非水溶剂Li-O2电池锂负极研究进展
2017-05-10张彦涛刘圳杰王佳伟王亮彭章泉
张彦涛 刘圳杰 王佳伟 王亮 彭章泉,*
(1中国科学院长春应用化学研究所,电分析化学国家重点实验室,长春130022;2中国科学院大学,北京100049)
非水溶剂Li-O2电池锂负极研究进展
张彦涛1,2刘圳杰1,2王佳伟1王亮1,2彭章泉1,*
(1中国科学院长春应用化学研究所,电分析化学国家重点实验室,长春130022;2中国科学院大学,北京100049)
非水溶剂Li-O2电池因其高的理论能量密度,近年来备受关注。非水溶剂Li-O2电池的典型结构为金属锂负极、含Li+的非水溶剂电解液和多孔氧气正极。目前,多数Li-O2电池研究集中在正极的氧气电极反应;金属锂负极极强的还原性导致的副反应使Li-O2电池中的化学和电化学反应变得更为复杂。因为,电解液和从正极扩散来的O2都会与金属锂发生反应;锂负极上生成的副反应产物同样会扩散到正极一侧,干扰正极的O2反应。此外,锂负极上可能生成锂枝晶,降低电池的安全性能,进而阻碍Li-O2电池的实用化。因此,研究并解决锂负极的电化学稳定性和安全问题迫在眉睫。本文综述了近年来国内外在非水溶剂Li-O2电池锂负极保护和修饰方面的最新研究进展,包括:可替代的对/参比电极的选择、电解液和添加剂、复合保护层与隔膜的研究、先进实验技术的开发与应用、并针对未来非水溶剂Li-O2电池的发展进行了展望。
锂-氧电池;金属锂负极;锂负极稳定性;固态电解质界面膜
Key Words:Lithium-oxygen battery;Lithium metalanode;Lianode stability;Solid electrolyte interphase layer
1 Introduc tion
Confronted with the rapidly grow ing demands forhigh density energy storage applications,the aprotic Li-O2batteries,which are based on the Li-O2reaction of 2Li+O2⇌Li2O2(EӨ=2.96V(vs Li/Li+))with anultra-high theoreticalspecificenergy of 3500Wh· kg-1,have been proposed to be a holy grail for electric energystorageapplications1-8.Asearly as1996,theaprotic Li-O2battery was devised by Abraham and Jiang9.Subsequently,incessant effortshavebeenmade to contribute its further research and development.Despite considerate progresses,abunch of scientific and technological issues still impede the realization of practical Li-O2 cells,which include degraded capacity,limited cycle life, low energy efficiency,and instability of cell com ponents. Sofar,themajority of Li-O2 studies,using a standard configuration of Li|aprotic Li+electrolyte|porous cathode(O2),focuson theO2 reactions in the cathode.To alleviate theabovementioned problems,novel catalysts10-16,electrolytes17-22 and binders23,24 have been used.In addition,fundamental studies of understanding of the reactionmechanismsunder variousoperating conditions25-28 have been reported.However,the stability of the Lianode isstill another crucial issueand should notbe ignored.Especially,the reactions arising from themetallic Li becomemuchmore complicated w hen oxygen and water are involved in the electrolyte. Therefore,O2 shuttle is oneof themajor culprits thatcan cause passivation/parasitic reaction on Limetalanode.Moreover,some of the side reaction products could cross over to the cathode then interferew ith O2 electrochem istry,and degrade the overall performanceof Li-O2 cells29-31.In this light,stabilizing the Limetal anode interface could be as challenging as addressing the reversibility in air-cathode.Besides,Limetal electrode has been plagued for decadesw ith the problem of the dendrite formation during repeated stripping/plating,which lead to serious safety concernsand poor Coulombic efficiency32,33.

ZHANG Yan-Tao,received his Bachelor degree of Applied Chemistry in Heibei University of Science and Technology in 2012.He earned his master degree from Tianjin University of Technology.Since 2015,he is pursuing a Ph.D.under the supervision of Prof.PENG Zhang-Quan at Changchun Institute of Applied Chem istry (CIAC),Chinese Academy of Sciences(CAS).His current research interest is focused on themetallic anode in rechargeable aproticmetal-oxygen batteries.

LIU Zhen-Jie,comes from Shandong province and obtained his Bachelor degree in Department of Materials Chem istry at University of Jinan in 2013.He isnow aMaster student(M 3)in Prof.PENG Zhang-Quan′s group at Changchun Institute of Applied Chemistry(CIAC),Chinese Academy of Sciences(CAS).Recently,he is interested in the lithium deposition and stripping in different solvents and lithium anode protection.

