pH响应型苯硼酸酯缀合物的制备、表征和智能释放研究
2017-11-10吴兴军魏英英何文涛袁建超
吴兴军,魏英英,何文涛,施 萍,袁建超
(西北师范大学 化学化工学院,甘肃省高分子材料重点实验室,甘肃 兰州 730070)
pH响应型苯硼酸酯缀合物的制备、表征和智能释放研究
吴兴军,魏英英,何文涛,施 萍,袁建超*
(西北师范大学 化学化工学院,甘肃省高分子材料重点实验室,甘肃 兰州 730070)
以葡聚糖(Dextran)作为主链,将罗丹明B(RhB,荧光智能释放药物模型/示踪显像)、3-羟基苯硼酸(PBA)酯化后,缀合到葡聚糖上,形成一种新型pH响应的高分子缀合物.由于该高分子缀合物中存在硼酸酯键,使得其负载模型药物到达肿瘤细胞的溶酶体之后释放出罗丹明B,不仅降低了药物毒性,还能够示踪药物的递送过程.体外模拟释放结果表明,缀合物在癌细胞的溶酶体/内涵体 (pH 5)内能很好地释放出罗丹明B,而在药物输送过程(pH 7.4)中有很少的罗丹明B释放出来.另外,高分子载体葡聚糖的存在,使聚合物体现出良好的生物相溶性,从而减少了对正常组织的伤害,在抗癌药物的智能释放方面具有很好的应用前景.
高分子缀合物;pH响应;硼酸酯键;智能释放
刺激响应材料对于各种应用显示出巨大的潜力,因为它们的物理化学性质可以根据生物系统中的内部变化或外部变化而经历动态变化.外部或生理刺激,如光[1],温度[2],pH[3-5],氧化还原性[6-7],超声波[8],磁力[9],酶[10],已被测试用于触发药物释放.刺激响应性聚合物和纳米材料已应用于基因或药物递送[11]. 由于肿瘤细胞以异常高的速率增殖,导致糖酵解的高速率并积聚乳酸,降低环境pH值.此外,还研究了细胞器(内涵体和溶酶体,pH 4.0~6.0)的酸性环境,用于设计细胞内释放的pH响应纳米载体[12].针对肿瘤细胞(内涵体/溶酶体)内的弱酸性环境,pH触发的抗癌药物智能释放越来越多的应用于药物的靶向传递,以提高抗癌药物的治疗效率并减少其毒副作用.
早期含硼酸的大分子已被用于许多生物医学领域,包括用于糖反应性水凝胶,传感器和纳米材料中,通常以糖尿病的检测和治疗为目标,其需要不断监测血糖水平和主动胰岛素管理. 如今科学研究发现,硼酸与二醇形成硼酸酯键后,显示出pH敏感性[13]. 1,2-二酚成功地用于中性溶液中形成硼酸酯,并且可以在温和的酸性条件下水解[14]. 硼酸酯可以合成智能释放的药物载体,起到药物缓释的作用,被广泛应用于抗肿瘤治疗.
葡聚糖是一种多糖用于药物输送的天然替代物,因为它具有大量的反应性基团,可控的分子量,可生物降解的特征[15]. 葡聚糖还具有生物活性,其中β-葡聚糖的活性最强[16-17],葡聚糖作为药物载体,具有很好的生物相容性,分解后可作为人体供能的单糖存在,无任何毒性.同时葡聚糖还可以激活机体的免疫系统从而提高免疫力[18]. 通常,通过疏水改性手段制备基于葡聚糖的纳米胶束.
以罗丹明B作为一种药物模型/示踪剂连接到3-羟基苯硼酸上,再与葡聚糖相连,制备了一种生物相容性好的pH响应性药物载体.在肿瘤细胞内的溶酶体中,载药缀合物的硼酸酯键断裂[19],罗丹明B得以释放(图1);并进行了体外pH引发的药物模型释放.

图1 pH 响应性缀合物的合成及细胞内RhB释放Fig.1 Synthesis of pH-responsive conjugates and intracellular RhB release
1 实验部分
1.1 试剂与仪器
二环己基碳二亚胺 (DCC, 99%), 4-二甲基氨基吡啶(DMAP, 99%), 三乙胺 (TEA, 99%), 罗丹明B (RhB, 99%), 3-羟基苯硼酸 (PBA,99%)和β-葡聚糖(Dex,相对分子量质量20 000)购自阿拉试剂公司.二甲基亚砜(DMSO),四氢呋喃(THF)和二氯甲烷(CH2Cl2)购自北京化工厂.
