提高现代超级稻产量潜力的栽培生理研究途径探讨
2017-07-18顾骏飞陈颖毛倚琦
顾骏飞陈颖 毛倚琦
(扬州大学江苏省作物遗传生理国家重点实验室培育点/粮食作物现代产业技术协同创新中心,江苏扬州225009;第一作者:gujf@yzu.edu.cn)
提高现代超级稻产量潜力的栽培生理研究途径探讨
顾骏飞陈颖 毛倚琦
(扬州大学江苏省作物遗传生理国家重点实验室培育点/粮食作物现代产业技术协同创新中心,江苏扬州225009;第一作者:gujf@yzu.edu.cn)
水稻籽粒灌浆期同化物供应不足会导致籽粒灌浆不充分,从而影响产量,这种现象在大穗型、高库容的现代超级稻品种中尤为明显,直接限制了超级稻品种产量潜力的发挥。灌浆期水稻同化物的供给主要来源于花前同化物的积累与花后同化物的合成,它们均依赖于冠层群体光合能力。因此,加强相关过程的生理生态研究对揭示制约现代超级稻产量潜力的关键性因素具有重要意义。作者综述了相关领域的研究进展,并从叶片光合生理、冠层光氮匹配和根-冠水分平衡等方面,对提高现代超级稻产量潜力的栽培生理途径进行了探讨。同时,作者总结了模型分析在综合栽培生理认识、发现超级稻产量潜力限制性因子及其生理机制等方面的作用。
光合作用;产量潜力;冠层光氮匹配;根-冠水分平衡
现代超级稻品种穗型大,穗粒数多,有极高的库容,产量潜力能够达到1 000 kg/667m2以上[1]。但是在实际生产中,以江苏为例,超级稻单产只在600~700 kg/667 m2之间,且表现出灌浆不充分、结实率低,影响了产量潜力的发挥。超级稻品种的结实率往往比常规稻品种低10%以上[2]。现代超级稻品种灌浆不充分、结实率低,主要原因是灌浆期同化物的供给不充分所造成。要是能突破灌浆期同化物供应的瓶颈,将有望进一步提高我国超级稻的产量。
灌浆期水稻同化物的供给主要来源于两个方面(图1):一是花前累积的同化物,它们来源于花前冠层群体的光合,主要以非结构性碳水化合物的形式贮存在茎鞘中,对产量的贡献为30%左右[3-4];二是花后冠层群体的光合,其中剑叶光合对产量贡献较大。以下将分别从叶片光合与冠层光合角度来分析提高水稻净同化力的限制性因子。
1 叶片光合作用
光合作用是指绿色植物通过叶绿素吸收光能,同化CO2和H2O,制造有机物并释放O2的过程,根据是否需要光的参与,分为光反应和暗反应两个过程。在光反应过程中,类囊体上的电子传递链捕获和利用光能,合成暗反应需要的能量ATP和还原剂NADPH。光反应形成的ATP和NADPH供给暗反应同化CO2形成碳水化合物。在暗反应中,CO2受体为1,5-二磷酸核酮糖(RuBP),在核酮糖二磷酸羧化酶(Rubisco)等一系列酶的作用下,CO2被还原成磷酸丙糖3-磷酸甘油醛(3-GAP)。磷酸丙糖不能直接透过叶绿体内膜,其必需由磷酸运转器与Pi对等交换才能出入叶绿体。当Pi不足或长期光照时,磷酸丙糖会在叶绿体内积累,形成淀粉粒。