Wang Jia-Wei,received his Ph.D.from Northeast Normal University in 2012 majored in Physical Chem istry.In 2012,he joined Professor PENG Zhang-Quan′s group at Changchun Institute of Applied Chemistry (CIAC),Chinese Academy of Sciences (CAS),and worked as a Research Associate. His current research interest is focused on the understanding of fundamentalmechanisms in metal-oxygen batteries through SERS technique and various traditional electrochem ical techniques.

WANG Liang,obtained his Bachelor degree in Instituteof PolymerMaterialsatSchoolof M aterials Science&Engineering,Shandong University in 2015.He is now a 2nd year Master student in Prof.PENG Zhang-Quan′s group at Changchun Institute of Applied Chemistry(CIAC),Chinese Academy of Sciences(CAS).His current research interest is focused on the fundamentalmechanisms of lithium anode in rechargeableaprotic lithium-oxygen batteries.

PENG Zhang-Quan,obtained his Bachelor in Wuhan University in 1997 and received his MSc and Ph.D.in Analytical Chem istry from Changchun Institute of Applied Chem istry (CIAC),Chinese Academy of Sciences (CAS)in 2000 and 2003,respectively.He followed up by working as a postdoctoral fellow at University of Dusseldorf,Germany and University of Aarhus,Denmark,and asa Research Associate under the direction of Prof.Peter G.Bruce at University of St Andrews,UK from 2004 to 2012.Hewas selected in Recruitment Program of Global Youth Experts in 2012.Now,his current research interests are associated w ith interfacial electrochem istry, in situ spectra electrochemistry to study the fundamentalmechanismsof oxygen electrode reactions in aprotic Li-O2batteries.
Toward a better understanding of the Li-O2reactions and realization of practical aprotic Li-O2batteries,it is critically necessary to develop novel strategies to im prove the stability of lithium metal electrode especially in the presence of O2.In this paper,the recentadvances in fundamentalscience of improving themetallic lithium stability have been reviewed,including(i) alternative counter/reference electrodes that reactw ith neither electrolyte nor O2;(ii)solid electrolyte interphase(SEI)modification,including in situ SEIformation and various artificial SEI; (iii)experimental techniques.Finally,we propose a perspective on challenges and opportunities for Limetalelectrode used in aprotic Li-O2batteries.
2 A lternative counter/re ference elec trodes
Limetal is recognized as themost promising anodematerial due to its high theoretical specific capacity(3860mAh·g-1)as wellas the lowestelectrochemicalpotential(-3.04V,vs SHE(the standard hydrogen electrode)).Theuseof the Limetalelectrode accompanied by safety concerns associated to itswell-known reactivity,possible dendrite formation leading to short circuit34-36, remainsgreat challenge on commercialization of Limetalbatteries (LMBs),Li-sulfur(Li-S)batteries and Li-oxygen(Li-O2)batteries2,4,37.M oreover,for Li-O2batteries operated under ambient atmosphere,the parasitic reactionsof Limetal and the permeated O2in theelectrolyte can notbeeliminated completely.The lithium metal can,in fact,reactwith theO2solubilized in theelectrolyte toform lithium oxideor hydroxide at themetalsurface,resulting in the increase of the cell resistance and polarization38,39.The replacementof the lithium metal by alternative lithium ion/alloy material represents one of the possible solutions to overcome the aforementioned issues.
To identify thealternative counter/referenceelectrodematerials, severalworks havebeen reported.In 2012,Bruce etal.40initially introduced lithium iron phosphate(LiFePO4)as counter electrode to replace Limetal used in Li-O2batteries due to instability of metallic lithium toward dimethylformamide(DMF)electrolyte. For the sim ilarly reason,M cCloskey et al.41also adopted LiFePO4as the anode to conduct the quantitative analysis of O2using differential electrochem ical mass spectrometry(DEMS)in acetonitrile(CH3CN)electrolyte.As shown in Fig.1a,the partially charged Li1-xFePO4acting asnotonly counterelectrode(Li+rich) but also reference electrode(stabilized at~3.45 V,vs Li/Li+), involving in a two-phase reaction of Li1-xFePO4and FePO4,can significantly reduce the contam inations from the electrolyte decomposition on Li anode42.Thus,the obtained clean electrochem ical system of Li-O2batteries can ensure a better understanding of the reactionmechanisms and quantitativemeasurement of the O2reversibility43,44.From this view,the Li-ion intercalation materials,which reactw ith neither electrolyte nor O2, display significantpromiseasalternative anodematerialsused in Li-O2batteries.In the study reported by Lee etal.45,graphite and Li4Ti5O12showing high Coulombic efficiencies in Li-ion batteries, were successfully demonstrated their cyclability and reversibility asanode in Li-O2batteries(Fig.1b and 1c).Butsuch kindsof Liion intercalationmaterialsarenotsuitable to replacemetallic Li for rechargeable Li-O2cells,because thehigh energy density of Li-O2batterywasseriously discounted.