Mercury plus-400 MHz 核磁共振波谱仪(Varian,美国); DUV-3700 紫外可见分光光度计(UV/Vis,日本岛津公司).
1.2 RhB-PBA的合成
称取罗丹明B (240 mg, 0.50 mmol)和N,N-二环己基碳二亚胺(110 mg, 0.58 mmol)溶于二氯甲烷(12 mL)中; 3-羟基苯硼酸(138 mg, 1.00 mmol)与 4-二甲氨基吡啶(140 mg, 1.15 mmol)溶解在四氢呋喃(4 mL)中;混合加入圆底烧瓶.将圆底烧瓶置于室温反应24 h.反应液过滤除去生成的盐,减压蒸馏除溶剂后进行硅胶柱层析[洗脱液为V(乙酸乙酯)∶V(甲醇)=6∶1]分离纯化,得红褐色固体RhB-PBA.
1.3 RhB-PBA-Dex的合成
称取RhB-PBA(181 mg, 0.29 mmol)和葡聚糖(50 mg, 0.003 mmol)置于Schlenk瓶中,用二甲基亚砜溶解(15 mL),再加入氢化钙(13 mg, 0.31 mmol),抽真空充氮气循环3~5次,密封Schlenk瓶,于30 ℃ 反应24 h.用冰乙醇沉淀,过滤,重复操作3次后,得橙色固体.
1.4 体外释放
取两份10 mg 缀合物,分别置于100 mL 的pH 7.4与100 mL的 pH 5.0的缓冲溶液中,透析48 h,按照一定的时间间隔(1,3,5 h 等)内,每次取3 mL 透析液测样,并立即放回以维持缓冲液的体积.用 UV/Vis可见光谱在552 nm 处检测透析液中罗丹明B的吸光度,并绘制体外释放曲线.
2 结果与讨论
2.1 缀合物的核磁共振氢谱表征
罗丹明B和3-羟基苯硼酸通过酯化,硅胶柱层析分离纯化等一系列过程得到RhB-PBA.并用它与葡聚糖硼酯化合成RhB-PBA-Dex. RhB-PBA和 RhB-PBA-Dex的1H NMR谱图如图2和图3所示.

图2 RhB-PBA的核磁共振氢谱Fig.2 1H NMR spectrum of RhB-PBA

图3 缀合物RhB-PBA-Dex的核磁共振氢谱Fig.3 1H NMR spectrum of RhB-PBA-Dex
图2 中δ7.37(e)和7.15(f, g, h)处都为间羟基苯硼酸中苯环(C-H)的质子吸收峰,8.39、7.81、7.77、7.67、6.84、6.71(a, b, c, d, i, g)处分别为RhB的苯环上质子吸收峰,3.58处为- CH2-的质子吸收峰,1.28处为- CH3-的质子吸收峰,表明成功合成了RhB-PBA.图3为共聚物RhB-PBA-Dex的1H NMR谱图,δ4.72、3.76、3.67、3.28(a、b、c、d、e、f)处分别为Dex的质子吸收峰,与图1相比,缀合物RhB-PBA-Dex中其他质子的位置基本保持不变.
2.2 pH响应与罗丹明的体外药物释放研究
研究表明,硼酸酯是一种pH敏感的化合物,当其所属的化学环境从中性向酸性改变过程中,硼酸酯开始断键,如缀合物从细胞质逐渐进入溶酶体中,酸性环境有所改变,从而硼酸酯断裂,如图4所示.
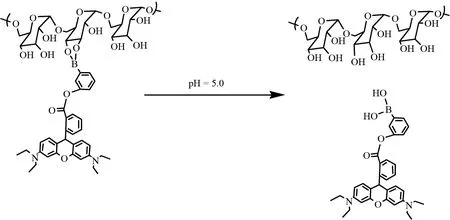
图4 硼酸醇的pH响应示意图Fig.4 Scheme of pH-response of borate ester
为了进一步研究缀合物对RhB的缓释行为,我们分别在pH=7.4(如图5)和pH=5.0(如图6)的缓冲溶液中模拟体外药物释放,释放曲线如图7所示.pH=7.4时,RhB的累积释放率十分缓慢,48 h后累计释放率仅为2.2%.而pH=5.0时,起初1 h内RhB的累计释放率为9.5%,出现明显的暴释现象, 48 h后累计释放率高达60.0%.这一释放结果说明缀合物对pH具有响应性,可以对细胞内酸性环境作出响应,实现细胞内药物的可控释放,同时有利于提高药物在肿瘤细胞中的累积,减少药物毒副作用.

