尼龙无铬粗化与无钯活化的金属化过程
2017-07-05贾志刚孔德龙王洺浩黎德育
贾志刚, 孔德龙, 王洺浩, 黎德育, 李 宁
(哈尔滨工业大学 化工与化学学院, 哈尔滨 150001)
尼龙无铬粗化与无钯活化的金属化过程
贾志刚, 孔德龙, 王洺浩, 黎德育, 李 宁
(哈尔滨工业大学 化工与化学学院, 哈尔滨 150001)
为取代传统的铬酐粗化和胶体钯活化的化学镀前处理工艺,通过一种绿色环保的方法实现尼龙(PA10T)表面金属层的制备. 利用傅里叶红外光谱仪、X射线光电子能谱仪研究前处理过程中尼龙表层官能团分布及价键变化,并建立模型阐述金属化过程. 结果表明:采用硫酸-乙醇的粗化体系可达到粗化效果,使PA10T中酰胺键断裂并生成氨基和羧基; 采用无钯活化工艺时通过Cu2+与氨基、羧基的配位使尼龙表面活化得以实现. 通过上述简易、环保的方法可以在PA10T表面得到结合力良好的镀层,采用电子万能材料试验机测试镀层结合力达到537 N·cm-2.
尼龙;无铬粗化;无钯活化;化学镀;镀层结合力
新型工程塑料PA10T具有耐热性好、吸水率低的特点,金属化后在电子电器、装饰材料和机器零件等领域具有广泛的应用[1]. 通过激光照射、真空蒸镀、气相沉积等方法实现金属化存在工艺复杂、设备昂贵等问题[2-4],而化学镀法是一种工艺简单、成本低廉、效果明显的实用化方法. 化学镀法的一般工艺流程为除油→粗化→敏化→活化→化学镀. 粗化时常用的铬酐严重危害环境,其使用受到国内外的严格限制;活化工艺主要是利用贵金属钯,而钯资源有限且价格昂贵.
为了解决粗化、活化过程中存在的制约,国内外对ABS[5-6]、PI[7-8]、PA[9-10]、PET[11]、PVC[12-13]、PC[14-15]等工程塑料无铬粗化和无钯活化的化学镀前处理工艺进行了大量的研究,针对不同材料粗化液主要分为MnO2[16-18]、H2SO4[19-20]、NaOH[21-22]三大体系,粗化后可直接进行无钯活化,也可在表面嫁接富含活性官能团的有机配体如壳聚糖[23-24]、聚丙烯酸[25]、聚吡咯[26-27]、硅烷偶联剂[28-29]等后再金属化. 但针对PA的无铬粗化主要通过激光照射[30-31]、氧低温等离子体处理[32],采用多种酸混合的方式也可实现无铬粗化[33],但使用多种易挥发的酸对环境和操作人员危害较大. 无钯活化主要通过在PA表面预制聚苯胺膜层[9]、聚丙烯酸膜层[34]、含氨基功能层[35]后实现,上述方法较为复杂且成本昂贵. 本文研究一种简易环保的针对PA10T的化学湿法无铬粗化工艺,粗化后无需嫁接配体可直接进行无钯活化,通过化学镀可得到结合力良好的镀层.
1 实 验
1.1 实验材料及试剂
PA10T中玻璃纤维含量约为50%. 胶体钯活化液、解胶剂、化学镀铜液和电镀酸性镀铜液采用的是广东致卓精密金属科技有限公司生产的IMT-8736、IMT-8746、IMT-8760和IMT-360H,离子钯活化液为自制.
1.2 金属化过程
裁取大小约为54 mm×15 mm×2 mm规格的试片,将其浸入由H2SO4和CH3CH2OH按不同体积配比组成的粗化液中,进行一定时间粗化后取出直接浸入乙醇中清洗,并辅助毛刷涮洗,用热风吹干待用. 无钯活化步骤:将洗净的试样在50 g·L-1的CuSO4溶液中于50 ℃下活化5 min,取出后直接进行热风吹干. 接着在组分为30 g·L-1NaBH4、20 g·L-1NaOH的还原溶液中于50 ℃下还原3 min,还原完毕用去离子水清洗干净,洗净后立即进行化学镀铜20 min,此时表面会镀覆厚度为0.8 ~ 1.0 μm的化学镀铜层. 试样清洗后进行电镀酸铜处理,在电流密度为2 A·dm-2的条件下镀覆20 min. 胶体钯活化步骤:将试样浸入胶体钯活化液中活化2 min后取出用去离子水洗净,再置于解胶剂中解胶20 s后即可清洗并进行化学镀. 离子钯活化步骤:将试样浸入离子钯活化液中活化5 min后即可取出清洗并进行化学镀.
1.3 表面分析及结合力测试
用深圳市高索数码科技有限公司生产的0-500×型USB电子显微镜观察粗化前后试样表面形貌;用英国Perkin Elmer公司的PE-983G型傅里叶红外光谱仪(FTIR)检测粗化前后试样表面官能团的种类;用美国Physical Electronics公司的PHI5700型X射线光电子能谱仪(XPS)检测试样表面原子间键合关系、元素含量,污染碳C1s按照284.6 eV作能量校正;用美国FEI公司的Quanta 200FEG型场发射环境扫描电子显微镜(ESEM)观察化学镀后试样表面形貌;按照GB/T 5270-2005与GB/T 9286-1998的要求,采用胶带粘拉法、热震试验测试镀层结合力,用日本岛津公司的AG-X型电子万能试验机获取镀层结合力值.