Moreover,Hassoun et al.46,38have reported lithium-alloying composites,LixSi-C and LixSn-C electrodes,to replace the unsafe lithium metalanode.Asshown in Fig.1d and 1e,the LixSi-O2and LixSn-O2batteriesdischarged around 2.7 and 2.4V respectively, attributing to the lower anode potentialof LixSi(~0.3 V(vs Li/Li+)) and LixSn(~0.5V(vs Li/Li+)),unlike the LiFePO4(~3.45V(vs Li/ Li+)).A lthough the alloy materials show up big prom ise to the practical lithium ion-O2batteries,slight voltage decay isobserved only after several cycles in Fig.1d and 1e.A functional uniform SEIfilm that can alleviate the attack ofO2,was formed on lithiated Al-carbon(LixAl-C)anode47.As shown in Fig.1f,the invariable dischargeand charge profiles also verified the interfacialstability between LixAl-C and electrolyte.We speculate that the interface stability toward the permeated O2in theelectrolyteand the volume expansion during repeated stripping/plating stillneed to be further improved.
3 SEIm od ification
Asearly as in 1979,Peled49firstly identified the conceptof SEI, which constitutes of various organic and inorganic components and displays the properties of electrically insulating and ionically conducting.The SEI layerw ith a thicknessof~20 nm is formed from thespontaneous reactionsbetweenalkalinemetaland solvent/ slatanions in liquid electrolyte48.Although the consumed Li+used for the formation of SEI leads to a low efficiency,the SEIcan effectively prevent the further physical contact and chemical reaction betw een the Liand electrolyte.In addition,Li ions can be evenly distributed via functionalized SEIduring repeated stripping/ plating and the dendrite-freemorphology can beobtained.In order to protect the Lianode for Li-O2battery better,it is significantly important tomodify SEI.The following summarized theadvanced strategies tomodify SEI layer including adopting new electrolyte systems,additives and some artificial composite protect layers (CPLs).
3.1 Electro ly te and add itives
The conventionalelectrolyte systems in Li-O2batterymostly contain solvents and Li salts3-5.The w idely used organic alkyl carbonate-based and esters-based electrolyteswere demonstrated susceptible to theattack of the superoxide radicalanion and severe decomposed during the chargeand discharge processes.So it is critically necessary to adoptmore chemically/electrochem ically stable ether and glymes-based,e.g.dimethoxyethane(DME), tetraglyme(TEGDME);nitrile-based,e.g.acetonitrile(CH3CN),trimethylacetonitrile(TMA);sulfone-based,e.g.dimethyl sulfoxide(DMSO),amide-based,e.g.N,N-dimethylacetamide(DMA), dimethylformam ide(DMF);ionic liquid-based(IL)and gel/solid state-based electrolytes5.Although theabove-mentioned solvents showed relative stability towards oxygen reduction species,the unstable interphase,such as the SEIformed on the surfaceof the Limetalanode,w ill result in the deterioration of battery performance.