图5 pH=7.4载药缀合物的体外药物释放荧光光谱图Fig.5 Release of RhB from RhB-PBA-Dex in buffer pH 7.4 (fluorescence spectra)

图6 pH=5.0载药缀合物的体外药物释放荧光光谱图Fig.6 Release of RhB from RhB-PBA-Dex in buffer pH 5 (fluorescence spectra)
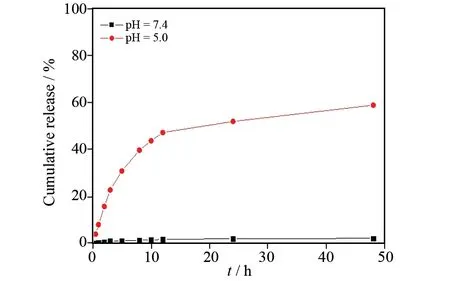
图7 pH响应性载药缀合物的体外药物释放图Fig.7 Release of RhB from RhB-PBA-Dex in buffers pH 5 and pH 7.4
硼酸酯键在中性溶液中十分的稳定,累积释放率不到3%;远远低于以前运用的腙键pH响应性药物的释放[20].当在细胞外药物载体受到影响时,传统药物载体受损,药物释放进入正常细胞组织,损坏正常机体;然而本实验药物载体RhB-PBA-Dex,很好地克服了传统缓释药物的缺点,当硼酸酯键断裂后,PBA具有靶向作用,能够识别肿瘤细胞上表达的唾液酸[21],从而使得药物模型/示踪显像被拉入肿瘤中,无损于正常细胞组织.本研究是通过化学反应,形成了一种缀合物,而不是胶束.比起胶束的包覆药物,此缀合物表现比较稳定.
3 结论
合成了一种简单的基于硼酸酯pH响应的缀合物RhB-PBA-Dex.该缀合物在水溶液中具有良好的溶解性与稳定性.pH=5.0时,连接PBA和Dex的硼酸酯键断裂,导致RhB解离.缀合物的体外模拟释放研究结果表明,pH=5.0时的药物累计释放率明显较高,48 h内RhB的累积释放率达60.0%,而pH=7.4时RhB的累积释放率为2.2%.总之,RhB-PBA-Dex缀合物为智能药物载体系统提供了一个高效的平台,在癌症治疗中有着极为广阔的应用背景.
[1] NIIKURA K, IYO N, MATSUO Y, et al. Sub-100 nm gold nanoparticle vesicles as a drug delivery carrier enabling rapid drug release upon light irradiation [J]. Applied Materials & Interfaces, 2013, 5(9): 3900-3907.
[2] YU E, GALIANA I, MARTNEZMEZ R, et al. Poly(N-isopropylacrylamide)-gated Fe3O4/SiO2core shell nanoparticles with expanded mesoporous structures for the temperature triggered release of lysozyme [J]. Colloids & Surfaces B Biointerfaces, 2015, 135: 652.
[3] CHEN X, YAO X, WANG C, et al. Mesoporous silica nanoparticles capped with fluorescence-conjugated cyclodextrin for pH-activated controlled drug delivery and imaging [J]. Microporous & Mesoporous Materials, 2015, 217: 46-53.
[4] DU P, ZHAO X, ZENG J, et al. Layer-by-layer engineering fluorescent polyelectrolyte coated mesoporous silica nanoparticles as pH-sensitive nanocarriers for controlled release [J]. Applied Surface Science, 2015, 345: 90-98.
[5] WU Y, CHEN W, MENG F, et al. Core-crosslinked pH-sensitive degradable micelles: a promising approach to resolve the extracellular stability versus intracellular drug release dilemma [J]. Journal of Controlled Release, 2012, 164(3): 338-345.
[6] BROADERS K E, GRANDHE S, FRÉCHET J M J. A biocompatible oxidation-triggered carrier polymer with potential in therapeutics [J]. Journal of the American Chemical Society, 2011, 133(4): 756-758.
[7] CHENG R, FENG F, MENG F, et al. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery [J]. Journal of Controlled Release, 2011, 152(1): 2-12.
[8] HERNOT S, KLIBANOV A L. Microbubbles in ultrasound-triggered drug and gene delivery [J]. Advanced Drug Delivery Reviews, 2008, 60(10): 1153-1166.
[9] HOARE T, TIMKO B P, SANTAMARIA J, et al. Magnetically triggered nanocomposite membranes: a versatile platform for triggered drug release [J]. Nano Letters, 2011, 11(3): 1395.