因此在低光强下,囊体上的电子传递受限,影响ATP的合成,从而影响RuBP再生(RuBP再生限制);在饱和光强下,暗反应关键酶Rubisco的数量与活性,及反应底物CO2的浓度是限制光合作用的最关键因素(Rubisco羧化限制);在长期光照下,大量形成的光合产物需要借助叶绿体被膜上的磷酸运转器及时运转出叶绿体,此时光合速率受细胞质中无机磷运转速率限制(磷酸丙糖运转限制)[5-6]。根据叶片光合的限制性因子,国内外学者开展了很多研究,希望突破叶片光合限制,提高同化物的供给,例如,通过提高Rubisco活化酶(RCA)的活性来改善Rubisco活性[7],通过提高Rubisco酶对CO2(相对于O2)的专一性(Sc/o)来改善Rubisco活性[8],降低光呼吸损耗[9],过表达SBPase基因改善RuBP的再生能力[10-11],通过增加细胞色素b6f复合体含量提高叶绿体电子传递[12],引入蓝细胞的CO2浓缩机制来提高反应底物CO2浓度[13],过表达水通道蛋白NtAQP1提高叶肉导度从而提高反应底物CO2浓度[14-15],将高效的C4途径引入水稻来消除光合作用的氧抑制[16]。但是将这些分子生物学成果运用于水稻的大田生产中,预计需要15年甚至更长的时间[16-17]。针对大田生产中广泛使用的粳型超级稻品种沈农265和农家旱稻品种毫格劳,我们对它们及它们杂交组合后代进行了遗传与生理分析,发现气孔与叶肉导度是高光强下影响光合最主要的限制性因子[18-19]。Adachi等[20-22]研究发现,水稻品种Takanari干物质积累比水稻品种Koshihikari高20%~30%,主要是因为Takanari根系具有较高的导水率,能够维持高的叶片水势,保持气孔开发,有较高的气孔导度。Yang等[23]也发现,目前大田高氮肥施用条件下氮素利用效率降低是由于水稻根系导水率低,吸水能力不足,气孔开放程度低,气孔导度小所致。因此,如何通过栽培措施调节根系、根系-叶片水分平衡,提高气孔导度,可能是提高水稻光合能力的一个潜在的靶标。
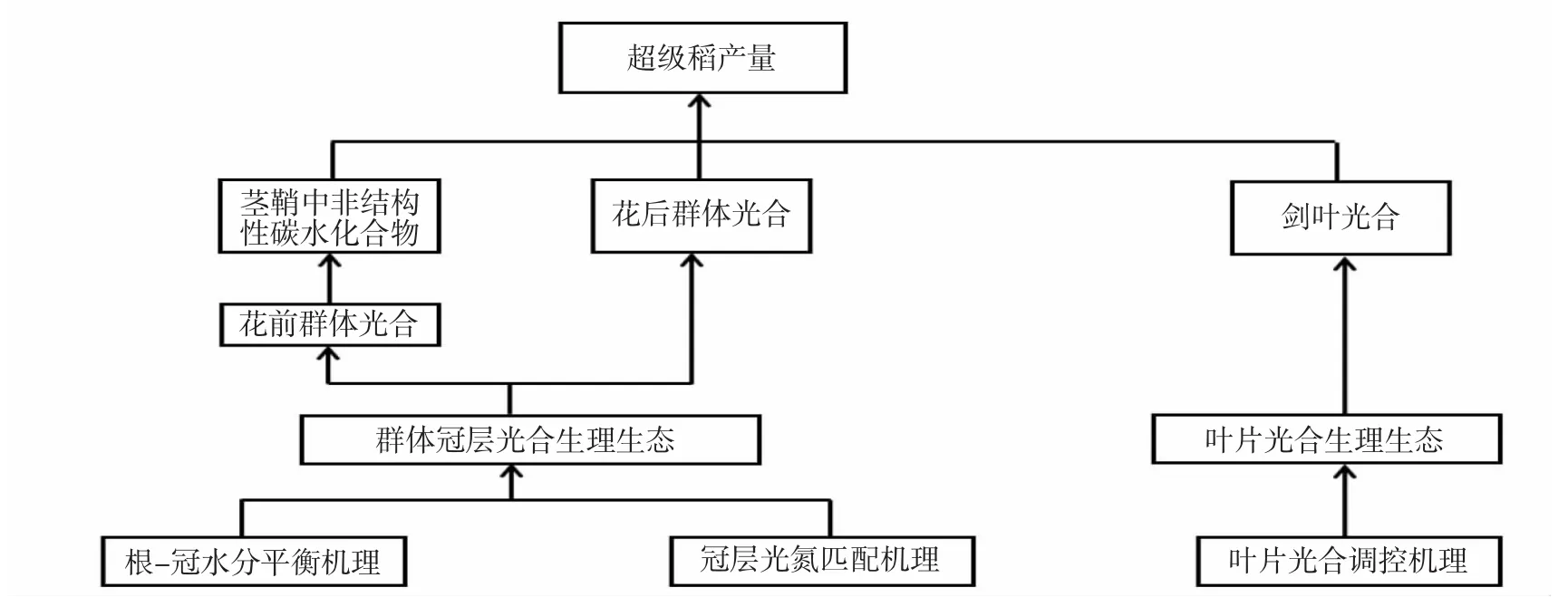
图1 提高现代超级稻产量潜力的栽培生理途径分解
2 冠层光合作用
相对于叶片光合,冠层群体的光合与水稻干物质的积累、同化物的供应联系更紧密[24]。冠层结构大大提高了作物光能利用效率。对于水稻等C3作物叶片来说,当光合有效辐射(Photosynthetically active radiation, PAR)达到700~1 000μmol/(m2·s)时,叶片的光合值达到上限,光合能力不再随着光强的提高而增加,称为光饱和点。而当光合有效辐射增加到2 000μmol/(m2s),冠层光合仍然没有达到饱和,此时,冠层光合是叶片光合的3倍[25-26]。这是因为除了冠层顶部的光饱和叶(如剑叶等),冠层内阴影部分70%的叶片能够吸收占总光能30%的散射光与漫射光,贡献冠层约50%的光合能力[27]。因此,研究整个冠层的光合生理生态对水稻的干物质积累非常重要。合理的冠层结构历来受到国内外育种和栽培生理专家的重视与关注[24,28-29]。相关研究普遍认为,茎叶夹角小、直立叶片构成的冠层有利于群体受光,对群体光合作用和物质生产有利。但在水稻高产实践中发现,基于株型选择的,具有矮秆、直立叶片等特性的新株型(New type plant-NTP)水稻品种的产量往往比基于产量选择的超级稻品种的产量低[29],说明人们对群体冠层结构和功能的认识仍然是片面的,不系统的,需要深入理解作物高产群体冠层结构的生理生态基础及其与光温等生态因子的匹配机理。