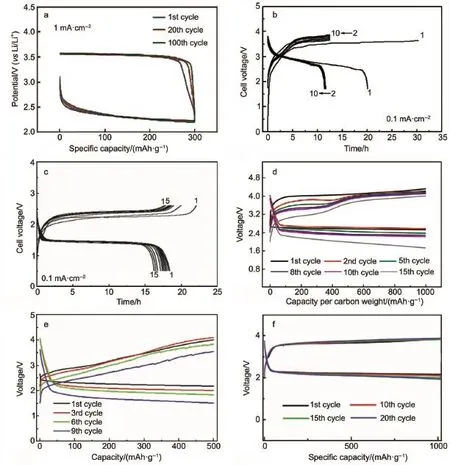
Fig.1 Galvanostatic d ischarge-charge curves of Li ion-O2batteries fab ricated w ith(a)LiFePO442,(b)graphiteand(c)Li4Ti5O1245, (d)LixSi-C46,(e)LixSn-C38and(f)LixAl-C47ascounter/reference electrode instead of Limetalcolor online
In order to enhance the interfacialstability of the straight-chain alkyl amidesw ith the Lianode,lithium nitrate(LiNO3),which previously was show n to improve battery performance by formation of a stable SEIin Li-S battery50-52,has been applied to enhance the interphase compatibility between Limetaland DMA electrolyte in Li-O2systems.Walker et al.53showed that some electroactive species generated from the reaction of Liand DMA, while the inclusion of LiNO3can substantially inhibit this reaction. The compatibility of thissystem with Limetal isattributed to the inertness of the am ide core combined with the nitrate anion contributing to the formation of a protective SEI.Thisassembled Li-O2battery show s com paratively high efficiency and stability over80 cycles(>2000 h)withminimal changes in the voltage in Fig.2a.Subsequently,Giordani et al.29demonstrated the synergistic effectof theO2and LiNO3on the formation of a stable SEI. The low overpotential and durable cyclic performance in symmetric Li-Li cellsunder O2isbetter than Ar as depicted in Fig.2b. Combined Fig.2b w ith 2c,we can conclude thatneither O2nor LiNO3alone is able to stabilize the Limetal interface in DMA. The further studiesabout theeffectof O2combiningw ith additives LiNO3and vinylene carbonate(VC)havebeen investigated on the typical1mol·L-1LiClO4/DMSO electrolyte by Roberts etal.30.As displayed in Fig.2d,the improved cyclenumbersof 25,27 and 33 wereachieved respectivelywith theaddition of O2,LiNO3and VC. It is obvious that the use of oxygen asan additive is sufficient to stabilize the surface,but this result isa little different from thatofGiordani etal.29,in DMA electrolyte.The positiveeffectiveabout theO2stabilizing effect is consistentwith Aurbach′s report54.

Fig.2 Voltage profilesof(a)Li-O2cells53in 1mol·L-1LiNO3/DMA;(b)symmetric Li/Licellunder O2and Ar(inset)in 1m ol·L-1LiNO3/DM A,(c)pressu reand voltage p rofi les of symm etric Li/Licell cycled under O2in 1m ol·L-1LiTFSI/DM A29; (d)Li-Cu cells in 1mol·L-1LiClO4/DMSO electrolytew ith and w ithoutadditives in thep resenceand absence of oxygen301 bar=0.1MPa,color online
However,contradictory resultswere proposed by Assary39and Younesi55et al.,Assary et al.39observed LiOH as well as carbonates rooting in theO2crossover from the cathode to anode in tetraglyme-based electrolyte.Younesi etal.55discussed the SEI components on the Lianodewith 1mol·L-1LiPF6/PC electrolyte used in Li-O2cell for the first time.The X-ray photoelectron spectra(XPS)analysis concludes that the SEI layer on the Li anode constantly changes during the charge and discharge processes in the presentO2,namely theonly existed O2isunable to stabilize the SEI.Theseworse impactsarebased on the unstable ether or carbonate-based electrolytes.The mechanism and reliability of oxygen stabilizing the SEIon the Limetal is complicated and isnot fully understand yet.We speculate that the partial pressureof oxygen,the identity of solvents,saltsand additivesact essential roleon fabricating a stable SEI.
Except those additivesmentioned above,Bryantsev et al.56has demonstrated 2%N,N-dimethyltrifl uoroacetamide(DMTFA)as additiveswhich can effectively stabilize the Lielectrode in 0.5 mol·L-1LiTFSIand 98%DMA.Five different types of fluorinated amidessolventswere investigated to stabilize the lithium/ electrolyte interface as shown in Fig.3a and 3b.The LiTFSI/ DMTFA electrolyte shows the lowest interfacial resistance and exhibits the best cyclic performance.Combined the calculated energy barriers w ith XPS analysis,LiF was recognized to be formed on the Limetal surfacewith littleorno activation energy. Although DMTFA shows reduced ability toform an effective SEI upon extended cycling due to the instability to the O2reduction reactions,the presence of DMTFA significantly im proves the stability of the unprotected Lianode in rechargeable Li-O2battery. Zhang et al.57also discovered LiF in an artificial SEI layer synthesized by pre-charging a symmetric Li|1mol·L-1LiF3SO3-TEGDME-fluoroethylene carbonate|Li cell within a voltage w indow of 0-0.7 V.The cyclic performance of Li-O2batteries assembled w ith thus obtained Limetal significantly improved. Both of the results reveal that LiF is essential composition for stabilizing SEI.