[10] LEE M R, BAEK K H, JIN H J, et al. Targeted enzyme-responsive drug carriers: studies on the delivery of a combination of drugs [J]. Angewandte Chemie International Edition, 2004, 43(13): 1675-1678.
[11] MURA S, NICOLAS J, COUVREUR P. Stimuli-responsive nanocarriers for drug delivery [J]. Nature Materials, 2013, 12, 991-1003.
[12] KAMALY N, YAMEEN B, WU J, et al. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release [J]. Chemical Reviews, 2016, 116(4): 2602.
[13] BROOKS W L, SUMERLIN B S. Synthesis and applications of boronic acid-containing polymers: from materials to medicine [J]. Chemical Reviews, 2016, 116(3): 1375.
[14] PAN G, GUO B, MA Y, et al. Dynamic introduction of cell adhesive factor via reversible multicovalent phenylboronic acid/cis-diol polymeric complexes [J]. Journal of the American Chemical Society, 2014, 136(17): 6203-6206.
[15] WU L, ZHANG L, SHI G, et al. Zwitterionic pH/redox nanoparticles based on dextran as drug carriers for enhancing tumor intercellular uptake of doxorubicin [J]. Materials Science & Engineering C, 2016, 61: 278.
[16] NANBA H, HAMAGUCHI A, KURODA H. The chemical structure of an antitumor polysaccharide in fruit bodies of grifola frondosa (maitake) [J]. Chemical & Pharmaceutical Bulletin, 1987, 35(3): 1162-1168.
[17] KAWAGISHI H, KANAO T, INAGAKI R, et al. Formolysis of a potent antitumor (1→6)-β-d-glucan-protein complex from Agaricus blazei, fruiting bodies and antitumor activity of the resulting products [J]. Carbohydrate Polymers, 1990, 12(4): 393-403.
[18] UKAWA Y, ITO H, HISAMATSU M. Antitumor effects of (1→3)-beta-D-glucan and (1→6)-beta-D-glucan purified from newly cultivated mushroom, Hatakeshimeji (lyophyllum decastes sing.) [J]. Journal of Bioscience & Bioengineering, 2000, 90(1): 98-104.
[19] LI L, BAI Z, LEVKIN P A. Boronate-dextran: An acid-responsive biodegradable polymer for drug delivery [J]. Biomaterials, 2013, 34(33): 8504-8510.
[20] YI W, LEI Z, ZHANG X, et al. Precise polymerization of a highly tumor microenvironment-responsive nanoplatform for strongly enhanced intracellular drug release [J]. ACS Applied Materials & Interfaces, 2016, 8(9): 5833-5846.
[21] DESHAYES S, CABRAL H, ISHII T, et al. Phenylboronic acid-installed polymeric micelles for targeting sialylated epitopes in solid tumors [J]. Journal of the American Chemical Society, 2013, 135(41): 15501-15507.
pH-sensitiveborateesterconjugate:preparation,characterizationandintelligentrelease
WU Xingjun, WEI Yingying, HE Wentao, SHI Ping, YUAN Jianchao*
(KeyLaboratoryofGansuPolymerMaterials,CollegeofChemistryandChemicalEngineering,NorthwestNormalUniversity,Lanzhou730070,Gansu,China)
Using Dextran (Dex) as a main chain rhodamine B (RhB) and 3-hydroxyphenylboronic acid (PBA) were esterified and then conjugated to dextran to form a novel pH-responsive polymer conjugate. Because of the presence of borate ester bonds in the polymer, the drug loaded conjugate can release rhodamine B in cancer cells, which not only reduces the toxicity of the drug, but also can be used to trace the drug delivery process. The results show that rhodamine B released well from the conjugate in lysosomes/endosomes of cancer cell (pH 5), and only little rhodamine B released in the process of drug delivery (pH 7.4). In addition, the presence of polymer carrier dextran made the polymer reflect a good bio-compatibility, thereby reducing the damage for normal tissue. The release of anti-cancer drugs would have a good application prospect.
polymer conjugate; pH response; borate ester bond; intelligent release
O632
A
1008-1011(2017)05-0639-06
2017-01-17.
国家自然科学基金资助项目(21364011, 20964003).
吴兴军(1989-), 男, 硕士生, 研究方向为靶向抗肿瘤高分子药物、生物高分子材料.*
, E-mail:jianchaoyuan@nwnu.edu.cn.
[责任编辑:张普玉]
