The role of motor network reorganization during rehabilitation
2017-06-05YueLi,EdmundR.HollisII
The role of motor network reorganization during rehabilitation
There are roughly 282,000 individuals living with spinal cord injury in the United States alone (National Spinal Cord Injury Statistical Center, Birmingham, AL, USA). Spinal cord injury often results in permanent functional impairments with only a limited capacity for spontaneous recovery. For the return of motor function, such as locomotion or hand and arm dexterity, rehabilitative training is the principal means to maximize the endogenous recovery of the central nervous system. The extent of rehabilitation-mediated motor recovery is likely dependent upon the extent of spared spinal cord tissue. However, the mechanisms supporting that recovery are not well understood. The primary focus of spinal cord injury research has been on inducing axonal growth and regeneration, with a lesser emphasis on how motor networks incorporate the changes induced by injury. An understanding of the role for motor learning mechanisms after spinal cord injury will provide means to maximize the functional recovery mediated by rehabilitation, stem cell treatments, regenerative therapies, or other interventions aimed at inducing plasticity of the neural circuits underlying motor function.
Cortical motor maps and motor learning. The motor cortex is composed of roughly topographical representations or maps of the body which arise with the development of manual dexterity and refinement of corticospinal axon collaterals. Motor maps are far from static, and can be dramatically reshaped throughout life in response to motor learning or injury. During motor learning, the size of motor representations for the musculature involved in the trained movement increases, at the expense of neighboring map areas. Connectivity changes within the motor cortex appear to underlie this plasticity. Corticospinal neurons that project to low cervical (C8) spinal cord levels governing movement of the distal forelimb show significantly greater dendritic spine density and branching in rodents trained on skilled forelimb reach than in untrained animals (Wang et al., 2011). In contrast, neighboring corticospinal neurons that project to the more rostral levels (C4), that are not engaged in distal forelimb dexterity, exhibit no significant structural modification upon completion of learning this new motor experience (Wang et al., 2011).In vivoimaging of a non-specific layer 5 pyramidal neuron population has revealed a rapid induction of dendritic spine formation during skilled reach (Fu et al., 2012). Multiple training sessions are required for the development of expertise. During these sessions, successively formed spines cluster along the dendrite, potentially amplifying the post-synaptic response to related task-specific inputs (Fu et al., 2012). In these studies, spine density in layer 5 pyramidal neurons returns to baseline levels through a subsequent increase in spine elimination. Despite the increased levels of elimination, spines that form in response to skilled learning are more stable than spontaneously formed spines in control mice and persist over the course of 4 months (Figure 1A). It is not clear how spine turnover is affected in corticospinal neurons projecting to specific forelimb motor circuits at discrete spinal levels. What is clear is that spine turnover in the apical dendrites of layer 5 neurons mirrors the plasticity observed in excitatory layer 2/3 neurons during motor learning (Peters et al., 2014). The potentiation of existing or newly-formed spines in layer 2/3 is a critical component of motor learning, as a photoactivated shrinkage of newly potentiated spines, using a novel Rac1 GTPase modified optoprobe, disrupts the learning of novel motor skills (Hayashi-Takagi et al., 2015).
In addition to structural changes, cortical neurons show a remarkable remodeling of activity patterns during motor learning. During the initial phase of learning an unskilled lever press task, different activity patterns of layer 2/3 excitatory neurons can give rise to similar forelimb movements. With repeated training, the variability of motor cortex activity patterns decreases and reproducible, spatiotemporal activity patterns gradually emerge (Peters et al., 2014). The development of expertise correlates with a transient increase in dendritic spine turnover, implicating changes in intracortical connectivity in the acquisition of novel motor learning. It is likely that this intracortical remodeling results in altered connectivity with corticospinal neurons, a potential mechanism underlying the changes in motor maps of evoked output observed with motor learning. Altering input, or patterns of input, to corticospinal neurons will be required for learning-induced changes as the spinal levels to which they project do not appear to change (Wang et al., 2011), short of injury.
