M icroscopic M echanism on the Incipient Pyrolysis of Insulation Paper
2017-07-19WANGXueleiWANGShuoZHUWenbingZHOUJiabinXUWei
WANG Xuelei,WANG Shuo,ZHUWenbing,ZHOU Jiabin,XUWei
(State Grid Shandong Electric Power Research Institute,Jinan 250003,China)
M icroscopic M echanism on the Incipient Pyrolysis of Insulation Paper
WANG Xuelei,WANG Shuo,ZHUWenbing,ZHOU Jiabin,XUWei
(State Grid Shandong Electric Power Research Institute,Jinan 250003,China)
By investigating the microscopic pyrolysismechanism of the insulation paper,especially on the incipient pyrolysis stage,it can reveal potential guiding values,such as further understanding on the pyrolysis process in internal insulation system of a transformer undergoing overheating defects and early evaluations of insulation deterioration and life expectancy.To this end,the way of experiment combined with simulation was adopted in this article.Firstly,the GC/MS experiment on cellulose under 350~900℃ was conducted,and it is found that allose was the product of the highest ration and it is also the most important product for diagnosing the degree of deterioration on the incipient stage.For further clarification of the formation mechanism and the following rules of the pyrolysis of allose,the high temperature reactive molecular dynamics simulation for the pyrolysis of cellobiose which is the constitutional unit of the paper cellulose was investigated and discussed in this article. By tracking the visual decomposition process of the cellulose and observing the splitting and forming rules of the bonds at different time,it is found that allose was generated by the split of C′4-O glucosidic bond and C1-O5bond at the 1-pyranoid ring.The small molecules generated during pyrolysis are measured by velocitron and can be used as micro-features in diagnosing thermal faults of the power transformers.
insulation paper;incipient pyrolysis;reactivemolecular dynamics;microscopicmechanism;allose
0 Introduction
With the rapid development of our power industry,electrical power transformer as a key element among the power transmission and transformation equipment,is developing towards the direction of the ultra-high voltage and large capacity.Once the transformer fails to work,it will ravage the electricity grid and cause huge economic losses.Oil and paper are the main insulating materials for oil-immersed transformers.When the transformer undergoes some partial defects,such as a local overheating defect,it will generate heat high enough to endanger the stability of the oil/paper insulating structure.If not dispatched in time,the heat is able to rise the local temperature to a high level in the transformer and cause oil/paper fastpyrolysis[1-2].The transformer oil/paper cracking is a very complicated process of chemical change,involving electrical,material,chemical,and many other disciplines.The existingmacroscopic experimental study on insulation defects is not sufficient to explain the indepth mechanism of the pyrolysis process of oil/paper insulation from the microscopic mechanism point of view.
For the past few years,with the quantum mechanics theory gradually improving,continuous development and update of the force field and the popularity of the computing speed and capacity promotion of the computers,the theory and methods ofmolecular simulation was developing rapidly.In the physical,chemical,material and life science and many other fields,it plays an increasingly important role,and has gradually formed a specialdiscipline-themolecularsimulation[3-5]. Simulation of the molecular structures and behaviors based on molecular model on the atomic level,it can not only precisely calculate the micro parameters of the insulatingmaterial,but also help analyzing themicroscopic mechanism of all kinds of complicated phenomenon,so as to explain the relationship betweenmicrostructure and macro properties for insulating material.
For themicroscopic degradationmechanism of the transformer oil/paper insulation,some explorations are already invested.The molecular dynamics simulation is carried out to investigate the thermal degradation process of cellulose molecules in insulation paper in paper[6-7].It is found that joint ofβ-1-4 glycosidic bond on the main chain of the cellulose molecules is mostly prone to rupture and lead to reduction the degree of polymerization.Thermal stability of cellulose molecular is studied in paper[8]and it is found that the stability of the structure and machinery of the insulation paper are significantly affected by cellulose internal hydrogen bond.Themolecular simulation of the oil conformation in the cellulosemolecules on different surfaces is analyzed in paper[9]and it is found that the oilmolecular on the surface of cellulose amorphous region appreciate vertical conformation and are most likely to produce charge at the interface.In paper[10],aided by Material Studio software and COMPASS force field,the effects of oxygen,water and hydronium on insulation paper aging degradation are analyzed from cellulose chain conformation,flexibility,and small molecule diffusion coefficient and other parameters.All the above studiesmainly focused on the physics calculations ofmolecular dynamics,and have not involved the chemical reactionmechanism of cellulose pyrolysis process.In paper[11],the cellulose pyrolysis process at high tem perature is simulated.To reduce the time consumption on the simulation,the temperature is set to 1 900 K in the simulation,in which,the cracking reaction is so fast that it is unable to elaborate pyrolysis law for early cracking.So it will be useful if a further study on themicro crackingmechanism of cellulose,especially the early cracking mechanism is carried out.This study will provide further understanding on the pyrolysis process of the transformer’s internal insulating system under overheating defects,potentially valuable in the early evaluation of insulation cracking and insulation life assessment.
