝宝石上生长石墨烯尺寸大,成本低,可能具有潜在的商业应用价值。
[1] LIU X, CHENG S T, LIU H,et al.A survey on gas sensing technology[J]. Sensors, 2012, 12: 9635-9665.
[2] CAPONE S, FORLEO A, FRANCIOSO L,et al.Solid state gas sensors: state of the art and future activities[J]. J. Optoelectron. Adv.Mater., 2003, 5: 1335-1348.
[3] GUPTA K C, ULSAMER A G, PREUSS P W. Formaldehyde indoor air: sources and toxicity[J]. Environ. Int., 1983, 8: 349.
[4] WANG X F, DING B, SUN M,et al.Nanofibrous polyethyleneimine membranes as sensitive coatings for quartz crystal microbalance-based formaldehyde sensors[J]. Sens. Actuators B,2010, 144: 11-17.
[5] HERSCHKOVITZ Y, ESHKENAZI I, CAMPBELL C E,et al.An electrochemical biosensor for formaldehyde[J]. J. Electroanal. Chem.,2000, 491: 182-187.
[6] ZHOU K W, JI X L, ZHANG N,et al.On-line monitoring of formaldehyde in air by cataluminescence-based gas sensor[J]. Sens.Actuators B, 2006, 119: 392-397.
[7] VARGHESE S S, VARGHESE S H, SWAMINATHAN S,et al.Two-dimensional materials for sensing: graphene and beyond[J].Electronics, 2015, 4: 651-687.
[8] LI X, ZHAO Y, WANG X,et al.Reduced graphene oxide (rGO)decorated TiO2microspheres for selective room-temperature gas sensors[J]. Sensors and Actuators B: Chemical, 2016, 230: 330-336.
[9] BASU S, BHATTACHARYYA P, Recent developments on graphene and graphene oxide based solid state gas sensors[J]. Sens. Actuators B: Chem., 2012, 173: 1-21.
[10] LEBEDEV A A, LEBEDEVA S P, NOVIKOVC S N,et al.Supersensitive graphene-based gas sensor[J]. Technical Physics, 2016,61(3): 453-457.
[11] CHEN G, PARONYAN T M, HARUTYUNYAN R. Sub-ppt gas detection with pristine graphene[J]. Appl. Phys. Lett., 2012, 101:652-655.
[12] YUAN W, SHI G. Graphene-based gas sensors[J]. Journal of Materials Chemistry A, 2013, l: 10078-10091.
[13] LI X, XIE D, DAI R,et al.Formadelyde-sensing properties of reduced graphene oxide by layer-by-Iayer self-assemble method[C]//2014 IEEE International Conference on Electron Devices &Solid-State Circuits. 2015: 1-2.
[14] MU H, ZHANG Z, ZHAO X,et al.High sensitive formaldehyde graphene gas sensor modified by atomic layer deposition zinc oxide films[J]. Appl. Phys. Lett., 2014, 105: 033107.
[15] HWANG J, KIM M, MPBELL D,et al. Van der waals epitaxial growth of graphene on sapphire by chemical vapor deposition without a metal catalyst[J]. ACS Nano, 2013, 7: 385-395.
[16] MIYASAKA Y, NAKAMURA A, TEMMYO J. Graphite thin films consisting of nanograins of multilayer graphene on sapphire substrates directly grown by alcohol chemical vapor deposition[J].Jpn. J. Appl. Phys., 2011, 50: 04DH12.
[17] FANTON M A, ROBINSON J A, PULS C,et al.Characterization of graphene films and transistors grown on sapphire by metal-free chemical vapor deposition[J]. ACS Nano, 2011, 5: 8062-8069.
[18] TERASAWA T O, SAIKI K. Growth of graphene on Cu by plasma enhanced chemical vapor deposition[J]. Carbon, 2012, 50: 869-874.
[19] FERRARI A C. Raman spectroscopy of graphene and graphite:disorder, electron-phonon coupling, doping and nonadiabatic effects[J]. Solid State Commun., 2007, 143: 47-57.
[20] NI Z H, WANG Y Y, YU T,et al.Raman spectroscopy and imaging of graphene[J]. Nano Res., 2008, 1: 273.
[21] FERRARI A C, MEYER J C, SCARDACI V,et al.Raman spectrum of graphene and graphene layers[J]. Phys. Rev. Lett., 2006, 97:187401.
[22] NI Z H, WANG H M, KASIM J,et al.Graphene Thickness determination using reflection and contrast spectroscopy[J]. Nano Lett., 2007, 7(9): 2758-2763.
[23] ROBINSON J A, WETHERINGTON M, TEDESCO J L,et al.Correlating Raman spectral signatures with carrier mobility in epitaxial graphene: a guide to achieving high mobility on the wafer scale[J]. Nano Letters, 2009, 9(8): 2873-2876.
[24] YU C, LI J, LIU Q B,et al.Buffer layer induced band gap and surface low energy optical phonon scattering in epitaxial graphene on SiC(0001)[J]. Appl. Phys. Lett., 2013, 102: 013107.
[25] YU C, LI J, LIU Q B,et al. Quasi-equilibrium growth of monolayer epitaxial graphene on SiC (0001)[J]. Acta Phys. Sin., 2014, 63:038102.
[26] ZHANG H, FAN L, DONG H,et al.Spectroscopic investigation of plasma-fluorinated monolayer graphene and application for gas sensing[J]. ACS Appl. Mater. Interfaces., 2016, 8: 8652-8661.
[27] LEE G, YANG G, CHO A,et al.Defect-engineered graphene chemical sensors with ultrahigh sensitivity[J]. Phys. Chem. Chem.Phys., 2016, 18: 14198.
[28] VARGHESE S S, LONKAR S, SINGH K K,et al.Recent advances in graphene based gas sensors[J]. Sensors and Actuators B, 2015, 218:160-183.
[29] WANG T, HUANG D, YANG Z,et al.A review on graphene-based gas/vapor sensors with unique properties and potential applications[J].Nano-Micro Lett., 2016, 8(2): 95-119.
[30] YU C, LIU Q B, LI J,et al.Preparation and electrical transport properties of quasi free standing bilayer graphene on SiC (0001)substrate by H intercalation[J]. Appl. Phys. Lett., 2014, 105: 183105.
date:2017-03-30.
Prof. FENG Zhihong, ga917vv@163.com
supported by the National Natural Science Foundation of China (61674131, 61306006).
PECVD growth of graphene on sapphire substrate and its gas sensor
YU Cui1, HE Zezhao1, LIU Qingbin1, LI Xian2, XIE Dan2, CAI Shujun1, FENG Zhihong1
(1National Key Laboratory of ASIC,Semiconductor Research Institute,Shijiazhuang050051,Hebei,China;2Institute of Microelectronics,Tsinghua University,Beijing100084,China)
Gas sensors play a significant role in the fields of environmental monitoring, industrial production,safety, medical diagnosis, military and aerospace. Solid state gas sensors possess advantages of small size, low power, high sensitivity and low cost. They can detect very low concentrations of a wide range of gases in the range of parts-per-million (mg·L-1). Graphene is a promising material for solid state gas sensors with high sensitivity due to its high carrier mobility, maximum surface-to-volume ratio, and low noise level. In this work, nano-grain graphene is grown on sapphire substrate by plasma enhanced chemical vapor deposition at a relative low temperature at 950℃ and a short time of 15 min. The as-prepared graphene shows bilayer structure with large amount of defects, which brings advantages for graphene gas sensors. The nano-grain graphene gas sensor on sapphire shows good response and recovery characteristics for formaldehyde gas, and exhibits good sensitivity of 3% response toward a concentration of formaldehyde gas of 20 mg·L-1at room temperature with a response time of 9.92 min. The recovery time of the nano-grain graphene sensor for formaldehyde gas of 20 mg·L-1is 17 min.Low energy barrier of the gas adsorption and desorption induced by large numbers of grain boundaries and wrinkles in the nano-grain graphene is the main reason for its good gas sense characteristics.
graphene; adsorption; membranes; measurement; formaldehyde; aeration
TQ 127. 1+1
A
0438—1157(2017)11—4423—05
10.11949/j.issn.0438-1157.20170331
2017-03-30收到初稿,2017-07-05收到修改稿。
联系人:冯志红。
蔚翠(1983—),女,博士,高级工程师。
国家自然科学基金项目(61674131,61306006)。