3 提高冠层光合作用的途径
光是绿色植物进行光合作用的能量来源。优良的冠层结构有利于光在群体中的分布,有利于群体受光。除了光的分布,氮素作为Rubisco酶与叶绿体的重要组成部分,它在冠层中的分布也是限制植物体内物质合成的关键因素[30-32]。和光一样,氮素在冠层内的分布也呈现顶部高、基部低的梯度分布。这种氮素的梯度分布相对于均匀分布,冠层光合提高了20%[33],这种现象被认为是植物对外界光环境的适应,是为了提高冠层光合同化力和氮素利用效率[33-35]。但是通常冠层中氮素的分布并不是最优的[36-38],Hikosaka等[39]通过研究发现,如果能够调控冠层中的氮素分布,使之与光的分布一致,达到最优,冠层光合能额外再提高20%。Dingkuhn等[40]在1991年就提出,优化冠层氮的分布(即加大氮素的分布梯度差,增加上部叶片含氮量,减少下部叶片含氮量)应该作为育种中的重要选择靶标。但是,氮素在冠层中的分布调控机理非常复杂,除了光强外,也受其他因素调控,如环境中红光/远红光比例[41]、植株体内激素[42]、叶龄[32]、库源关系[31]等。在栽培调控措施方面,Hikosaka等[34,43-44]的研究表明,高施氮水平能够改善氮素与光的梯度分布,但是受发育时期影响[45]。而Sinclair等[46-47]却持不同观点,认为氮肥水平对光、氮的分布没有影响。因此,在生产中栽培措施能否优化光、氮分布,充分利用植株体内氮素与光合效率空间梯度特点,实现作物群体光合效率的最大化,值得深入探讨。鉴于冠层光合的复杂性,冠层中的光、氮分布及其对冠层光合的贡献研究常常借助于模型模拟分析[27,39,48-50]。同时,植物冠层群体是动态变化的,苗期叶片的快速生长与冠层结构的建成,有利于光的有效截获和花前干物质的积累[40,51]。开花后作物氮素吸收、分配与植株体内氮素代谢影响着冠层光合持续期长短及其与光温资源的合理匹配,最终决定品种产量潜力是否能充分发挥。以往的作物模型研究结果[50,52]为定量评价这些因子对提高超级稻产量潜力的影响打下了很好的基础。
综上所述,作者建议从叶片光合生理(光合电子传递、Rubisco酶含量与活性、气孔与叶肉导度、叶绿体发育状况、磷酸丙糖运转等)、冠层光氮匹配(冠层光氮分布、氮素代谢、激素调控等)和根-冠水分平衡(根茎叶木质部发育、根系导水率、水通道蛋白表达等)这几个方面开展下一步研究,并在此基础上利用模型分析方法为辅助,揭示现代超级稻品种实现产量潜力的关键制约因素及其生理调控基础,并提出相应的栽培调控手段。
[1]央广网.农业部通报:“超级稻”亩产已过千公斤[EB/OL].http:// china.cnr.cn/NewsFeeds/201410/t20141010_516575617.shtml.
