Superhydrophobic modification of ceramic membranes for vacuum membrane distillation☆
2017-06-01YanhuiYangQianqianLiuHaizhiWangFushengDingGuoshanJinChunxiLiHongMeng
Yanhui Yang,Qianqian Liu,Haizhi Wang,Fusheng Ding,Guoshan Jin,Chunxi Li,Hong Meng*
College of Chemical Engineering,Beijing University of Chemical Technology,Beijing 100029,China
1.Introduction
Membrane distillation(MD)is an emerging low-cost,energyefficient membrane separation technology,requiring a robust membrane with porosity and hydrophobicity[1,2].For the membrane materials used in MD process,generally polymeric membranes are easily fabricated,and have a high packing density in membrane module[3-7].However,these polymeric membranes perform poorly and are always restricted for use under some harsh conditions,because of their low mechanical strength,short lifespan and poor in heat resistibility.Inorganic membranes(i.e.,ceramic membrane)are always prepared from metal oxides such as Al2O3,TiO2,ZrO2,and SiO2,having many advantages over organic membranes,such as the high temperature resistance,high chemical stability,high mechanical strength,strong antimicrobial activity,adjustable pore structure and high separation efficiency[8-10].The inorganic membrane is always hydrophilic because of the hydroxyl groups existing on the surface,which is not conducive to the MD process[11].In order to improve the hydrophobicity of ceramic membranes made of metal oxides,it is necessary to modify membrane with organic groups such as fluoroalkylsilanes.Picard[12]fabricated a hydrophobic ceramic membrane with a high surface water contact angle of 145°.Similarly,Krajewski[2]obtained a membrane with a water contact angle close to 120°by surface modification using fluoroalkylsilanes.Khemakhem[13]performed experiments on grafting fluoroalkylsilanes onto a clay mineral membrane,and the obtained water contact angle on grafted membrane surface was 141°.The fabricated membrane had a flux of 17.0 kg·m-2·h-1and a retention ratio of 93%for desalination of a 0.1 mol·L-1NaCl solution.The group of Zhang[14]prepared silicon nitride hollow- fiber membranes with a water contact angle of 136°for seawater desalinationviaa direct contact membrane distillation experiment.Experimental results showed that the membrane flux was 27.77 kg·m-2·h-1,and its retention ratio was about 99%.Ren and coworkers[15]fabricated hydrophobic alumina planar membranes for water desalination.They found that the contact angle on their grafting membrane was 133°,the MD flux was 19.1 kg·m-2·h-1,and its retention ratio was about 99.5%for a 2 wt%NaCl solution.

Fig.1.Reaction mechanism for the superhydrophobic modification of ceramic membrane.
In this experiment,1H,1H,2H,2H-perfluorooctyltrichlorosilane(PFAS)and 1H,1H,2H,2H-perfluorodecyltriethoxysilane(PFDS)were selected as modifiers.The modifier molecules are stable since their end groups contain fluorine,which has low surface energy and high hydrophobicity.The modifier grows perpendicular to the membrane[16].All of the above characteristics enable the construction of a rough surface.Molecules containing highly active groups such as¯¯Cl and¯¯OCH2CH3easily undergo hydrolysis and substitution by hydroxyl groups and subsequent dehydration.The morphological and physicochemical changes of the resulting membranes were analyzed by scanning electron microscopy(SEM)and X-ray diffraction(XRD).Hydrophobicity was characterized by a contact angle analyzer.The resulting membrane was used for vacuum membrane distillation of 1 wt%NaCl solutions.The variations of VMD performance with modi fication conditions were intensively investigated.
2.Experimental
2.1.Materials
Al2O3ceramic membranes(mean pore size:200 nm,thickness:2.5 mm,porosity:35%;water contact angle:46°)were kindly provided by the Materials Laboratory of Nanjing University of Technology(Nanjing,China).PFAS and PFDS were obtained from Sigma-Aldrich(Shanghai,China).NaCl,anhydrous ethanol,and benzophenone were obtained from Aladdin Chemistry Company(Shanghai,China).Deionized(DI)water was prepared by the Beijing University of Chemical Technology(Beijing,China).
2.2.Preparation of hydrophobic membranes
To improve the membrane performance in vacuum membrane distillation(VMD),PFAS and PFDS were used as reagents for hydrophobic modifications and absolute ethanol as solvent.An alkali-pretreated ceramic membrane was placed in a Petri dish and then combined with the hydrophobic modification reagent.The recommended immersion depth for the membrane was 0.5 mm.The culture dish was covered with a quartz plate,and the reaction was carried out under ultraviolet irradiation for 30 min to produce the grafted polymer membrane.The membrane was then washed with anhydrous ethanol and DI water to remove unreacted modification reagent and dried in a vacuum oven at 100°C for 45 min.The aforementioned grafting steps were done several times.After each grafting step,the modification regent was replenished for the next immersion.Finally,the dried grafted membrane was stored under a dry,clean atmosphere.
2.3.Characterization
The contact angle on the membrane samples was measured with a Data-Physics OCA-20 contact angle analyzer(DataPhysics Instruments GmbH,Stuttgart,Germany).Scanning electron microscope(SEM)imaging of the membrane was carried out on a Hitachi s-4300 scanning electron microscope(Hitachi Limited,Tokyo,Japan).X-ray photoelectron spectroscopy(XPS)of surface elements was done by using an ESCA250(Thermo Fisher Scientific,Shanghai,China)XPS system.