Spinal cord injury. It is well established that cortical motor and sensory maps are dramatically affected by spinal cord injury. Both animal and human studies have demonstrated that functional reorganization occurs rapidly after injury, with intact regions above the level of injury expanding into de-efferented cortical areas. Blood-oxygen-level-dependent functional magnetic resonance imaging (BOLD-fMRI) and optogenetic stimulation demonstrate that shifts in rodent sensory and motor representations, respectively, occur rapidly and persist at more chronic time points (Endo et al., 2007; Hollis II et al., 2016). The immediate effects on cortical representations are likely due to the loss of sensory input and reduced lateral inhibition within the motor networks. Within a few days, however, structural changes in cortical networks can be observed. As early as 3 days after spinal cord injury, spine loss is detectable on axotomized corticospinal neurons (Ghosh et al., 2012). Spine loss continues and after one week is apparent in pyramidal neurons within both layer 5 and layer 2/3 (Figure 1B) (Ghosh et al., 2012). After an incomplete lesion of the mid-to-low cervical spinal cord, more proximal motor representations initially expand into de-efferented areas. This expanded cortical territory is eventually relinquished to more distal, injury affected motor representations during behavioral recovery (Hollis II et al., 2016). Using optogenetic activation of channelrhodospin to stimulate motor output of cortical pyramidal neurons over time, this cortical reorganization was found to be depen-dent upon a low level of rehabilitative training on a skilled forelimb task (Hollis II et al., 2016). The cortical plasticity observed did this result in the return of hindlimb evoked movements in this study. Another study using similar optogenetic mapping techniques found that after a high cervical injury, which significantly reduces both forelimb and hindlimb motor maps, recovery of cortical representations can occur through the spared minor components of the corticospinal tract (Hilton et al., 2016). In this study, the recovered area and position of cortical maps corresponding to forelimb and hindlimb movements are relatively similar to the intact maps prior to injury. The absence of spared proximal muscle innervation in this model likely prevents the cortical reorganization observed with more caudal injuries to the midto-low cervical spinal cord.
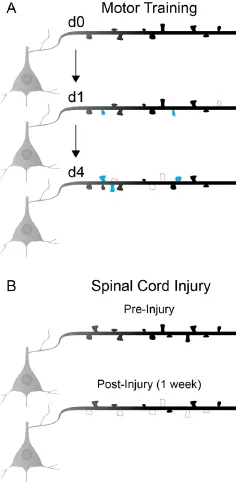
Figure 1 Changes in layer 5 dendritic spine dynamics during motor learning and after spinal cord injury.
Is motor map remodeling necessary for recovery of previously learned skills? Given the relationship between cortical map reorganization and motor learning, it is a critical question whether or not reorganization of motor maps is also required for the recovery of previously learned skills. After partial motor cortex lesions, the recovery of skilled forelimb function corresponds with training-induced remodeling of cortical motor maps. The depletion of cholinergic input from the basal forebrain prevents this remodeling of cortical forelimb representations and blocks skilled forelimb recovery (Conner et al., 2005). Ablation of cholinergic neurons also impairs rehabilitation-mediated increases in spine density and dendritic complexity after motor cortex lesion, limiting motor map remodeling and functional recovery (Wang et al., 2016).
It is perhaps surprising then that a complete aspiration of motor cortex, bilaterally, shows no impact on the performance of a previously learned simple motor skill (Kawai et al., 2015). While motor cortex plays an essential role as a tutor to subcortical structures in acquiring learned lever press sequences, it is dispensable for the execution of this simple learned motor behavior (Kawai et al., 2015). In the absence of any intervention, aspiration of the motor cortex fails to impact the kinematics of learned, simple lever press movements, indicating that subcortical structures in rodents are sufficient for the maintenance of previously learned motor sequences of unskilled behavior (Kawai et al., 2015). While the learning of stereotyped, spatiotemporal sequences of unskilled lever press movements results in the refinement of networked firing patterns of layer 2/3 excitatory neurons (Peters et al., 2014), training on a similar unskilled task fails to induce the large-scale motor map remodeling observed during the learning of skilled, dexterous movements (Kleim et al., 1998). This raises questions regarding the relationship between motor cortex and subcortical structures in the recovery of motor function after injury, and what relevance motor map reorganization has to the recovery of skilled function.
In order to address the role of motor map reorganization in the recovery of skilled function, the cortical response to corticospinal axon remodeling was determined after a mid-cervical spinal cord injury at level 5 (C5) (Hollis II et al., 2016). In both mice and rats, respectively, conditional knockout of, or infusion of function blocking antibody against, the repulsive Wnt receptorRykresults in increased axon growth proximal to the injury site and a greater recovery of function than in control animals. Increased axonal growth with corticalRykdeletion results in larger forelimb motor maps, which are disrupted by a subsequent, higher injury at C3 that interrupts much of thede novocorticospinal circuitry and reducesRykknockout-mediated skilled forelimb reach performance to control levels. Selective transection of the corticospinal tract at the level of the pyramid both eliminates unilaterally-evoked motor maps and completely impaired skilled forelimb reach (Hollis II et al., 2016).