[2]Yang J,Zhang J.Grain-filling problem in‘super’rice[J].JExp Bot, 2010,61:1-5.
[3]Gebbing T,Schnyder H.Pre-anthesis reserve utilization for protein and carbohydratesynthesis in grainsofwheat[J].PlantPhysiol,1999, 121:871-878.
[4]TakaiT,Fukuta Y,Shirawa T,etal.Time-relatedmappingofquantitative trait loci controlling grain-flling in rice[J].JExp Bot,2005, 56:2 107-2 118.
[5]Farquhar GD,von Caemmerer S,Berry JA.A biochemicalmodel of photosynthetic CO2assimilation in leaves of C3species[J].Planta, 1980,149:78-90.
[6]von Caemmerer S,FarquharG,Berry J.BiochemicalmodelofC3photosynthesis[C]//Laisk A,Nedbal L,Govindjee G,etal.Photosynthesis in silico:Understanding complexity frommolecules toecosystems. Dordrecht,The Netherlands:Springer,2009:209-230.
[7]Kumar A,Li C,Portis Jr A R.Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis atmoderately high temperatures [J].Photosynth Res,2009,100:143-153.
[8]Raines C A.Increasing photosynthetic carbon assimilation in C3plants to improve crop yield:current and future strategies Photosynthesis[J].Plantphysiol,2011,155:36-42.
[9]Kebeish R,Niessen M,Thiruveedhi K,etal.Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana[J].NatBiotechnol,2007,25:593-599.
[10]Miyagawa Y,TamoiM,Shigeoka S.Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth[J].Nat Biotechnol,2001,19: 965-969.
[11]Stitt M,Lunn J,Usadel B.Arabidopsis and primary photosynthetic metabolism-more than the icing on the cake[J].Plant J,2010,61: 1 067-1 091.
[12]YamoriW,TakahashiS,Makino A,etal.The rolesof ATPsynthase and the cytochrome b6/f complexes in limiting chloroplast electron transportand determining photosynthetic capacity[J].Plant Physiol, 2011,155:956-962.
[13]Lieman-Hurwitz J,Rachmilevitch S,Mittler R,etal.Enhanced photosynthesis and growth of transgenic plants thatexpress ictB,a gene involved in HCO3-accumulation in cyanobacteria[J].PlantBiotechnol J,2003,1:43-50.
[14]Flexas J,Ribas-CarbóM,Hanson D T,et al.Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2in vivo[J]. Plant J,2006,48:427-439.
[15]Sade N,Gebretsadik M,Seligmann R,et al.The role of tobacco Aquaporin1 in improvingwater useefficiency,hydraulic conductivity,and yield production under salt stress[J].Plant Physiol,2010, 152:245-254.
[16]von Caemmerer S,Quick W P,Furbank R T.The developmentof C4rice:currentprogressand future challenges[J].Science,2012,336: 1 671-1 672.
[17]Sheehy JE,Ferrer A B,Mitchell P L,etal.How the rice crop works and why itneedsanew engine[C]//Sheehy JE,Mitchell PL,Hardy B,eds.Charting new pathways to C4rice,Los Ban~os,Philippines: InternationalRice Research Institute,2008:3-26.
[18]Gu J,Yin X,Struik PC,etal.Using chromosome introgression lines to map quantitative trait loci for photosynthesis parameters in rice(Oryza sativa L.)leavesunder droughtand wellwatered field conditions[J].JExp Bot,2012,63:455-469.
[19]Gu J,Yin X,Stomph T J,et al.Physiological basis of genetic variation in leafphotosynthesisamong rice(Oryza sativa L.)introgression linesunder droughtand well-watered conditions[J].JExp Bot,2012, 63:5 137-5 153.
[20]Adachi S,Baptista L Z,Sueyoshi T,et al.Introgression of two chromosome regions for leaf photosynthesis from an indica rice into the genetic background ofa japonica rice[J].JExp Bot,2014,65:2 049-2 056.
[21]Adachi S,Tsuru Y,Nito N,etal.Identification and characterization of genomic regions on chromosomes 4 and 8 that control the rate ofphotosynthesisin rice leaves[J].JExp Bot,2011,62:1927-1938.
[22]Taylaran R D,Adachi S,Ookawa T,etal.Hydraulic conductance as well as nitrogen accumulation plays a role in the higher rate of leaf photosynthesis of themost productive variety of rice in Japan[J].J Exp Bot,2011,62:4 067-4 077.
[23]Yang X,LiY,Ren B,etal.Drought-induced rootaerenchyma formation restrictswater uptake in rice seedlings supplied with nitrate[J]. PlantCell Physiol,2012,53:495-504.
[24]Long SP,Zhu X,Naidu SL,etal.Can improvement in photosynthesis increase crop yields?[J].Plant,Celland Environ,2006,29:315-330.
[25]Zhu XG,Song Q,Ort D R.Elements of a dynamic systemsmodel of canopy photosynthesis[J].CurrOpin PlantBiol,2012,15:237-244.
[26]Evans JR.Improving photosynthesis[J].Plant Physiol,2013,162: 1 780-1 793.
[27]Song Q,Zhang G,Zhu X G.Optimal crop canopy architecture to maximise canopy photosynthetic CO2uptake under elevated CO2-a theoreticalstudyusingamechanisticmodelof canopy photosynthesis [J].FunctPlantBiol,2013,40:108-124.
[28]Duvick DN.Genetic ratesofgain in hybridmaize yieldsduring the past40 years[J].Maydica,1977,22:187-196.
[29]Peng S,Khush GS,Virk P,etal.Progress in ideotypebreeding to increase rice yield potential[J].Field Crop Res,2008,108:32-38.
[30]Grindlay D JC.Towards an explanation of crop nitrogen demand based on theoptimization of leafnitrogen per unit leafarea[J].JA-gric Sci,1997,128:377-396.
[31]DreccerM F,SlaferGA,RabbingeR.Optimization ofverticaldistribution of canopy nitrogen:an alternative trait to increase yield potential inwinter cereals[J].JCrop Prod,1998,1:47-77.
[32]Hikosaka K.Leafcanopy asadynamic system:ecophysiology and optimality in leaf turnover[J].Ann Bot,2005,95:521-533.
[33]Hirose T,Werger M JA.Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy[J]. Oecologia,1987,72:520-526.
[34]Hikosaka K,Terashima I,Katoh S.Effectsof leafage,nitrogen nutrition and photon flux density on the distribution of nitrogen among leavesofa vine(Ipomoea tricolor Cav.)grown horizontally toavoid mutualshadingof leaves[J].Oecologia,1994,97:451-457.
[35]Drouet JL,Bonhomme R.Do variations in local leaf irradiance explain changes to leaf nitrogen within row maize canopies?[J].Ann Bot,1999,84:61-69.
[36]Pons T L,Schieving F,Hirose T,et al.Optimization of leaf nitrogen allocation for canopy photosynthesis in Lysimachia vulgaris[C]//H Lambers,M L Cambridge,H Konings,et al.Causes and consequencesof variation in growth rate and productivity of higher plants. The Hague,TheNetherlands:SPBAcademic,1989:175-186.
[37]Anten N,Schieving F,Werger M.Patterns of light and nitrogen distribution in relation towhole canopy carbon gain in C3and C4monoand dicotyledonousspecies[J].Oecologia,1995,101:504-513.
[38]Yin X,Lantinga EA,Schapendonk A H CM,etal.Some quantitative relationships between leaf area index and canopy nitrogen contentand distribution[J].Ann Bot,2003,91:893-903.
[39]Hikosaka K.Optimalnitrogen distributionwithin a leafcanopy under direct and diffuse light[J].Plant Cell Environ,2014,37:2 077-2 085.
[40]Dingkuhn M,Penning de Vries FW T,De Datta SK,etal.Concepts foranew plant type for directseeded flooded tropical rice[C].//InternationalRice Research Institute.Directseeded flooded rice in the Tropics.Los Ban~os,Philippines,1991:17-38.
[41]Rousseaux M,Hall A,Sánhez R.Light environment,nitrogen content,and carbon balance of basal leaves of sunflower canopies[J]. Crop Sci,1999,39:1 093-1 100.
[42]Boonman A,Prinsen E,Gilmer F,et al.Cytokinin import rate as a signal for photosynthetic acclimation to canopy light gradients[J]. PlantPhysiol,2007,143:1 841-1 852.
[43]GrindlayD JC,Sylvester-Bradley R,ScottRK.The relationship between canopygreen areaand nitrogen in the shoot[J]//G Lemaire,IG Burns,eds.Diagnostic Procedures for Crop NManagement.Poitiers, France:INRA,1995,82:53-60.
[44]L. o.tscher M,Stroh K,Schnyder H.Vertical leaf nitrogen distribution in relation to nitrogen status in grassland plants[J].Ann Bot,2003, 92:679-688.
[45]DreccerM F,Van Oijen M,Schapendonk A H CM,etal.Dynamics of vertical leaf nitrogen distribution in a vegetative wheat canopy: impacton canopy photosynthesis[J].Ann Bot,2000,86:821-831.
[46]Sinclair TR,Shiraiwa T.Soybean radiation-use efficiency as influenced by nonuniform specific leaf nitrogen distribution and diffuse radiation[J].Crop Sci,1993,33:808-812.
[47]Milroy SP,BangeM P,Sadras VO.Profilesof leafnitrogen and light in reproductive canopiesof cotton(Gossypium hirsutum)[J].Ann Bot,2001,87:325-333.
[48]Prieto JA,Louarn G,Perez Pena J,etal.A leafgasexchangemodel thataccounts for intra-canopy variability by considering leafnitrogen content and local acclimation to radiation in grapevine(Vitis vinifera L.)[J].PlantCellEnviron,2012,35:1 313-1 328.
[49]Chen TW,Henke M,de Visser PH,etal.What is themost prominent factor limiting photosynthesis in different layersofa greenhouse cucumber canopy?[J].Ann Bot,2014,114:677-688.
[50]Gu J,Yin X,Stomph T J,etal.Can exploiting natural genetic variation in leaf photosynthesis contribute to increasing rice productivity? A simulation analysis[J].PlantCell Environ,2014,37:22-34.
[51]Richards R A.Selectable traits to increase crop photosynthesis and yield ofgrain crops[J].JExp Bot,2000,51:447-458.
[52]Gu J,Yin X,Zhang C,etal.Linkingecophysiologicalmodellingwith quantitative genetics to supportmarker-assisted crop design for improved yieldsof rice(Oryza sativa)under droughtstress[J].Ann Bot, 2014,114:499-511.
Approaches to Improve Yield Potential of Super-rice from a Crop Physiological Perspective
GU Junfei,CHEN Ying,MAO Yiqi
(Jiangsu Key Laboratory of Crop Genetics and Physiology/Co-Innovation Center for Modern Production Technology of Grain Crops,Yangzhou University,Yangzhou,Jiangsu 225009,China;1st author:gujf@yzu.edu.cn)
Rice yield production is limited by the carbohydrate supply during grain-filling,which is unable to fill the large number of florets of rice plants,especially in the newly bred super-ricewith numerous spikelets in a panicle.During grain-filling stage,carbohydrate supply depends on carbon from two sources:current photosynthetic assimilates and pre-stored assimilates in culms and leaf sheaths of rice plants.It is necessary to conduct the ecophysiological study on the sources of carbohydrate supply,which would enhance our understanding of limitations to yield potential inmodern super-rice.The author summarized recent progresses in this field, and proposed that yield potential ofmodern‘super’rice could be improved by exploring ecophysiological properties of leaf photosynthesis,interaction of light and nitrogen distribution within canopy,and the relationship between root water uptake and leaf water potential.In the end,the author emphasized the role ofmodelling in integrating crop physiological knowledge to find the limitations to realizing super-rice yield potential and its physiological basis.
photosynthesis;yield potential;light and nitrogen distribution within canopy;relationship between root water uptake and leafwater potential
S511.048
:A
:1006-8082(2017)03-0001-05
2016-11-20
国家重点基础研究发展计划(“973”计划)(2015CB150400);国家自然科学基金(31501254);江苏省自然科学基金(BK20140480);中国博士后基金(2014M550312,2015T80590);江苏省高校自然科学基金(14KJB210007)