To this end,this paper combines the experiment and simulation method.Firstly,cellulose pyrolysis experiment at 350~900℃ is carried out to analyze the cracking process,especially the main product at the early stage.Then,based on the experimental study,the targeted pyrolysis simulation of cellulose is carried out. Through tracing the paths of themain products during the early stage,the law of cellulose pyrolysis is analyzed and detailed in this paper.
1 Insulation Paper Pyrolysis Experim ent Based on Py-GC/MS Technology
In the recent few years,pyrolysis chromatography mass spectrometry(Py-GC/MS)is an effective tech nology to study the pyrolysismechanism[12-13].Py technology has the advantage of ultra-fast heating of the sample to the specified temperature and low speed of the continuous decomposition in the process of heating.Meanwhile,the technology of GC/MS can analyze the components of the pyrolysis products more accurately and obtain the semi-quantitative data.Therefore,using Py-GC/MS to analyze the components and relative contents of the transformer insulation paper’s thermal cracking products can not only verify the effectiveness of the previous simulation of reaction kinetic thermal cracking in paper[11],butalso have great significance for further exploring insulation paper’s thermal cracking mechanism.
1.1 Experimentalequipments and materials
The Py-GC/MSused in this experiment is generated by CDS 5000 pyrolysis apparatus manufactured by CDS Company and Agilent 7890A-5975C gas chroma tograph-mass spectrometer made by Agilent Company in the United States.The equipment and lab setup is shown in Figure 1.The wire cracking is used in CDS 5000 pyrolysis apparatus for fast pyrolysis. Pyrolysis probe on the platinum filament can be quickly heated to the specified temperature for thermal cracking,up to the highest temperature of 1 500℃. The pyrolysis products are analyzed on line through HP-5MS elastic capillary column(60m×0.25 mm× 0.25 m)in Agilent 7890A-5975C GC/MS.The temperature of the connector between chromatography and mass spectrum is 300℃.The mass-to-charge ratio in mass spectrometer is 35~400 amu.The sampling frequency is 4 Hz.NIST database is used for production determination.

Figure 1.Pyrolysis-GC/MS analysis meter
The insulation paper used in this experiment is from transformer made by Weidmann.The thickness of the paper is 0.5mm.Main components in the insulation paper includeα-cellulose mass fraction 89%~91%,hemicellulose 6%~7%and lignin 3%~4%.
1.2 Experiment procedure
During the experiment,about 5 mg of insulation paper powder was placed in a quartz tube,and both ends of the tubewere fixed by quartz cotton.The quartz tube filled with insulation paper was placed in platinum coil,as shown in Figure 2.And then start the thermal crack ing test under high-purity nitrogen(99.999% )environment in the cracking chamber. Comparisons among the insulation paper’s cracking products at different temperatures are made in this experiment.The cracking temperature is holding at 350℃,500℃,700℃ and 900℃ on different stage during the test.The initial temperature of the plat inum wire is 150℃ and then rising to 350℃,500℃,700℃ and 900℃ with the rising rate 10℃/ms,holding for 12 s at each target temperature.The insulation paper begins to crack at the target temperature and forms fugitive constituent.The product will first enter the adsorption vessel and then be desorbed until the cracking reaction is completed.Then all the cracking fugitive constituent are moved into the connected chromatographic mass spectrometer to analyze the products,the whole process shown in Figure 3.To prevent the cracking product from condensing,the temperature of the external transfer line is maintained at 250℃.
Figure 2.The pyrolysis probe

Figure 3.Experimental flow diagram
1.3 Thermal cracking products analysis of insulation paper
The GC/MS spectrum of the pyrolysis products for the insulation paper at the set temperature is shown in Figure 4.The X-axis represents the retention time correspondent with the products,in which the heavier the relative molecular mass of the products is,the longer retention time.The Y-axis represents relative value of ion current which is proportional to the content of related product.Since the relative peak area is linearly related to the gas production,in this paper,the relative peak area is used to characterize the change of the product composition during the pyrolysis.At first,area normalization method is used to calculate the peak area of each component and then sum them,and calculate its percentage in total peak area,which can contribute to the qualitative analysis of the pyrolysis products’GC/MS spectrum.
Itcan be seen from Figure4 that the decomposition is insignificant when the temperature is 350 ℃ . However when the temperature rises to 500℃,the amount of pyrolysis product is evidently more than that in 350℃.During temperature rising from 500℃to 900℃,the amount ofmicromolecule pyrolysis product is increasing significantly and the amount of macromolecule is reducing gradually.
As shown in Figure 4,the main products from insulation paper pyrolysis include allose,CO2,glycolic aldehyde,formic acid,furfural,1-Hydroxy-2-butanone,5-hydroxymethylfurfural,which are marked at the corresponding peak position in Figure 4.As the main production,CO2,glycolic aldehyde and formic acid are analyzed inpaper[11]bymoleculardynamicssimulation and their generating paths are traced.None of these is the focuse in this paper.From the statistics obtained from the experiment,it is found that allose is the highestquantity of products in the experiments,but the content declines rapidly with the temperature increasing especially after 700℃.This indicates that allose is extremely unstable at high temperature and break down into smallermolecules quickly.This is why it is not found in the previous researches of high temperature simulations[11].