Zhang and co-workers58discovered a self-healing electrostatic shield mechanism by emp loying the Cs+additives in 1mol·L-1LiPF6/PC electrolyte.These additive cations form a positively charged electrostatic shield around the initial grow th tip of the protuberances to alter dendrite formation used in LMBs.On the basic of the above ingenious ideas,Lee and Park59successfullyintroduced CsIasamultifunctional redoxmedium,which coupled with the synergistic function of I-and Cs+,to reduceoverpotential
and im prove the cyclic performance of Li-O2cells.Besides,the addition of the LiNO3salt in theelectrolyteactivates thesemultieffects and strengths the protection ofmetallic Li.Theobviously smooth surfacewithoutcracksand holeseven Lidendrites can be observed in Fig.4a-4d after disassembled the cell cycling with CsI.Thisw ork proposed a strategy by integrating the redoxmedium,addictives or salt tofacilitate the decomposition of the discharge product(e.g.Li2O2)and improve the stability of the metallic Li.Ishikawa etal.60provided anovelapproach that the additivealuminum iodide(AlI3,smallAl3+concentration of 600× 10-6(w,mass fraction))wasutilized asa pre-modification reagent for Limetalanode in binary electrolytes of propylene carbonate (PC)and dimethyl carbonate(DMC).Such type additive contributes tofabricating a durable Li-alloy layeron Limetal interface.But this formula stillneed to be provedwhether it is feasible in Li-O2battery.

Fig.3 Tim eevolution of the in terfacial resistance(a)and cyclic per form ance(b)of symm etric Li/electrolyte/Licellsw ith 0.5m ol·L-1LiTFSI in five different solvents at 30°C under A r56color online
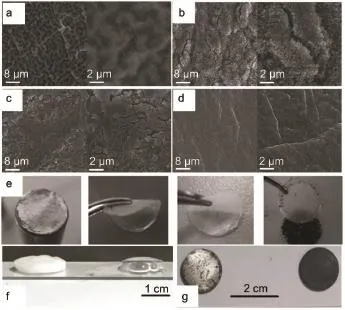
Fig.4 SEM and op tical im agesof them etallic Lianodebefore and after treated w ith differentelectrolytes(a)pristine,aftercycling in(b)1mol·L-1LiTFSI,(c)mixtureof LiNO3and LiTFSI(50:50),(d)0.05mol·L-1CsIwith 0.5mol·L-1LiNO3and 0.5mol·L-1LiTFSI in TEGDME59;(e)from left to rightshows the lithium foil,PEO-based membrane and super Pcarbon grid after CV testin Li-O2cell67;Lisheet(f)w ith awaterdrop,(g)exposed in airw ith(left)andw ithout(right)gelelectrolyte75
Currently,the solid-state electrolytes(SSEs),such as ceram ic solid electrolyte(CSE)and solid polymer electrolyte(SPE),are considered as competitive alternatives to the flammable and volatile liquid electrolytes in termsof safety issues.In contrast, unwanted chemical cross-over actions,severe anion and cation concentration gradientand dendrite formation can be effectively overcome by making use of the SSE.In addition,the robust mechanicalstrength,elevated thermal stability,uneasy-leaked and lower cost properties are pushing itsw idely application5.Now, moreandmoreworkshavebeen done to integrate theSSE into Li-O2batteries.
The lithium-ion conducting glass ceramic of Li-Al-Ge-PO4(LAGP)61,62and Li-A l-Ti-PO4(LATP)63,64systemsw ith high ionic conductivity(in the range of 10-4-10-3S·cm-1)have been em ployed in Li-O2battery.Most lithium solid electrolytes are thermodynam ically unstable against Limetal.Various kindsof Liion conductors,such as lithium-nitride(Li3N),lithium phosphorous oxynitride(LiPON)and poly(ethylene)oxide(PEO),are applied as the protective interlayer to separate the CSE and Li metalelectrode.Kumar etal.61investigated one kind of solid state electrolyte combined LAGPwith polymer-ceramic(PC)membranes comprised of PC(BN),PC(AIN),PC(Si3N4)and PC(Li2O) in Li-O2batteries.Using PCmaterial can reduce the cell impedance and enhance the electrochem ical compatibilityw ith lithium. Another sim ilar optimized componentand configuration of SSE fabricated from glass-ceramic(GC)LAGPand polymer-ceramic (PC)containing Li2O and BN was assembled in Li-O2cells as shown in Fig.5a65.This sandwiched SSE exhibited excellent thermal stability and cyclic performance used in Li-O2cells operated in the tem perature range of 30-105°C and physically isolated lithium from air andmoisture.However,the limited cycle life(less than 40 cycles)needs to im prove further.A long-term cyclic performance(150 cycles)super-hydrophobic quasi-solid electrolyte Li-O2cell coupled with RuO2/MnO2/SPcathodewas proposed asdepicted in Fig.5b66.This specialsuper-hydrophobic SiO2-based composite solid-state electrolyte can resist hum idatmosphere(RH of 45%),which also displayed higher thermal tolerance,w ider operation electrochem icalw indow(>5.5 V)and bettermechanical flexibility in contrastw ith the traditional liquid electrolyte.