2 结果与讨论
2.1 粗化对试样表面状态的影响规律
借鉴刻蚀聚酰胺高聚物的方法,主要的刻蚀剂确定为硫酸,溶剂选用与硫酸有很好互溶性的乙醇,且乙醇对基体有一定的润胀作用,对粗化产物有很好的分散作用,这也是清洗步骤中选用乙醇作为清洗剂的主要原因. 图1(a)为硫酸浓度为20% ~ 70%时刻蚀量随时间的变化趋势,可知浓度为20%左右时短时间内刻蚀量很小,而乙醇对试样有润胀作用导致其表层结构疏松,对镀层与基体的结合不利. 当硫酸浓度为70%时(如图1(b)所示),粗化速率又高达1.32 mm·h-1(3 mg·cm-2·min-1),试样刻蚀较快. 粗化过程要求温度适中(40~60 ℃),保证刻蚀时试样不会受热变形;酸浓对刻蚀量的影响较大,刻蚀严重易造成试样强度等性能下降;刻蚀时间可作为控制刻蚀量的工具.
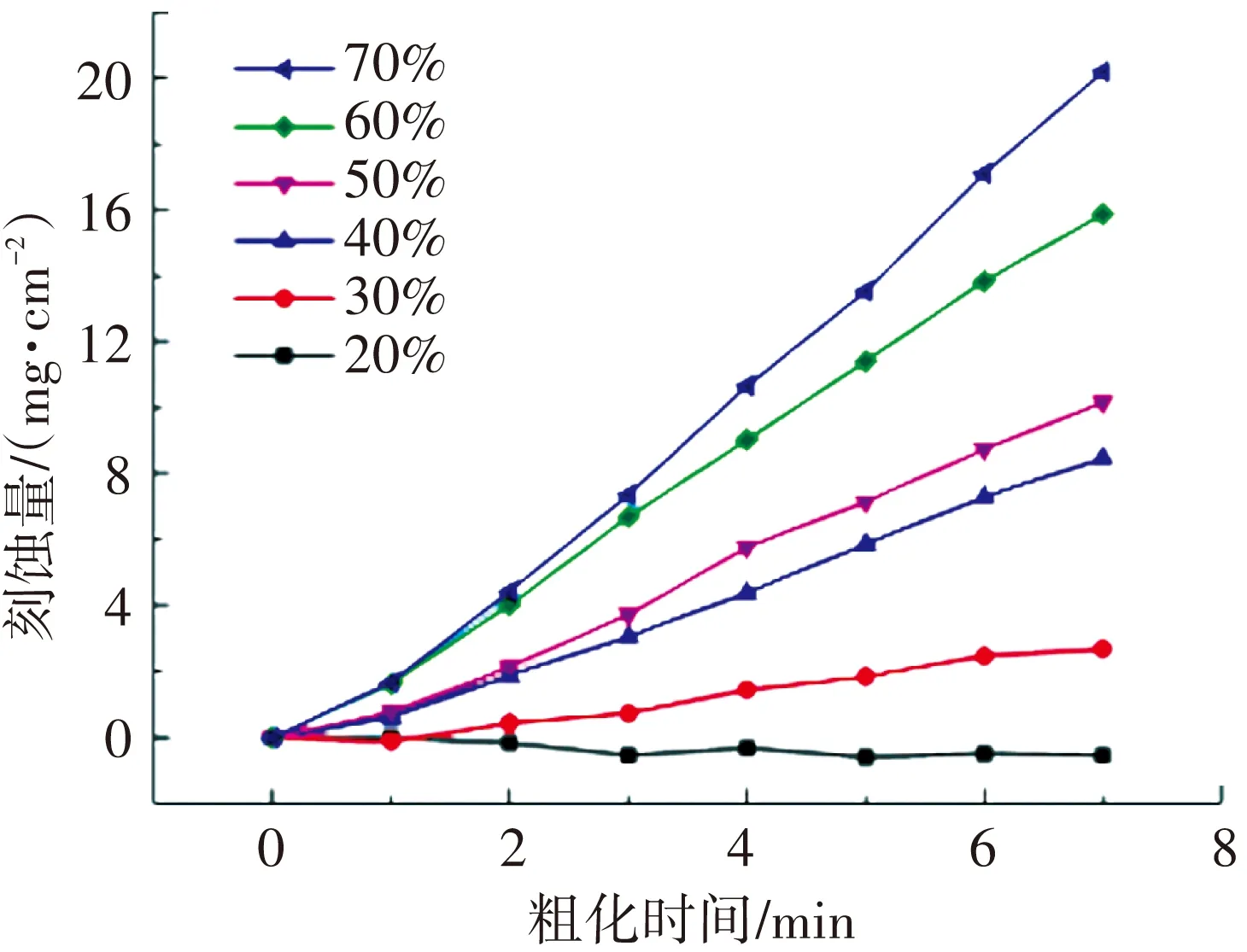
(a)刻蚀量随时间的变化趋势)

(b)刻蚀速率随酸浓的变化趋势
图2(a) ~ (f)分别是刻蚀量为0、-0.5、2.7、8.5、10.2、15.9 mg·cm-2的试样. 图2(a)为原板,表面基本光滑,无明显的玻璃纤维裸露;图2(b)试样在刻蚀过程中受到乙醇的润胀作用而增重,故刻蚀量为负数,刻蚀后试样表面的部分玻纤裸露;图2(c)显示在30%酸浓下刻蚀后单根玻纤的形貌清晰可见;图2(d)显示刻蚀量达到8.5 mg·cm-2时玻纤出现互相搭接的现象,说明表层玻纤可以实现完全裸露;图2(e)显示随着刻蚀量进一步增大至10.2 mg·cm-2时玻纤之间互相交错且密集度增加;图2(f)显示刻蚀量增大至15.9 mg·cm-2时由于聚酰胺成分进一步被刻蚀导致试样表面玻纤翘起,玻纤与基体平面的夹角增大. 不同刻蚀量的试样金属化后表面形貌见图2(g) ~ (i),经胶带粘拉法测试发现其镀层均无明显脱落.
2.2 粗化对活化作用的影响
粗化过程本质是硫酸的强氧化性对PA10T分子链的破坏,了解粗化的机理对于设计合理的活化方案十分重要,本文首先通过傅里叶红外光谱研究了粗化产物与原试样基体表面官能团的区别,结果如图3所示. 基体的光谱中观察到N-H的伸缩振动(3 306 cm-1)、-CH2的伸缩振动(2 928 cm-1和 2 856 cm-1)、酰胺中C=O的伸缩振动(1 639 cm-1)、酰胺中N-H弯曲振动及C-N的伸展振动(1 543 cm-1)[36]. 产物的光谱中3 378 cm-1处吸收峰为-NH2伸展振动,2 606 cm-1、1 682 cm-1可能为芳香酸羧基的伸缩振动,2 500~3 100 cm-1为羟基的伸缩振动[37-38],此外还出现与基体图谱中类似的一些吸收峰. 基体中的C=O、C-N的吸收峰对应的透过率值低于产物,这可能意味着基体中这两种价键的含量更高. 而产物中N-H峰(3 378 cm-1)对应的透光率更低,则是因为氨基的出现导致N-H键含量增加. 产物中羧基和氨基的出现以及酰胺键的C-N含量减少可以推测粗化会对PA10T中酰胺键产生破坏并生成带有氨基和羧基的产物.

图2 各条件下试样的USB电子显微镜图
Fig.2 The USB electronic microscope images of sample under different roughening conditions

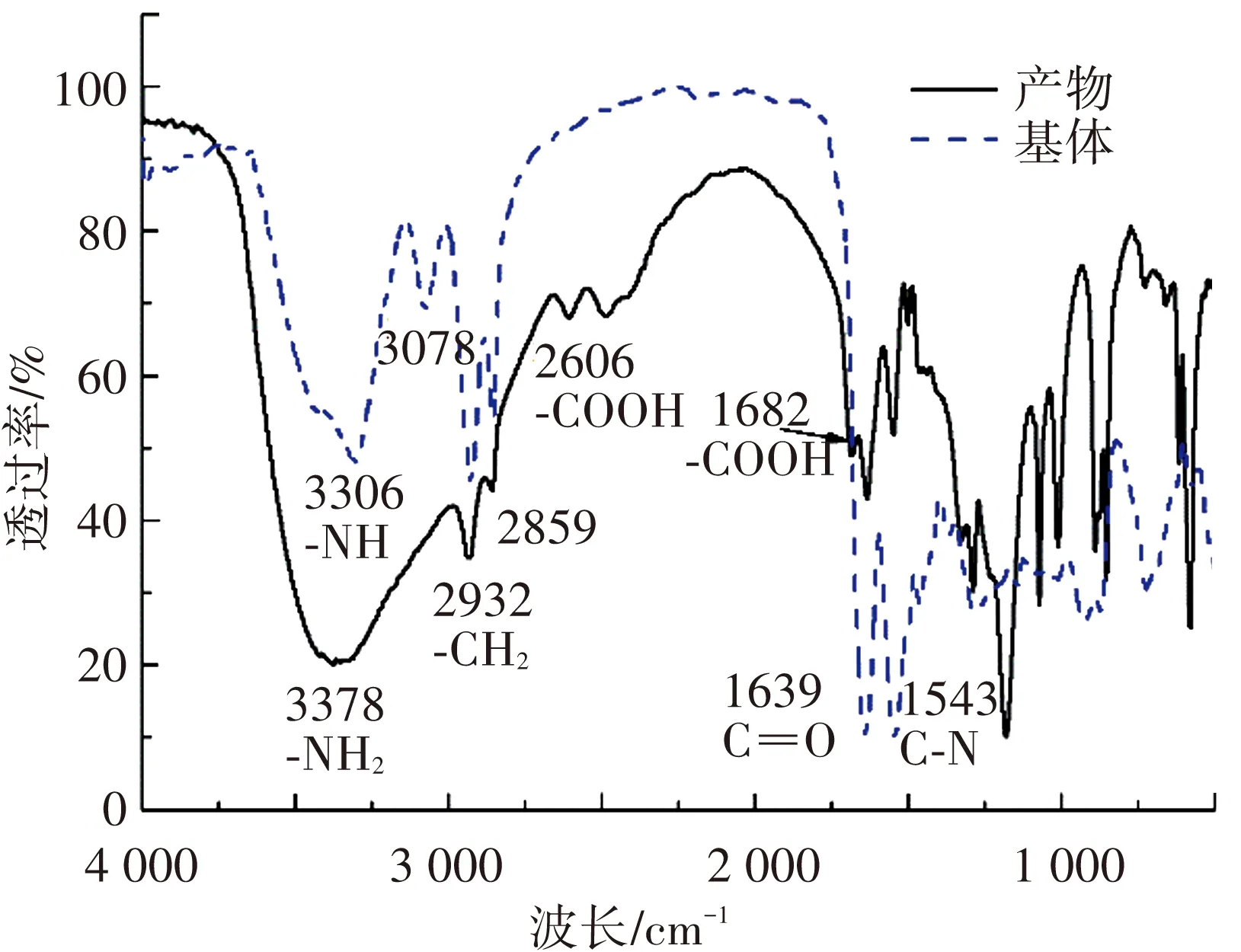
图3 粗化产物及基体的红外谱
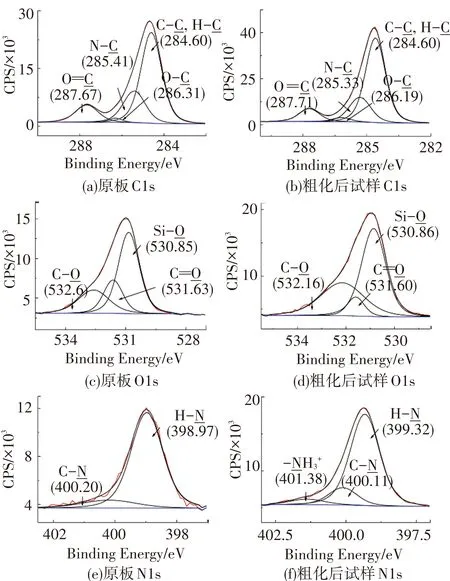
图4 粗化前后试样的XPS窄谱图
Fig.4 Narrow acanning spectra of XPS for the sample before and after coarsening
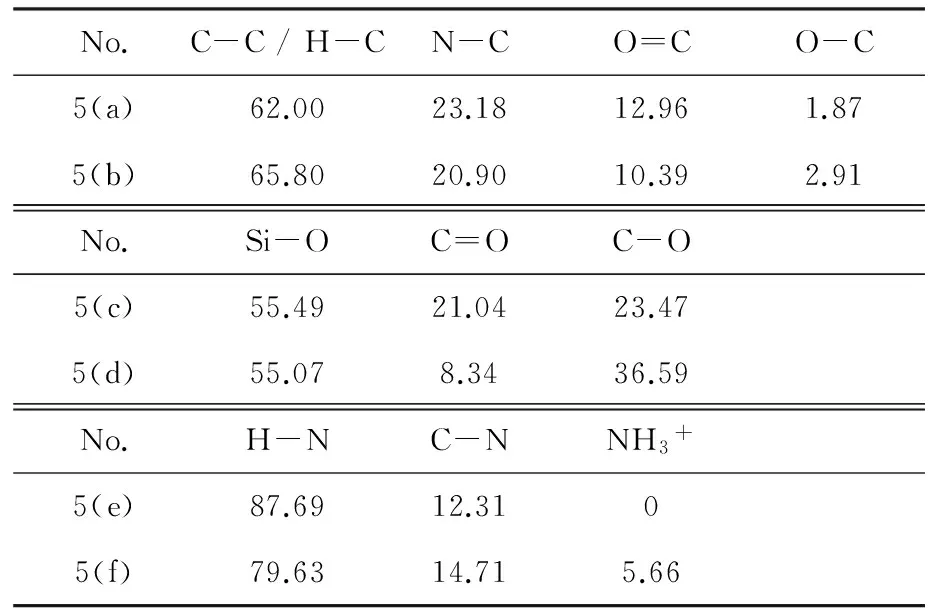
表1 不同状态下原子的含量
通过上述讨论得知粗化后试样表面含有大量的氨基和羧基,氮原子的孤对电子使其有很强的给电子能力,故氨基对金属离子有很强的配位能力. 羧基中的羰基氧和羟基氧最外层有孤对电子,羰基氧本身有配位能力而羟基氧失去氢质子也有较强的配位能力,很容易与金属离子形成配位键. 以Cu2+为例,Cu2+的杂化轨道容易接受电子,所以粗化后的PA10T浸在含Cu2+的溶液中时,Cu2+容易与N、O原子形成配位键. 表面吸附Cu2+的试样浸在含NaBH4的溶液中时,Cu2+被NaBH4还原成铜从而完成活化. 基于上述理论,可以设计活化和还原步骤实现试样表面的铜粒子的沉积,铜粒子为化学镀提供催化中心. 鉴于化学镀铜液中主盐为CuSO4,以CuSO4作为活化液中Cu2+的供应者可以避免杂质被带入到化学镀液中. 在还原液NaBH4溶液中添加NaOH可以使其更稳定,同时质子氢可以被中和,提高了羧基的配位能力.
图5为粗化前后以及通过胶体钯活化、离子钯活化、离子铜活化后试样的XPS全谱图. 从图5可以发现:试样1、2中检测出主要有C1s、N1s、O1s和Si2p四种元素的吸收峰,其中C、N、O为PA10T的组成元素,Si为试样中玻璃纤维的组成元素. 试样3中检测出了Cu2p吸收峰,试样4、5中检测到Pd3d吸收峰,说明试样3~5表面实现活性金属粒子的沉积,即达到了活化效果. 因为试样5经过了胶体钯活化,而Pd和Sn是胶体钯的主要成分,因此试样5中还检测出了Sn3d元素吸收峰. 多种活化效果的实现说明表面活性官能团的存在并且对金属离子存在络合作用.
图6为离子铜活化还原后试样的N1s、O1s、Cu2p元素的窄谱图. 图6(a)中出现Cu与N配位键的吸收峰在399.37 eV[12];图6(b)中出现Cu与O配位键的吸收峰在530.04 eV[8];图6(c)中出现Cu0的Cu 2p3/2和Cu 2p1/2的吸收峰分别在931.67 eV和951.48 eV,Cu与O配位键中Cu 2p3/2和Cu 2p1/2的吸收峰分别在933.65 eV和953.58 eV. 明显的Cu与N配位键吸收峰说明基体表面的氨基与Cu实现共价键络合,Cu与O配位键吸收峰也说明Cu与O实现共价键络合. 通过上述分析验证了氨基、羧基这两种活性官能团对金属离子的络合作用,达到了预期活化和还原步骤的设计要求.

A—原板,B—粗化后试样,C—离子铜活化还原后试样,D—离子钯活化还原后试样,E—胶体钯活化解胶后试样
图5 各试样的XPS全谱
Fig.5 XPS survey spectra of all kinds of sample
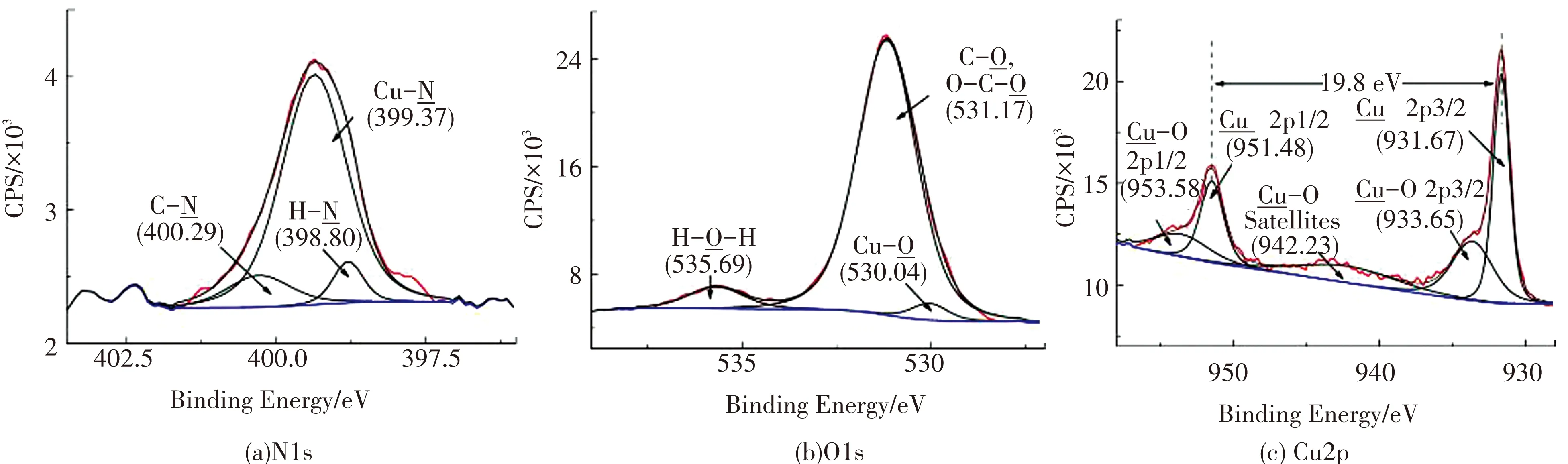
图6 离子铜活化还原后试样的XPS窄谱
2.3 金属化过程模型
为了进一步阐述活化过程,提出一个可能的模型如图7所示. 首先,粗化过程中酰胺键断裂并生成氨基和羧基,表面形成不规则形状的缺口,同时有大量的玻璃纤维裸露出来. 完成粗化后,将试样浸入CuSO4溶液中,氨基和Cu2+会首先配位,试样取出后不水洗直接吹干使表面残留CuSO4. 再浸入NaBH4和NaOH混合溶液中,羧基中质子氢被中和,残留的CuSO4与羧酸钠盐发生离子交换生成羧酸铜盐[22, 40],羧基与氨基络合的Cu2+共同被NaBH4还原为铜原子,且铜原子与基体通过共价键络合. 试样表面均匀覆盖具有催化活性的铜粒子后,在化学镀铜液中可以催化铜的化学沉积,最终试样表面完成金属化.
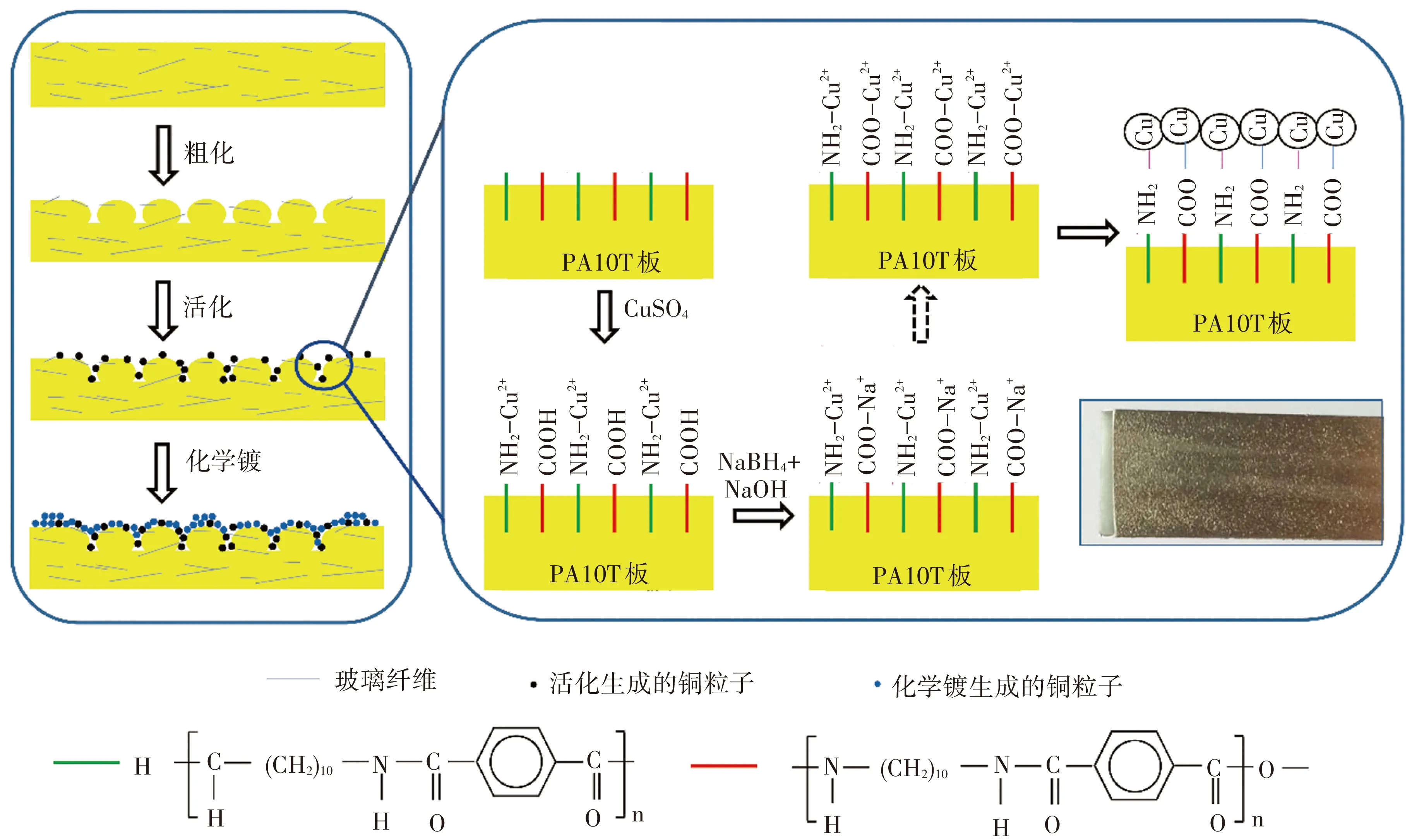
图7 PA10T金属化过程示意
2.4 镀层性能表征
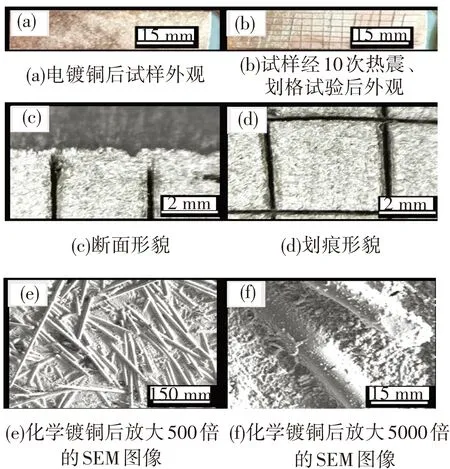
图8 50 ℃下在50%的硫酸乙醇溶液中粗化6 min后,经离子铜活化并金属化后的试样形貌Fig.8 Surface morphologies of sample etched for 6 min under the acid concentration of 50% and via ionic copper activation, electroless copper plating, copper electroplating
镀层结合力是衡量金属化性能好坏的重要标志,是决定产品的使用范围和寿命的首要因素. 因此,对镀层结合力的测试显得尤为重要. 断面观察法是通过打磨将试样截面磨出,从截面观察镀层与基体之间有无不良接触,如起泡、断裂等. 经观察采用硫酸-乙醇体系粗化、离子铜活化的前处理工艺金属化后镀层均没有出现起泡、断裂等现象,见图8. 热震试验后划痕处镀层也没有翘起,显示镀层结合力良好.
将两试样保持一定的接触面积并粘结,与接触平面呈水平方向上利用电子万能材料试验机分别拉伸两试样至镀层脱落并记录结合力值为537 N·cm-2. 对铬酐粗化的ABS表面单位面积的金属复合材料层结合力进行测试[41-42],在平面垂直方向上使用拉伸附着力测试仪测得其值约为2 800~3 000 N·cm-2. ABS经化学湿法无铬粗化后化学镀铜,将试样表面镀层切割成10 mm×50 mm形状,用90°剥离实验仪以25 mm·min-1的速度测试其剥离强度约1.2 ~1.4 kN·m-1[5]. 利用超临界CO2处理PA6并化学镀镍磷合金后测试其剥离强度可达到1 kN·m-1[43].
鉴于材料本身特性不同且测试方法有一定差异,PA10T镀层结合力测试值虽然低于ABS,但可以通过胶带粘拉法、热震试验等测试,证明其仍然可以有广泛的用途.
3 结 论
1) 采用硫酸-乙醇的无铬粗化体系可以对含玻纤增强的PA10T产生有效的粗化效果.
2) PA10T粗化后酰胺键会断裂并生成氨基和羧基,这两种基团在活化时会与金属离子配位.
3) 采用CuSO4溶液作为活化液、NaBH4和NaOH混合溶液作还原液可以实现Cu与氨基、羧基的有效配位,进而实现PA10T的无钯活化.
[1] 张凯, 赵建青, 刘述梅, 等. 半芳香族尼龙的结构性能及其应用[J]. 石油化工, 2015, 44(5): 536-42.
ZHANG Kai, ZHAO Jianqing,Liu Shumei, et al. Structure, properties and applications of semi-aromatic nylons[J]. Petrochemical Technology, 2015, 44(5): 536-542.
[2] MIN H, LEE B, JEONG S, et al. Laser-direct process of Cu nano-ink to coat highly conductive and adhesive metallization patterns on plastic substrate[J]. Optics and Lasers in Engineering, 2016, 80: 12-16. DOI: 10.1016/j.optlaseng.2015.12.007.
[3] STRUPPERT T, HEFT A, GR NLER B. Thin functional films by combustion chemical vapour deposition (C-CVD)[J]. Thin Solid Films, 2012, 520(12): 4106-9. DOI: 10.1016/j.tsf.2011.06.048.
[4] DE BRUYN K, VAN STAPPEN M, DE DEURWAERDER H, et al. Study of pretreatment methods for vacuum metallization of plastics[J]. Surface & Coatings Technology, 2003, 163: 710-715. DOI: 10.1016/s0257-8972(02)00684-9.
[5] OLIVERA S, MURALIDHARA H B, VENKATESH K, et al. Plating on acrylonitrile-butadiene-styrene (ABS) plastic: a review[J]. Journal of Materials Science, 2016, 51(8): 3657-3674. DOI: 10.1007/s10853-015-9668-7.
[6] ZHAO W X, WANG Z L. Adhesion improvement of electroless copper to ABS resin by low environmental pollution surface etching system[J]. Transactions of the IMF, 2013, 91(3): 149-155. DOI: 10.1179/0020296713z.00000000099.
[7] LI Libo, MA Yue, XIE Jingchen, et al. Metallization process of a polyimide surface with palladium-free activation for electronic field applications[J]. Journal of Electronic Materials, 2015, 44(10): 4042-4051. DOI: 10.1007/s11664-015-3874-6.
[8] YOON S S, KIM D O, PARK S C, et al. Direct metallization of gold patterns on polyimide substrate by microcontact printing and selective surface modification[J]. Microelectronic Engineering, 2008, 85(1): 136-142. DOI: 10.1016/j.mee.2007.04.142.
[9] SARITHA CHANDRAN A, NARAYANANKUTTY S K. Preparation and characterization of conducting nylon 6 fibers[J]. Journal of Materials Research, 2011, 24(8): 2728-2735. DOI: 10.1557/jmr.2009.0329.
[10]ANDRI E E. Chrome-free method of conditioning and etching of a thermoplastic substrate for metal plating: UNITED STATES, US 2010/0159260 A1 [P]. 20100624.
[11]LU Yinxiang, XUE Longlong, LI Feng. Silver nanoparticle catalyst for electroless Ni deposition and the promotion of its adsorption onto PET substrate[J]. Surface and Coatings Technology, 2010, 205(2): 519-524. DOI: 10.1016/j.surfcoat.2010.07.020.
[12]WANG Mingqiu, YAN Jun, DU Shiguo, et al. Adsorption characteristic of copper ions and its application in electroless nickel plating on a hydrogel-functionalized poly(vinyl chloride) plastic[J]. Journal of Materials Science, 2013, 48(20): 7224-7237. DOI: 10.1007/s10853-013-7539-7.
[13]WANG Mingqiu, YAN Jun, DU Shiguo, et al. Electroless plating of PVC plastic through new surface modification method applying a semi-IPN hydrogel film[J]. Applied Surface Science, 2013, 277: 249-256. DOI: 10.1016/j.apsusc.2013.04.035.
[14]ZHAO Wenxia, WANG Zenglin. Adhesion improvement of electroless copper to PC substrate by a low environmental pollution MnO2-H3PO4-H2SO4-H2O system[J]. International Journal of Adhesion and Adhesives, 2013, 41: 50-56. DOI: 10.1016/j.ijadhadh.2012.10.002.
[15]ZHAO Wenxia, LI Zhixin, WANG Zenglin. A study of the environmentally friendly polycarbonate surface etching system containing H2SO4-MnO2 colloid[J]. Journal of Adhesion Science and Technology, 2013, 27(13): 1455-1463. DOI: 10.1080/01694243.2012.745052.
[16]ZHAO Wenxia, DING Jie, WANG Zenglin. Improvement in the etching performance of the acrylonitrile-butadiene-styrene resin by MnO2-H3PO4-H2SO4 colloid[J]. Langmuir, 2013, 29(20): 5968-5973. DOI: 10.1021/la400321k.
[17]YUAN X L, YANG Z F, HE Y, et al. Pd-free surface activation technique for ABS surface metallisation[J]. Transactions of the IMF, 2013, 89(4): 202-205. DOI: 10.1179/174591911x13077162687419.[18]MA Qian, ZHAO Wenxia, LI Xirong, et al. Study of an environment-friendly surface pretreatment of ABS-polycarbonate surface for adhesion improvement[J]. International Journal of Adhesion and Adhesives, 2013, 44: 243-249. DOI: 10.1016/j.ijadhadh.2013.03.010.
[19]LI Dapeng, YANG Chenlu. Acidic electroless copper deposition on aluminum-seeded ABS plastics[J]. Surface and Coatings Technology, 2009, 203(23): 3559-3568. DOI:10.1016/j.surfcoat.2009.05.026.
[20]TEIXEIRA L A C, SANTINI M C. Surface conditioning of ABS for metallization without the use of chromium baths[J]. Journal of Materials Processing Technology, 2005, 170: 37-41. DOI: 10.1016/j.jmatprotec.2005.04.075.
[21]INAGAKI N, KIMURA H. Electroless copper plating on acrylonitrile butadiene styrene material surfaces without chromic acid etching and a palladium catalyst[J]. Journal of Applied Polymer Science, 2008, 111: 1034-1044. DOI: 10.1002/app.29144.
[22]WANG Yanqing, LI Ning, WANG Xianliang, et al. High-definition conductive silver patterns on polyimide film via an ion exchange plating method[J]. RSC Adv, 2016, 6(9): 7582-7590. DOI: 10.1039/c5ra23694k.
[23]TANG Xuejiao, WANG Jingang, SHEN Boxiong. The Preparation and Application of Chitosan Derivatives for Ni Coating on ABS[J]. Advanced Materials Research, 2011, 199-200: 1999-2004. DOI: 10.4028/www.scientific.net/AMR.199-200.1999.
[24]TANG Xuejiao, WANG Jingang, WANG Chengjun, et al. A novel surface activation method for Ni/Au electroless plating of acrylonitrile-butadiene-styrene[J]. Surface and Coatings Technology, 2011, 206(6): 1382-1388. DOI: 10.1016/j.surfcoat.2011.08.064.
[25]GARCIA A, BERTHELOT T, VIEL P, et al. ABS polymer electroless plating through a one-step poly(acrylic acid) covalent grafting [J]. ACS applied materials & interfaces, 2010, 2(4): 1177-1183. DOI: 10.1021/am1000163.
[26]BAZZAOUI M, MARTINS J I, BAZZAOUI E A, et al. A simple method for acrylonitrile butadiene styrene metallization[J]. Surface and Coatings Technology, 2013, 224: 71-76. DOI: 10.1016/j.surfcoat.2013.02.050.
[27]BAZZAOUI M, MARTINS J I, BAZZAOUI E A, et al. Environmentally friendly process for nickel electroplating of ABS[J]. Applied Surface Science, 2012, 258(20): 7968-7795. DOI: 10.1016/j.apsusc.2012.04.146.
[28]SUN Zhiping, HUANG Junun, LIU Qi, et al. Effects of temperature on Ni coating on poly(ethylene terephthalate) substrate modified with primer[J]. Journal of Materials Science: Materials in Electronics, 2016, 27(6): 5892-5898. DOI: 10.1007/s10854-016-4507-4.
[29]SUN Zhiping, HUANG Junun, WANG Libao, et al. Method for electroless nickel plating on poly(ethylene terephthalate) substrate modified with primer and self-assembled monolayer[J]. Journal of Materials Science: Materials in Electronics, 2015, 26(12): 10132-10137. DOI: 10.1007/s10854-015-3698-4.
[30]KIM J, KIM H S, PARK C H. Contribution of surface energy and roughness to the wettability of polyamide 6 and polypropylene film in the plasma-induced process [J]. Textile Research Journal, 2015, 86(5): 461-471. DOI: 10.1177/0040517515580511.
[31]RYTLEWSKI P, ZENKIEWICZ M, TRACZ A, et al. Surface morphology studies of laser irradiated and chemically metalized polyamide composites[J]. Surface and Coatings Technology, 2011, 205(23/24): 5248-5253. DOI: 10.1016/j.surfcoat.2011.04.105.
[32]TAO Dan, WEI Qufu, CAI Yibing, et al. Functionalization of polyamide 6 nanofibers by electroless deposition of copper[J]. Journal of Coatings Technology and Research, 2008, 5(3): 399-403. DOI: 10.1007/s11998-008-9118-4.
[33] 连华景, 吴子豹, 颜文艺, 等. 一种尼龙材料的粗化液及粗化方法: 201310302127.4[P]. 2013-07-18.
LIAN Huajing, WU Zibao, YAN Wenyi, et al. An roughening liquid and method for nylon material: 201310302127.4[P]. 2013-07-18.
[34]GARCIA A, BERTHELOT T, VIEL P, et al. Microscopic study of a ligand induced electroless plating process onto polymers[J]. ACS applied materials & interfaces, 2010, 2(11): 3043-3051. DOI: 10.1021/am100907j.
[35]ALONSO M H, MCCARTHY T J, JIA X. Nylon surface modification: 2. nylon-supported composite films[J]. Langmuir, 2006, 22:1646-1651. DOI: 10.1021/la0526737.
[36]WANG Wenzhi, WANG Xianwen, LI Ruixue, et al. Environment-friendly synthesis of long chain semiaromatic polyamides with high heat resistance[J]. Journal of Applied Polymer Science, 2009, 114(4): 2036-2042. DOI: 10.1002/app.30774.
[37] 金盈, 曾广赋, 朱丹阳, 等. 聚酰胺酸结构及其亚胺化的红外光谱分析[J]. 应用化学, 2011, 28(3): 258-262.
JIN Ying, ZENG Guangfu, ZHU Danyang, et al. Analysis of structure and imidization of poly(amic acid) using FTIR spectroscopy[J]. Chinese Journal of Applied Chemistry, 2011, 28(3): 258-262.
[38]KARIUKI V M, ZHANG J, PARLINSKA M, et al. 3D π-conjugated poly(amic) acid polymer as support matrices for ethanol electro-oxidation on palladium and platinum catalysts[J]. Electrocatalysis, 2016, 7(4): 317-325. DOI: 10.1007/s12678-016-0307-0.
[39]ZHAO Wenxia, MA Qian, LI Lisha, et al. Surface modification of ABS by photocatalytic treatment for electroless copper plating[J]. Journal of Adhesion Science and Technology, 2013, 28(5): 499-511. DOI: 10.1080/01694243.2013.845356.
[40]HSIAO Y S, CHEN C P, CHAO C H, et al. All-solution-processed inverted polymer solar cells on granular surface-nickelized polyimide[J]. Organic Electronics, 2009, 10(4): 551-61. DOI: 10.1016/j.orgel.2009.01.012.
[41]ARAI S, KANAZAWA T. Electroless deposition and evaluation of Cu/multiwalled carbon nanotube composite films on acrylonitrile butadiene styrene resin[J]. Surface and Coatings Technology, 2014, 25: 224-229. DOI:10.1016/j.surfcoat.2014.06.017.
[42]ARAI S, SATO T, ENDO M. Fabrication of various electroless Ni-P alloy/multiwalled carbon nanotube composite films on an acrylonitrile butadiene styrene resin [J]. Surface and Coatings Technology, 2011, 205(10): 3175-81.DOI:10.1016/j.surfcoat.2010.11.030.
[43]TENGSUWAN S, OHSHIMA M. Environmentally benign electroless nickel plating using supercritical carbon-dioxide on hydrophilically modified acrylonitrile-butadiene-styrene[J]. Applied Surface Science, 2014, 311: 189-200.DOI:10.1016/j.apsusc.2014.05.040.
(编辑 王小唯, 苗秀芝)
Metallization process of PA10T with chrome-free roughening and palladium-free activation
JIA Zhigang, KONG Delong, WANG Minghao, LI Deyu, LI Ning
(School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150001, China)
In order to replace the traditional pretreatment process for electroless plating of chromic acid roughening and colloid palladium activation, the metal layer was prepared on the surface of nylon (PA10T) through a green method. The distribution of functional groups and the change of valence bonds on the nylon surface were characterized by Fourier transform infrared spectrometry and X ray photoelectron spectrometry, and then a model was established to illustrate the metallization process. The results show that the roughening system of sulphuric acid-ethanol can be used to achieve the effect of roughening, make the amide bond rupture and generate amino and carboxyl in PA10T. When the palladium-free activation process is adopted, the surface activation of nylon could be achieved by the coordination of Cu2+with amino groups and carboxyl groups. The simple and environmentally friendly method mentioned above could get a good adhesion of the metal coating on the PA10T surface, the coating binding force reaches 537 N·cm-2which is tested by electronic universal material testing machine.
nylon; chrome-free roughening; palladium-free activation; electroless plating; coating binding force
10.11918/j.issn.0367-6234.201611017
2016-11-03
贾志刚(1993—),男,硕士研究生; 李 宁(1953—),女,教授,博士生导师
李 宁,lininghit@263.net
TQ153.3
A
0367-6234(2017)05-0042-07