Growing evidence supports a close relationship between motor cortex reorganization and rehabilitation from spinal cord injury. Motor map reorganization after injury appears to have at least two distinct phases, an immediate shift in spared motor representations immediately after spinal cord injury, followed by a use-dependent or rehabilitation-mediated phase that can drive dynamic map changes on a timescale of weeks to months after injury, in rodent models. It is obvious that the therapeutic alteration of injured spinal cord circuits will require a commensurate level of reorganization within supraspinal motor centers in order to support the recovery of function. It is conceivable that the induction of cortical circuit plasticity is a viable strategy for supporting recovery from spinal cord injury, however, the functional relevance of cortical reorganization after injury and the mechanisms supporting it require further study. The use of systems-level imaging and optogenetics approaches allow for these motor networks to be studied and manipulated over time, which will undoubtedly be an immense advantage in the development of therapeutic interventions for motor impairments due to spinal cord injury or other neurological injuries.The work was supported by funding to Edmund R. Hollis II (The Winifred Masterson Burke Foundation). We would like to acknowledge the contribution of Sydney Agger in providing the illustration.
Yue Li, Edmund R. Hollis II*
Burke Medical Research Institute, White Plains, New York, NY, USA (Li Y, Hollis II ER)
Brain and Mind Research Institute, Weill Cornell Medical College, New York, NY, USA (Hollis II ER)
*Correspondence to:Edmund R. Hollis II, Ph.D., edh3001@med.cornell.edu.
Accepted:2017-05-05
orcid:0000-0002-4535-4668 (Edmund R. Hollis II)
How to cite this article:Li Y, Hollis II ER (2017) The role of motor network reorganization during rehabilitation. Neural Regen Res 12(5):745-746.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Open peer reviewer:Samit Chakrabarty.
Additional file:Open peer review report 1.
Conner JM, Chiba AA, Tuszynski MH (2005) The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron 46:173-179.
Endo T, Spenger C, Tominaga T, Brene S, Olson L (2007) Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain 130:2951-2961.
Fu M, Yu X, Lu J, Zuo Y (2012) Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 483:92-95.
Ghosh A, Peduzzi S, Snyder M, Schneider R, Starkey M, Schwab ME (2012) Heterogeneous spine loss in layer 5 cortical neurons after spinal cord injury. Cereb Cortex 22:1309-1317.
Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H (2015) Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525:333-338.
Hilton BJ, Anenberg E, Harrison TC, Boyd JD, Murphy TH, Tetzlaff W (2016) Re-establishment of cortical motor output maps and spontaneous functional recovery via spared dorsolaterally projecting corticospinal neurons after dorsal column spinal cord injury in adult mice. J Neurosci 36:4080-4092.
Hollis II ER, Ishiko N, Yu T, Lu CC, Haimovich A, Tolentino K, Richman A, Tury A, Wang S-H, Pessian M, Jo E, Kolodkin A, Zou Y (2016) Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat Neurosci 19:697-705.
Kawai R, Markman T, Poddar R, Ko R, Fantana Antoniu L, Dhawale Ashesh K, Kampff Adam R, Ölveczky Bence P (2015) Motor cortex is required for learning but not for executing a motor skill. Neuron 86:800-812.
Kleim JA, Barbay S, Nudo RJ (1998) Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol 80:3321-3325.
Peters AJ, Chen SX, Komiyama T (2014) Emergence of reproducible spatiotemporal activity during motor learning. Nature 510:263-267.
Wang L, Conner JM, Rickert J, Tuszynski MH (2011) Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proc Natl Acad Sci U S A 108:2545-2550.
Wang L, Conner JM, Nagahara AH, Tuszynski MH (2016) Rehabilitation drives enhancement of neuronal structure in functionally relevant neuronal subsets. Proc Natl Acad Sci U S A 113:2750-2755.
10.4103/1673-5374.206641
杂志排行
中国神经再生研究(英文版)的其它文章
- Cerebral mechanism of puncturing at He-Mu point combination for functional dyspepsia: study protocol for a randomized controlled parallel trial
- Therapeutic opportunities and challenges of induced pluripotent stem cells-derived motor neurons for treatment of amyotrophic lateral sclerosis and motor neuron disease
- Inhibition and enhancement of neural regeneration by chondroitin sulfate proteoglycans
- Collapsin response mediator protein-2 plays a major protective role in acute axonal degeneration
- Hypoxia inducible factor-1 alpha stabilization for regenerative therapy in traumatic brain injury
- Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury