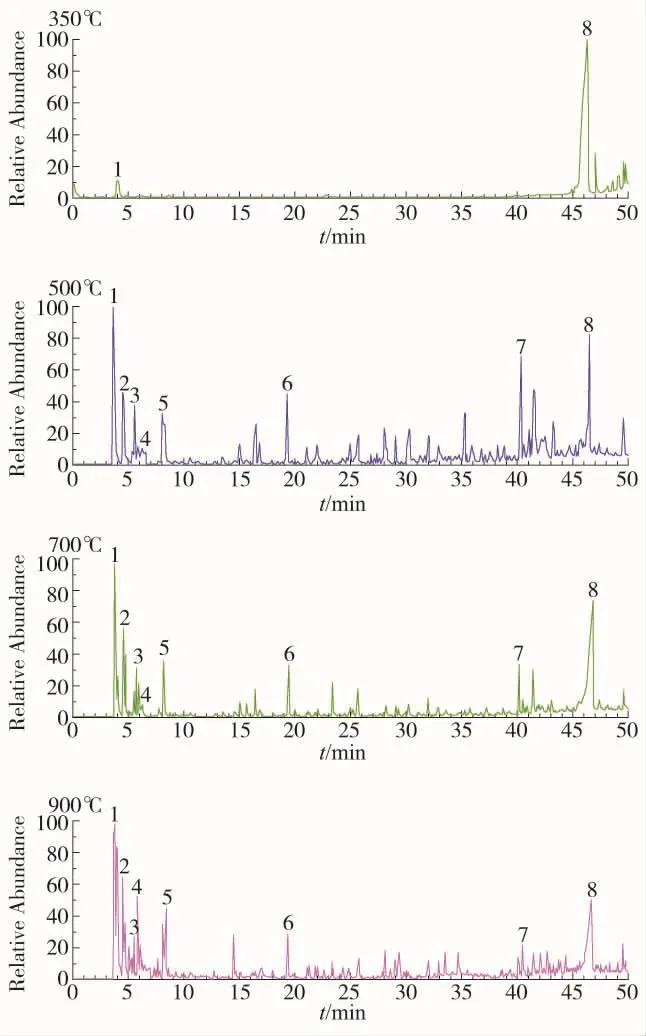
Figure 4.GC/MS spectrogram for insulation paper pyrolysis
For the early evaluation of insulation cracking,it isofvital importance to clarify thegeneratingmechanism of allose and explore the early stage cracking law of cellulose.To this end,reaction molecular dynamics simulation method is used in this research to simulate the early stage cracking of cellulose under high temperature,aiming at exploring the generating ways of allose and the initial crackingmechanism of insulation paper at the atomic level.
2 Molecular Dynam ics Simulation of Cellulose Pyrolysis at High Temperature
2.1 Molecularmodel construction
The main component of insulation paper in the transformer is cellulose,which is a linear polymer compound combined withβ-glycosyl group and D-glycosyl group under the help of 1-glycosidic bond and 4-glycosidic bond.Its repeating unit is cellobiose. After using different length of cellulose chains for simulations,it is found that amorphous area models composed with different lengths have no significant difference regardless the molecular conformation,physical and chemical properties[14-18].As cellobiose is the repeating unit of cellulose and for the time sake,cellobiose is used for themodel as shown in Figure 5(a).For easy understanding,this paper is going to name each atom of cellobiose using the method introduced in[19].As shown in the figure,in order to distinguish the two pyran rings of cellobiose,on the right side of the figure is 4-pyran ring which is connected with glycosidic bond’s O atoms via C′4,on the left side is 1-pyran ring which is connected with glycosidic bond’s O atoms via C1.Builder Model in the ADF software package is used to build the molecular model.The molecular model of cellobiose is shown in Figure 5(b),in which the white atoms represent H,the red atoms representO,and the gray atoms represent C.
2.2 Pyrolysis simulation methods and details
In this paper,ADF software is used to simulate the thermal degradation mechanism of cellulose.After using Builder command establish amorphous cells,the simulation of cracking reaction for cellobiose can be done under the ReaxFF force field.Different force fields are set in ReaxFF according to the differences of simulation elements.And cellobiose includes C,H and O three types of atoms,so CHO.ff force field is chosen in this paper.The canonical ensemble with the fixed N(number of particles)V(volume)T(temperature)is used during the simulation process.

Figure 5.Sturctural formula and moecularmodel
In order to balance the time and the accuracy of the simulation,and make sure the time consumption of the entire simulation is in a reasonable range,the parameters are set following the guidance of paper[11],in which,the pyrolysis process of the amorphous cells (simulation unit)containing three cellobiose molecules is emulated.The density of the unit is set to 1.599/cm3(actual cellulose density),air pressure is 0.1 MPa,and the temperature is 1 700 K.The simulation is repeated 16 times to ensure the reliability of the simulation results.For each simulation,the pyrolysis time is 100 ps,time step 0.1 fs,and thetrajectory file is saved for each 0.01 ps.
2.3 Simulation results analysis
According to the pyrolysis experiment results in chapter 1.3,the percentage of the peak area for each product is calculated,in which the main products at 900℃are shown in table 1,and are compared with the simulation results.
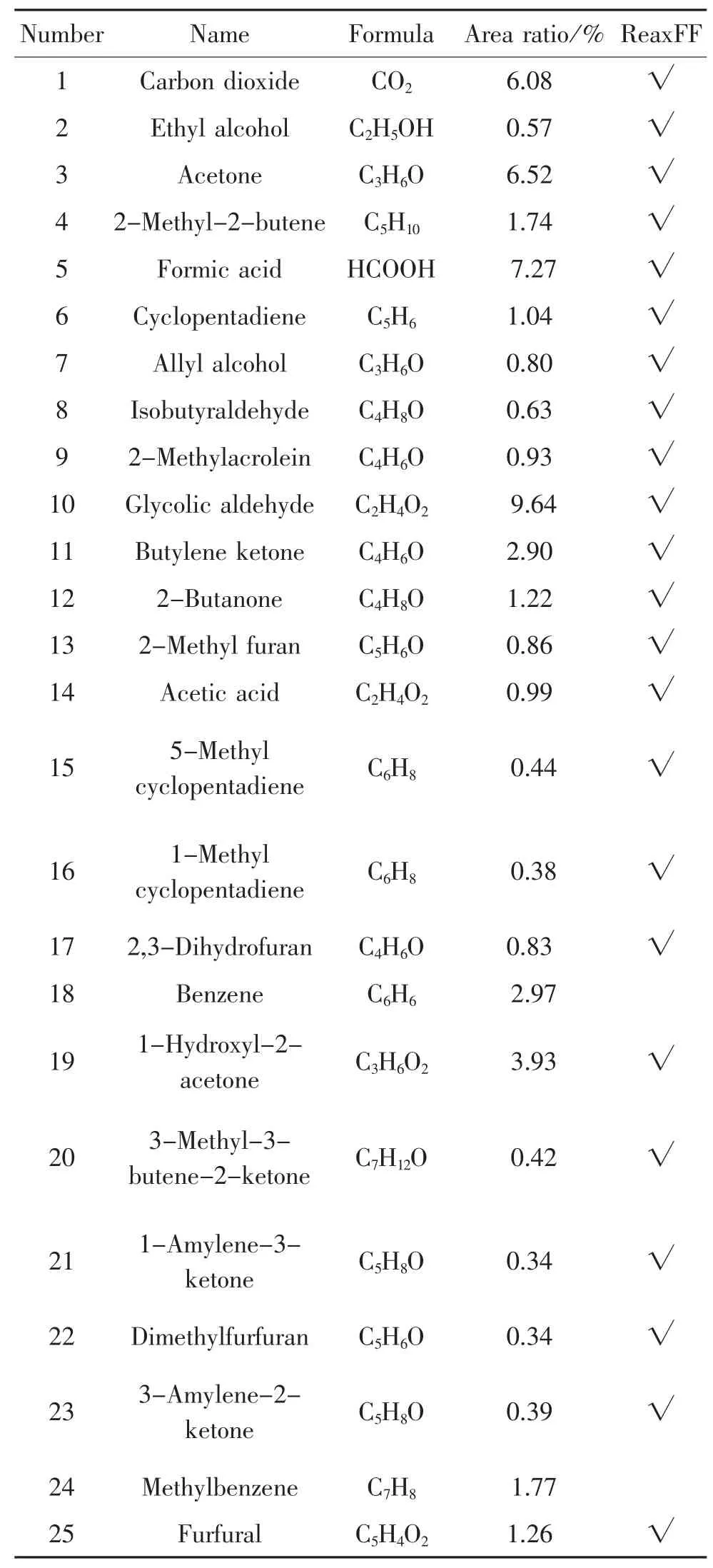
Table 1.Main products ofinsulation paperpyrolysis in 900℃
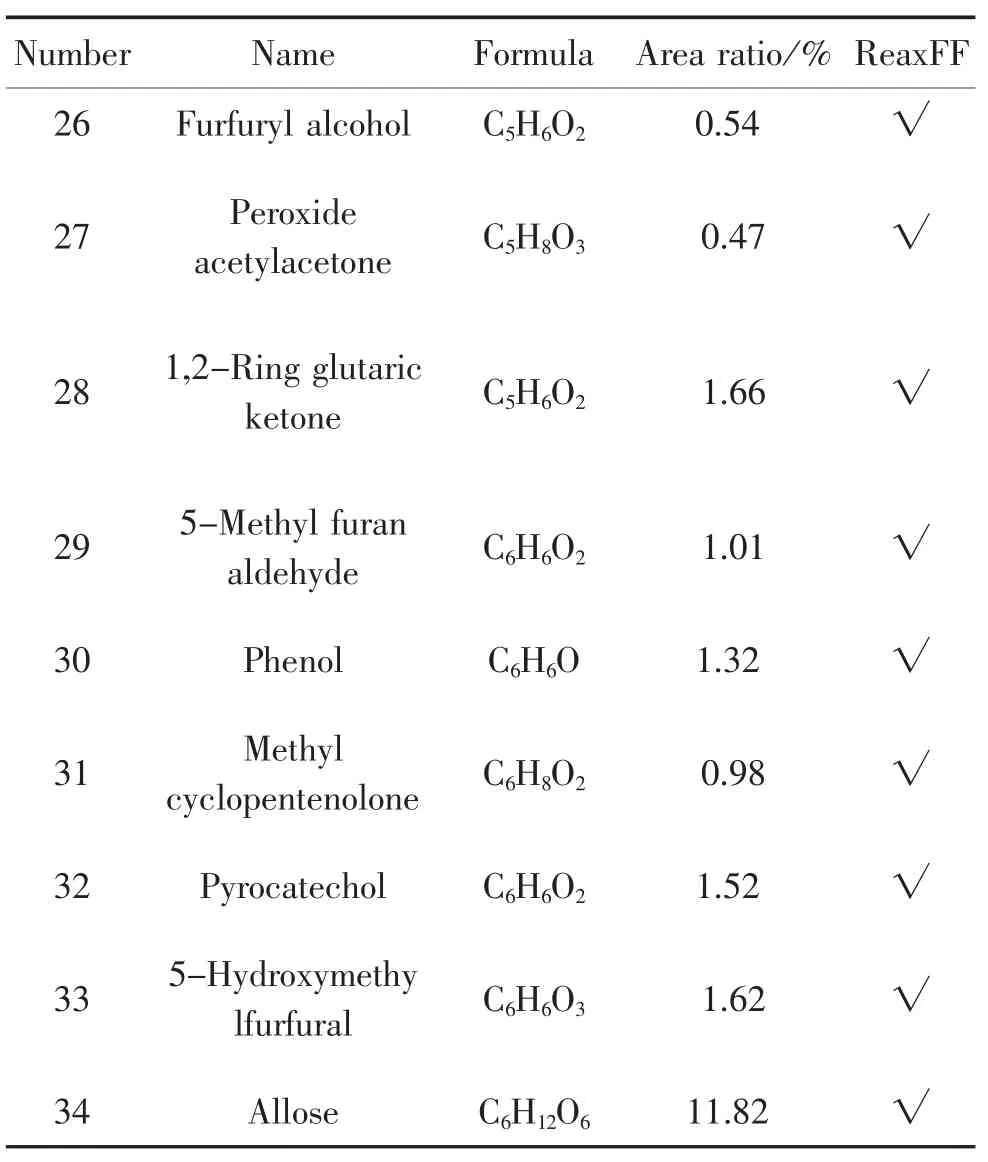
34 Allose C6H12O6 11.82 √32 Pyrocatechol C6H6O2 1.52 √33 5-Hydroxymethy lfurfural C6H6O3 1.62 √30 Phenol C6H6O 1.32 √31 Methyl cyclopentenolone C6H8O2 0.98 √28 1,2-Ring glutaric ketone C5H6O2 1.66 √29 5-Methyl furan aldehyde C6H6O2 1.01 √26 Furfuryl alcohol C5H6O2 0.54 √27 Peroxide acetylacetone C5H8O3 0.47 √Number Name Formula Area ratio/%ReaxFF
It can be seen from the statistics shown in the table above that 34 main products measured in the experiment,except benzene and toluene,are all observed in the simulations.This indicates that simulation is highly consistent with the experiment results and the simulation is reliable and very accurate.
From the previous experimental study it is found that allose is the product with the most generation amount during the early pyrolysis of cellulose.In order to clarify its pyrolysis mechanism and the later cracking law,by observing brakeage and generation of glycosidic bond and following the visible decomposition of cellulose,the main generating paths of allose are made clear in this paper,which is shown in Figure 6 and Figure 7.The atoms of the micromolecule products are identified from their colors.The blue ones are C atoms,the pink ones are O atoms,and the green ones are H atoms.Dotted line shows the broken bond’s location during the generating process of the main products.Red subscripts represent the atomic number in the chemical structure.