Fig.2.Schematic of the experimental setup for vacuum membrane distillation:(1)thermostatic water bath;(2)material tank;(3)peristaltic pump;(4)membrane module;(5)condenser;(6)water collector;(7)vacuum pump;(8) flow meter;(9,10)thermometers;(11)vacuum gauge.
2.4.VMD experiment
The PFAS grafted planar ceramic membranes were used in the experiment on seawater VMD.Fig.2 shows the setup for the experiment.Ahydrophobic modified Al2O3ceramic membrane(effective membrane area=4.91×10-4m2)was placed and then fastened in the membrane module.The module was then connected to a material tank and peristaltic pump.Next,a coiled condenser and condensate water collector were connected.One end of the former was connected to the steam outlet of the membrane module,and the latter was connected to a vacuum pump.The feed solution was heated in a thermostatic water bath to the target temperature(75°C)and then held at that temperature.The peristaltic pump was then opened,and the feed solution was circulated between the thermostatic water bath and membrane module.Subsequently,the vacuum pump was switched on.The cold side of the membrane module,coiled condenser,and water collector form a stable vacuum:steam is forced from the hot side of the membrane to the cold side and then condensed by the condenser and collected into a water collector.The temperature of the cold side was kept at-10°C.
2.5.Membrane characterizations
The products were analyzed by ICS-900 Ion Chromatography.The experimental flux(J)could be calculated as follows[17]:


Fig.3.SEM images of the membrane(A,C)before and(B,D)after modification.

Fig.4.XPS spectra of the membrane surface.
wherem,Aandtwere the mass of the permeate,membrane area and operation time,respectively.
中共中央、国务院日前发布《关于建立更加有效的区域协调发展新机制的意见》(以下简称《意见》),要求“加快形成统筹有力、竞争有序、绿色协调、共享共赢的区域协调发展新机制”。
The rejection rate(R)could be expressed by:

wherecfwas the initial concentration of the component in the feed solution andcpwas the concentration of the component in the rejection.
3.Results and Discussion
3.1.Morphological and physicochemical changes
SEM images of the original and grafted membranes are shown in Fig.3.As shown in Fig.3 A,C,the pore sizes and particle sizes on the surface of original Al2O3membrane were distributed uniformity.The contact angle on the original membrane was 46°because of the even distribution of hydroxyl groups.Fig.3 B,D showed that the grafted membrane had a polymer layer above a layer with prominent clusters,resulting in high surface roughness of the membrane.The contact angle of the grafted ceramic membranes was 159°,which was much higher than those without ultraviolet grafting and reached superhydro phobic range.It was clearly observed that they had a polymer layer formed by monomer dehydration condensation between PFAS and the ceramic membrane surface.In addition to the fluorocarbon chain itself having a good hydrophobic property,the formation of needle like and cluster structure was also an important factor in superhydrophobicity of the membrane.Before and after modification,the pore structure of the membrane changed little,and the range of the membrane was concentrated in 25%-32%by desorption of nitrogen method.
Surface X-ray photoelectron spectra of the ungrafted and grafted ceramic membranes are shown in Fig.4.Characteristic O1s and Al2p peaks at 531.1 and 74.62 eV,respectively,were due to the ungrafted ceramic membrane.The F1s peak at 687.46 eV indicated an organic polymer membrane on the surface of the ceramic channels.The peaks at 860 and 833 eV were Auger peaks for F.Relaxation of fluorine upon irradiation of the membrane surface implied its presence on the membrane surface.The C1s absorption peak consisted of overlapping peaks at 285.5 and 290.5 eV.X-ray photoelectron spectra of the ungrafted and grafted ceramic membranes may be compared in Fig.4.The absorption peak of fluorine of the grafted ceramic surface was distinct,in contrast to that of the ungrafted membrane.The degree of absorption by the other major elements changed mainly because of grafting of PFAS onto the ceramic membrane surface through its fluorocarbon chain.
3.2.Variations of hydrophobicity with modifier,photoinitiator and ultraviolet irradiation
Ceramic membranes were grafted with PFAS and PFDS in alcohol solution at room temperature.The concentrations of both modifier solution and diphenylketone(BP)were 0.01 mol·L-1,and modification was done five times.Contact angles of the membranes modified with PFAS and PFDS(Fig.5)were both 143°.The molecular structures of fluorine-containing carbon chains of PFAS and PFDS were very similar.The results thus indicated that although the chain of the latter was longer than that of the former,the properties and surface morphology of the grafted membranes were very similar(Fig.5).
The modifier concentration was a key factor affecting the modification.As shown in Fig.6,the contact angle first increases and then decreased with the increase in modifier concentration.When the modifier concentration was 0.0005 mol·L-1,the contact between monomers in solution and active sites on the membrane surface was influenced by shielding by solvent molecules and photosensitive molecules,which decreases the density,uniformity of grafted molecules on the membrane surface,and contact angle.When the modifier concentration increased from 0.0005 to 0.01 mol·L-1,the amount of monomers in the graft solution increased gradually and reached moderate density,which prevented self-polymerization and ensured maximum probability of contact between monomers and active sites.When the modifier concentration was 0.01 mol·L-1,the hydrophobicity increased,and the contact angle on the grafted membranes reached maximum.The largest contact angle on the grafted membranes was 159°.When the modifier concentration increased from 0.01 to 0.1 mol·L-1,the modifier concentration was too high.This excess led to selfpolymerization,forming polymerchains.F on the surface of the polymer chains buried the Si¯¯O bond in the middle of each polymer chain.Consequently,the probability of contact between monomers and active sites on the membrane surface increased drastically,thereby interfering with grafting.
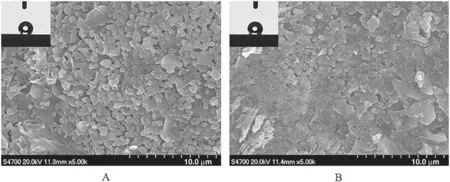
Fig.5.Effect of the surface modifiers(A)1H,1H,2H,2H-perfluorooctyltrichlorosilane and(B)1H,1H,2H,2H-perfluorodecyltriethoxysilane on the water contact angle;SEM images of membranes modified with(A)1H,1H,2H,2H-perfluorooctyltrichlorosilane and(B)1H,1H,2H,2H-perfluorodecyltriethoxysilane).

Fig.6.Effect of modifier concentration on the contact angle.
The effect of the modification cycles on membrane hydrophobicity was also investigated.As shown in Fig.7,the contact angle increased rapidly from 126°to a maximum of 159°as the number of cycles of modification increased from one to five,thereafter decreasing with increasing number of times.The contact angle decreased to 153°and 150°,respectively,after the eighth and tenth modification cycles.

Fig.7.Effect of the number of cycles of modification on the contact angle.
Ultraviolet photografting led to the formation of chemical bonds between modifier molecules and¯¯OH active sites on the membrane surface,thus forming a permanentand stable hydrophobic layer.Each PFAS molecule was anchored to the surfaceviareaction with at least one hydroxylgroup.The number of hydroxyl groups on the membrane surface and their uniformity was directly related to the modification effect.In the first modification,PFAS partially was bonded to surface hydroxyl groups.With the increasing number of modification cycles,modifier molecules were bonded to unreacted hydroxyl groups,thus increasing the hydrophobicity of the membrane.When all of the hydroxyl groups on the membrane surface had bound to modifier molecules,Si¯¯O bonds became incorporated into the membrane surface and long chains containing fluorine moved toward the exterior,thereby increasing the surface roughness.When the surface was covered with fluorinecontaining long chains,modifier molecules could not bond with the membrane surface.The contact angle therefore increased with increasing number of modifications.After five cycles of modification,a hydrophobic polymer layer completely covered the membrane surface,and further accumulation of the modifier decreased the surface roughness.Consequently,the contact angle no longer increased with increasing number of modifications,slightly decreasing instead.
Different amounts of the photoinitiator BP,which aids reaction were added to the modifier solution(0.01 mol·L-1).The ceramic membrane was immersed in the hydrophobic modifier solution at room temperature and then irradiated with ultraviolet light for 30 min.Afterward,it was cleaned and dried at 100°C for 45 min.Contact angles at various BP concentrations are listed in Table 1.

Table 1Variations in contact angle with the BP concentration
The experimental data indicated that at certain BP concentrations(0-0.01 mol·L-1),the contact angle increased with increasing concentration.However,it decreased with increasing BP concentration when the BP concentration was higher than 0.01 mol·L-1.The maximum contact angle was 159°,which was obtained at 0.01 mol·L-1.
The effect of BP was greater at low concentration,activating hydroxyl groups on the membrane surface and improving reactivity.However,when the BP concentration was too high,BP slowed the grafting reaction and decreased reactivity,thus decreasing the grafting efficiency.
In the experiment,the solutions of the modifiers PFAS and BP alcohol at three different concentrations were used.The concentrations of PFAS were 0.0005,0.005,and 0.01 mol·L-1and BP was 0.01 mol·L-1.The effect of ultraviolet irradiation on the contact angle at different concentrations is depicted in Fig.8.At the three concentrations,contact angles on the surface of the grafted membrane under ultraviolet irradiation were 143°,145°,and 159°respectively,and those on the membrane without ultraviolet irradiation were 137°,140°,and 152°.The number of hydroxyl groups and the grafting density of modifier monomers on the membrane were high.Treatment of the ultraviolet-irradiated membrane with BP solution generated surface free radicals and transferred ungrafted modifier monomers and hydroxyl group to the surface of membrane,thereby increasing the reaction rate.Increasing the grafting density increased coverage of the membrane surface by the grafting molecules.The grafting efficiency improved because of the increase in reaction rate.

Fig.8.The influence of ultraviolet irradiation on the contact angle.
3.3.Saline-water desalination by VMD
The fluxes of the MD process were related to the temperature difference and average temperature of the feed side and permeate side.A high average temperature difference between the feed side and permeate side led to greater permeate flux in the MD process.The relationships between the flux through the ceramic membrane,rejection rate,and temperature in the desalination process are depicted in Fig.9.The permeate flux in the VMD desalination process increased exponentially with the increase in temperature(Fig.9).This trend was due to the exponential relationship between the equilibrium vapor pressure of solution and the temperature:increasing the temperature caused an increase in equilibrium vapor pressure difference across the membrane,that is,the driving force.For PFAS modified ceramic Al2O3ceramic membrane(the contact angle of 159 °),at feed temperatures of 35 °C and 75°C,the VMD fluxes in the desalination process were 2.5 and 23.22 kg·m-2·h-1,as indicated in Fig.9.There were slight differences between the experimental data and the theoretical linear relationship probably because of the effect of the temperature difference and concentration polarization.

Fig.9.Dependence of the permeate flux and rejection rate on the feed temperature.
Theoretically,water in the gas phase permeated through pores during VMD desalination,whereas NaCl,which was nonvolatile,cannot.Thus,the rejection rate of the experimental study(99.9%)may be the result of minimal defects in the membrane.
Fig.10 depicts the effect of the feed concentration on the flux and on the interception rate.The feed temperature was 75°C,and the initial water flux was 33.0 kg·m-2·h-1.When the feed concentration was 3 wt%,the water flux decreased to 10.39 kg·m-2·h-1.This was because the higher concentration NaCl solution caused the concentration polarization on the feed side.With the increase of feed concentration on membrane surface,the activity of water reduced.Therefore,both the membrane surface temperature and saturated steam pressure decreased.However,this effect was relatively weak.Although the increase in concentration led to a decrease in flux,the flux remained high.The reason for the effect of concentration polarization was the decrease in the transfer rate due to the existence of the boundary layer in the membrane[18].
The interception rate did not decrease with the increase in feed concentration,staying at>99.95%.This result suggested that the membrane pore sizes were distributed uniformly,which led to stable performance.

Fig.10.Dependence of the permeate flux and rejection rate on the feed concentration.
The dependence of the VMD flux and rejection rate on the vacuum pressure is described in Fig.11.When the vacuum pressure in the experiment was less than 0.09 MPa,the flux was too small to cause condensate to accumulate on the cold side,preventing us from obtaining any data.When the vacuum pressure was 0.098 MPa,the flux was 23.22 kg·m-2·h-1at a feed temperature of 75 °C.When the vacuum pressure was 0.090 MPa,the flux decreased by 32.8% to 15.6 kg·m-2·h-1.In contrast to other MD processes,negative pressure on the permeate side in the VMD process was produced by applying a vacuum,and steam was removed from the membrane module under negative pressure.The higher vacuum degree in the permeate side would cause the higher the steam pressure difference,which was the mass transfer driving force through the membrane,subsequently led to the higher the flux[19].Energy loss due to heat transfer without the heat-transfer medium,which decreased the effect of temperature polarization and energy loss,could be neglected.
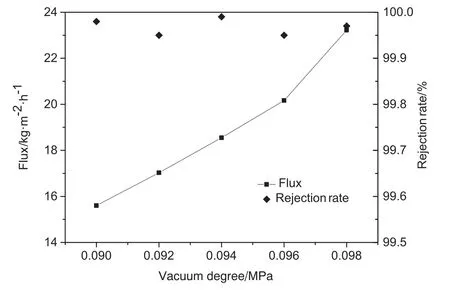
Fig.11.Dependence of the permeate flux and rejection rate on the vacuum pressure.
We therefore examined the effect of vacuum pressure on the process under the operating conditions.Upon analysis of the factors in the experiment,such as feed temperature,feed velocity,and cold vacuum pressure,we found that the optimal vacuum pressure is 0.098 MPa.
The feed flow rate affected the extent of mixing of the feed flow in the membrane module in the VMD process.With increasing feed flow rate,the heat-transfer coefficient increased and the thickness of the boundary layer decreased,thereby inhibited temperature polarization and concentration polarization.Under the experimental conditions,crystals on the membrane surface formed when the feed flow rate was below 16 L·h-1.The highest flux in the saline-water desalination experiment was 27.28 kg·m-2·h-1when the feed flow rate was 24 L·h-1.With increasing feed flow rate,a few crystals appeared on the membrane surface(Fig.12).Their appearance may be due to a difference in the boundary layers formed at high and low concentration and concentration polarization,with the effect being more significant at high concentration.This was consistent with the conclusions of a study by Tunet al.[20]on membrane fouling with NaCl solutions at high concentrations.They found that the flux decreased slowly before the point of the desired supersaturation.Using ion chromatography to transform Cl-of the condensate,they found that the Cl-content was(1-3)×10-6and that the membranes'retention rate was>99.8%.Although the retention rate decreased slightly with the increase in feeding rate,the decrease was small.
Fig.12.Images of the membrane used in vacuum membrane distillation.
4.Conclusions
The membrane surface hydrophobicity was one of the most important factors influencing the membrane distillation.In this work,we applied the ultraviolet irradiation to the fluoroalkylsilane modification and tune the ceramic surface to superhydrophobicity.It was noted that water contact angle could dramatically increase from 46°to 159°after ultraviolet grafting with fluoroalkylsilane.The effects of modification conditions on membrane performance,such as modifier,photoinitiator and ultraviolet irradiation were intensively investigated.The optimum concentration of modifier was 0.01 mol·L-1and the optimum number of modification cycles for the membrane was five.Moreover,the uses of this superhydrophobic ceramic membrane for membrane distillation were systematically evaluated under various operating conditions.It was noted that the superhydrophobic membrane exhibited an acceptable selectivity and high flux.For the 1%NaCl solution(75°C),the flux reached a maximum of 27.28 kg·m-2·h-1,and the retention rate was>99.8%(maximum of 99.99%).Therefore,the ultraviolet grafting with fluoroalkylsilane is proven to be a facile approach to tune the ceramic surface to superhydrophobicity,which opens a new way to achieve high performance membrane for saline-water distillation.
[1]M.S.El-Bourawi,Z.Ding,R.Ma,M.Khayet,A framework for better understanding membrane distillation separation process,J.Membr.Sci.285(2006)4-29.
[2]S.R.Krajewski,W.Kujawski,M.Bukowska,C.Picard,A.Larbot,Application of fluoroalkylsilanes(FAS)grafted ceramic membranes in membrane distillation process of NaCl solutions,J.Membr.Sci.281(2006)253-259.
[3]P.Peng,A.G.Fane,X.D.Li,Desalination by membrane distillation adopting a hydrophilic membrane,Desalination173(2005)45-54.
[4]F.Edwie,M.M.Teoh,T.S.Chung,Effects of additives on dual-layer hydrophobichydrophilic PVDF hollow fiber membranes for membrane distillation and continuous performance,Chem.Eng.Sci.68(1)(2012)567-578.
[5]M.Gryta,Influence of polypropylene membrane surface porosity on the performance of membrane distillation process,J.Membr.Sci.287(2007)67-78.
[6]M.Khayeta,J.I.Menguala,T.Matsuurab,Porous hydrophobic/hydrophilic composite membranes application in desalination using direct contact membrane distillation,J.Membr.Sci.252(2005)101-113.
[7]J.Zhang,J.D.Li,M.Duke,Z.Xie,S.Gray,Performance of asymmetrical hollow fibre membranes in membrane distillation under various configurations and vacuum enhancement,J.Membr.Sci.362(2010)517-528.
[8]M.Arzani,H.R.Mahdavi,O.Bakhtiari,T.Mohammadi,Preparation of mullite ceramic micro filter membranes using Response surface methodology based on central composite design,Ceram.Int.42(7)(2016)8155-8164.
[9]J.Malzbender,Mechanical aspects of ceramic membrane materials,Ceram.Int.42(7)(2016)7899-7911.
[10]J.-W.Wang,N.-X.Li,Z.-R.Li,J.-R.Wang,X.Xu,C.-S.Chen,Preparation and gas separation properties of Zeolitic imidazolate frameworks-8(ZIF-8)membranes supported on silicon nitride ceramic hollow fibers,Ceram.Int.42(7)(2016)8949-8954.
[11]M.M.Gentleman,J.A.Ruud,Role of hydroxyls in oxide wettability,Langmuir26(2010)1408-1411.
[12]C.Picard,A.Larbot,E.Tronel-Peyroz,R.Berjoan,Characterisation of hydrophilic ceramic membranes modified by fluoroalkylsilanes into hydrophobic membranes,Solid State Sci.6(2004)605-612.
[13]S.Khemakhem,R.B.Amar,Grafting of fluoroalkylsilanes on micro filtration Tunisian clay membrane,Ceram.Int.37(2011)3323-3328.
[14]J.W.Zhang,H.Fang,J.W.Wang,L.Y.Hao,X.Xu,C.S.Chen,Preparation and characterization of silicon nitride hollow fiber membranes for seawater desalination,J.Membr.Sci.450(2014)197-206.
[15]C.Ren,H.Fang,J.Gu,L.Winnubst,C.Chen,Preparation and characterization of hydrophobic alumina planar membranes for water desalination,J.Eur.Ceram.Soc.35(2015)723-730.
[16]D.Schondelmaier,S.Cramm,R.Klingeler,J.Morenzin,C.Zilkens,W.Eberhardt,Orientation and self-assembly of hydrophobic fluoroalkylsilanes,Langmuir18(2002)6242-6245.
[17]W.Cao,I.M.Mujtaba,Simulation of vacuum membrane distillation process for desalination with Aspen Plus,Ind.Eng.Chem.Res.54(2)(2015)672-680.
[18]K.W.Lawson,D.R.Liold,Membrane distillation.II:direct contact MD,J.Membr.Sci.120(1996)123-133.
[19]A.O.Imdakm,M.Khayet,T.Matsuura,A Monte Carlo simulation model for vacuum membrane distillation process,J.Membr.Sci.306(2007)341-348.
[20]C.M.Tun,A.G.Fane,J.T.Matheickal,R.Sheikholeslami,Membrane distillation crystallization of concentrated salts— flux and crystal formation,J.Membr.Sci.257(2005)144-155.
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Heat transfer and friction factor of Therminol liquid phase heat transfer fluid in a ribbed tube☆
- A numerical study on heat transfer enhancement and design of a heat exchanger with porous media in continuous hydrothermal flow synthesis system
- Computationalanalysis of the flow ofpseudoplastic power-law fluids in a microchannel plate
- Investigation of droplet breakup in liquid-liquid dispersions by CFDPBM simulations:The influence of the surfactant type
- Mixed convection characteristics in lid-driven cavity containing heated triangular block
- Synthesis,characterization and application of PVA/ionic liquid mixed matrix membranes for pervaporation dehydration of isopropanol