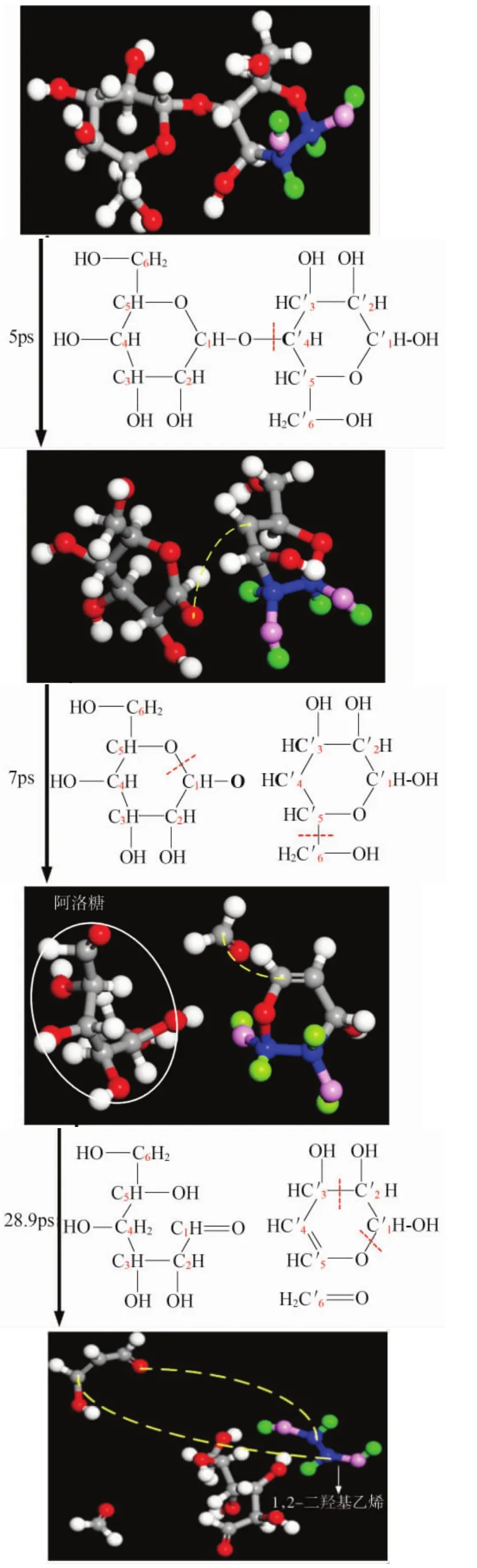
Figure 6.Generate path 1 for allose
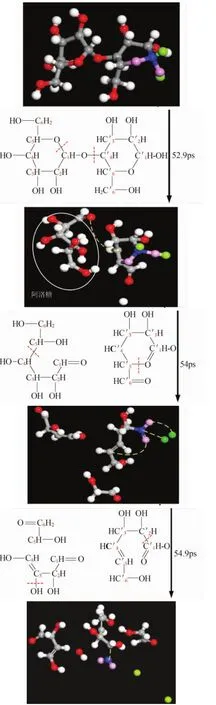
Figure 7.Generate path 2 for allose
As shown in Figure 6 and Figure 7,allose are all generated after glucosidic bond C′4-O breakage (5 ps in Figure 6 and 52.9 ps in Figure 7)and 1-pyranoid ring C1-O5bond breakage(7 ps in Figure 6 and 52.9 ps in Figure 7).As the decomposition continued,the final products are 1,2-dihydroxy ethylene(the generating path is shown in Figure 6:C′5-C′6bond breakage,generate a double bond between C′4and C′5,and C′1-C′2separate from 4-pyranoid ring),CO2(the generation path is shown in Figure 7:C′5-O′5separate from 4-pyranoid ring to creat C′1=O′5).
In addition,taking the statistics of cellobiose bond rupture at the initial cracking stage,the location data of the bond breakage is shown in Table 2.
The table above shows that the initial breakage position ismainly at O atoms of glycosidic bond,which shows that the glycosidic bond is a weak point in cellobiose structure.And this is consistent with the theoretical derivation that glycosidic bond is the first breaking point during cellulose’s pyrolysis process,which is mentioned in paper[20].Jiang Yuanye[21]used density functional theory to calculate the thermodynamic energy of cellobiose pyrolysis in different location.His conclusion indicates that glycosidic bonds cracking is superior to other positions in thermodynamics,which is consistent with the simulation results presented in this paper.
The table also shows that except glycosidic bonds,the oxygen atoms at the two pyran rings are easy to break free from the bond of C′5-O′5and C1-O5. This is also consistent with the conclusion of paper[13]which believes that pyran ring’s C1-O5is a breakage point from bond energy point of view.Because C1-O5on glycosidic bond and pyran rings is easy to break,and allose is generated after the breakage.This also supported the fact of allose’s mass production during the early stage of pyrolysis.
The research above shows that allose is similar to furfural that they are both the product during the cracking process of the insulation paper.In practical applications,there are two ways to test allose during transformer insulation aging process—off-line and online test.The off-line test requires the oil samples from the site for analysis using mass spectrometer in the laboratory.The on-line test can be realized by installing the built-in sensors for monitoring purposes aiming at specific chemicals.Because of that the producing of allose is at an earlier stage compared to the furfural during insulation paper pyrolysis,it is more accurate to assess the insulating aging process from the trend of the allose production,especially for early stage.
3 Conclusions
In this article,the way of experiment combined with simulation was adopted to analyze the cellulose’s initial pyrolysis mechanism,the distributing pattern and generating ways of main products from atomic level.
Insulation paper pyrolysis experiment shows that the main products include allose,CO2,glycolic aldehyde,formic acid,furfural,1-Hydroxy-2-butanone,5-hydroxymethylfurfural,etc.Among them,allose is the highest generating product but with the temperaturerising,the amount of allo se reduces rapidly.This indicates that allose is the most important product to characterize the degree of the early insulating pyrolysis.
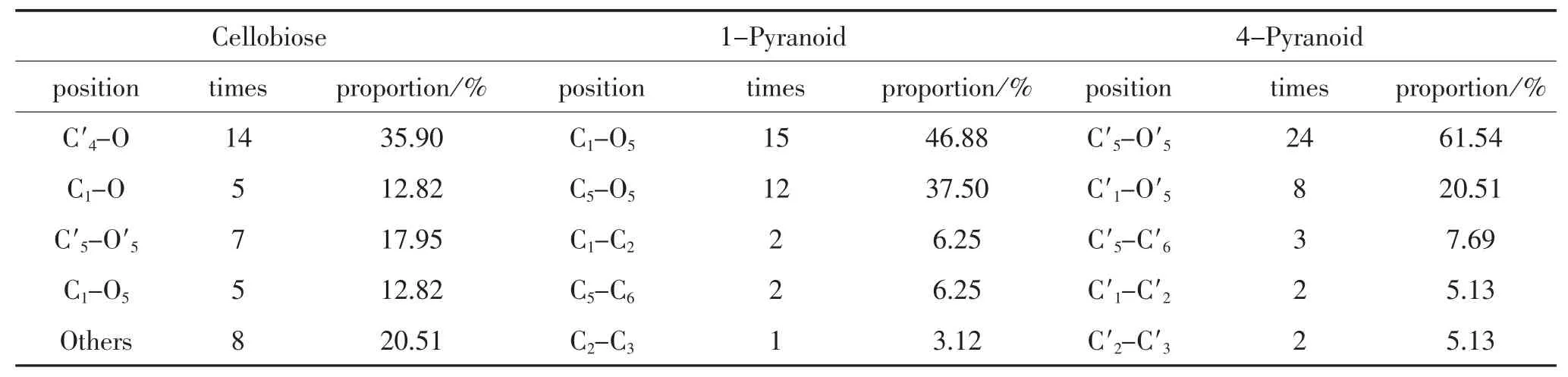
Table 2.Statisticaloverview of bond initial rupture location for the cellobiose
Reactive molecular dynamic simulation at high temperature is done for researching the pyrolysis mechanism of cellobiose in insulation paper.Theways of generating allose isanalyzed and detailed by tracing the visible cellulose decomposition process and observing bonds’breakageand generation atdifferent times.
The micro mechanism of cellulose’s pyrolysis from the atomic level is explored in this paper.It can be a theoretical support for deeper understanding to the pyrolysis process of the insulation paper during overheating defect in transformer and assessment of the insulation aging.
[1]WU G.N.,CUIY.G.,WANG X.J.“Evaluation of Water Content in Insulation Paper of Different Aging Degrees Using Acid Value in Oil”,High Voltage Engineering,Vol.41,No.1,pp.115-122,2015.
[2]ZHOU Y.X.,HUANG J.W.,SHA Y.C.“Electrical Properties of Oil-Paper Affected by Water Conductivity During Paper-Making Process”,High VoltageEngineering,Vol.41,No.2,pp.382-387,2015.
[3]SCHERAGA H A,KHALILI M,LIWO A.“Protein-Folding Dynamics:Overview of Molecular Simulation Techniques”,Annual Review of Physical Chemistry,Vol.58,No.1,pp.57-83,2007.
[4]CHENOWETH K,VANDUIN A C T,GODDARD W A.“Reaxff Reactive Force Field for Molecular Dynamics Simulations of Hydrocarbon Oxidation”,The Journal of Physical Chemistry A,Vol.112,No.5,pp.1 040-1 053,2008.
[5]WANG Q D,WANG J B.“Reactive Molecular Dynamics Simulation and Chemical Kinetic Modeling of Pyrolysis and Combustion of N-Dodecane”,Combustion and Flame,Vol.158,No. 2,pp.217-226,2011.
[6]LU Y.C.“Study on diffusion behavior of gas and mechanics of oilpaper aging using molecular simulation”,Chongqing,Chongqing University,2007.
[7]YANG L.,LIAO R.,SUN C.“Influence of Vegetable Oil on The Thermal Aging of Transformer Paper and Its Mechanism”,IEEE Transactions on Dielectrics and Electrical Insulation,Vol.18,No. 3,pp.692-700,2011.
[8]LIAO R.J.,ZHU M.Z.,ZHOU X.“Molecular Dynamics Simulation of the Diffusion Behavior ofWater Molecules in Oil and Cellulose Composite Media”,Acta Physico-Chimica Sinica,Vol.27,No.4,pp.815-824,2011.
[9]LIAO R.J.,HU J.,YANG L.J.“Molecular Simulation for Thermal Degradative Micromechanism of Power Transformer Insulation Paper”,HighVoltageEngineering,Vol.35,No.7,pp.1565-1570,2009.
[10]HU J.“Molecular Simulation Studies on Aging Mechanism in Crazing Zone of the Insulation Paper in Power Transformers”,Chongqing,Chongqing University,2009.
[11]YAN J.,WANG X.,LIQ.“Molecular Dynamics Simulation on the Pyrolysis of Insulation paper”,Proceedings of the CSEE,Vol.35,No.22,pp.5 941-5 949,2015.
[12]LIAO Y.,LUO Z.,WANG S.“Mechanism of Rapid Pyrolysis of CelloluseⅠ.Experimental Research”,Journal of Fuel Chemistry and Technology,Vol.31,No.2,pp.133-138,2003.
[13]WANG S.,LIAO Y.,TAN H.“Mechanism of Rapid Pyrolysis of CelloluseⅡ.Mechanism Analysis”,Journal of Fuel Chemistry and Technology,Vol.14,No.31,pp.317-321,2003.
[14]ZHU M.“Molecular dynamics study of thermal aging of oilimpregnated insulation paper”,Chongqing,Chongqing University,2011.
[15]ZHANG L.,Zybin S V,van Duin A C T.“Carbon Cluster Formation During Thermal Decomposition of Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine and 1,3,5-Triamino-2,4,6-Trinitrobenzene High Explosives from Reaxff Reactive Molecular Dynamics Simulations”,The Journal of Physical Chemistry A,Vol. 113,No.40,pp.10 619-10 640,2009.
[16]STRACHAN A,VAN DUIN A C T,CHAKRABORTY D.“Shock Waves in High-Energy Materials:The Initial Chemical Events in Nitramine RDX”,Physical Review Letters,Vol.91,No.9,pp. 10 619-10 640,2003.
[17]GUO F.,ZHANG H,CHENG X.“Molecular Dynamic Simulations of Solid Nitromethane under High Pressures”,Journal of Theoretical and Computational Chemistry,Vol.9,No.1,pp.315-325,2010.
[18]CHENOWETH K,DUIN A C T,PERSSON P.“Development and Application of A Reaxff Reactive Force Field for Oxidative Dehydrogenation on Vanadium Oxide Catalysts”,The Journal of Physical Chemistry C,Vol.112,No.37,pp.14 645-14 654,2008.
[19]PEI J.“Chemistry of Plant Fiber”,Beijing:China Light Industry Press,2012.
[20]RICHARDS G N.“Glycoaldehyde from Pyrolysis of Cellulose”,Journal of Analytical and Applied Pyrolysis,Vol.10,No.2,pp.251-255,1987.
[21]JIANG Y.,YU H.,FU Y.“Theoretical Study on Thermodynamic Properties of Pyrolysis of Cellulose Dimer Model Compound”,Acta Chimica Sinica,Vol.71,No.12,pp.1 611-1 619,2013.
Accepted date:2017-04-01
绝缘纸热裂解初期微观机理研究
王学磊,王 硕,朱文兵,周加斌,许 伟
(国网山东省电力公司电力科学研究院,山东 济南 250003)
探究绝缘纸的微观热裂解机理,特别是初期裂解机理,对于深入认识变压器内部绝缘系统过热缺陷下的裂解过程以及绝缘裂化的早期评价与绝缘寿命评估具有潜在的指导价值。为此,采取实验与仿真相结合的手段,首先,对纤维素进行了350~900℃下的气相色谱/质谱联用实验,研究表明,阿洛糖是实验中生成量最高的产物,是绝缘裂化初期表征裂化程度最重要的产物。为了进一步厘清阿洛糖裂解生成机理以及后续裂解规律,对绝缘纸纤维素中的结构单元纤维二糖的裂解进行了高温反应分子动力学模拟。通过跟踪可视化的纤维素分解过程,观测不同时刻键的断裂和生成规律,发现阿洛糖生成由最易发生断裂的糖苷键C′4-O键断裂和1-吡喃环上C1-O5键两化学键断裂而来。裂解小分子产物作为新的微观特征量,可通过质谱仪测得并进行趋势分析,在变压器过热故障诊断中具有潜在应用价值。
绝缘纸;初始裂解;反应分子动力学;微观机理;阿洛糖
TM211;TM853
A
1007-9904(2017)06-0008-09
ei(1986)
the B.S.M.E.and Ph.D.degree in electrical engineering from Shandong University,China,during 2005-2015.He is now working in the State Grid Shan Dong Electric Power Research Institute.His main research interest is transformer fault diagnosis and condition evaluation.