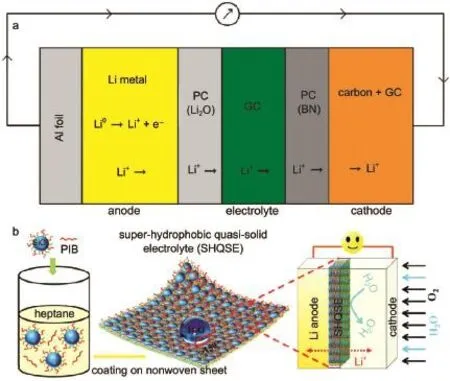
Fig.5 Schematicsof(a)the sandw iched PC/GC/PC SSEmodel65and(b)the proposed super-hyd rophobic quasi-solid electrolyte66used in Li-O2cells color online
Solvent-free polymer electrolytew ith a highmolecularweight PEO-LiCF3SO3was reported by Scrosati etal.67.The dissembled cell(Fig.4e)shows that the electrodesespecially the lithium is still in excellent condition,which confirms that the PEOmatrix can effectively protect lithium metal.Electrolytes with polymer additivesare reported as prom ising electrolyte systems thatpossess many advantagesover non-aqueous liquid or ionic liquid-based electrolytes in terms of electrochemicalstability.Recently Tokur et al.68reported that the selective amount of poly(vinylidene fluoride)(PVDF)and PEO additives can significantly increase the cyclability of Li-O2batteries.Another feature is visible from Fig.6a and 6b,in which PEO-based electrolyte exhibitsmore stable cyclic performancew ith lower overpotential than thatof PVDF.Itwassuggested thatpolymeradditivesnotonly provided high conductivity but also protected the Limetal against the corrosion.In order to improve the cyclic life,they69developed another highly reversible Li-O2batterywith addition of polymer (PEO)and ceramic(Al2O3)fillers in 1mol·L-1LiPF6/NMP-based composite polymer electrolyte.The voltage of the discharge scarcely changesparticularly after35 cyclesw ith a deep cycling capacity of 2.54 mAh in Fig.6c and 6d.The excellent electrochem ical properties indicate the stabile SEI formed on the Li anode.This result further demonstrates that the polymer electrolyte is prom ising to overcome the problem of anode corrosion and dendrite formation70.Such ceram ic fillers include Al2O371, TiO272,SiO273and ZrO274etc.Peng et al.75proposed a coaxialflexible-fiberarchitectureof all-solid-state Li-O2batteries coupled with gel electrolyte which mixed with lithium triflate(LiTF), tetraethyleneglycol dimethyl ether(TEGDME),poly(vinylidene fluoride-co-hexafluoropropylene)(PVDF-HFP),N-methyl-2-pyrrolidinone(NMP),2-hydroxy-2-methyl-1-phenyl-1-propanone (HMPP),and tri-methylolpropaneethoxylate triacrylate(TMPET). Fig.4f and 4g present that this coating layerwith gelelectrolyte can effectively prevent the lithium anode from corrosion bywater, oxygen,etc.
Theexploration of stableand compatible interphase established between SSE/CSE and electrodes becomes significant critical. Zhou etal.76reported a composite polymer electrolyte synthesized as polypropylene(PP)-supported poly(methyl methacrylate) (PMMA)-blend-poly(styrene)(PSt)with doping nanofumed SiO2. This novel gel polymer electrolyte displayed smaller interfacial resistance w ith Limetal than that of the liquid electrolyte.The better compatibility w ith Limetal anode contributes to durable cycling life comparedwith the traditional liquid one in Fig.7aand 7b.Besides,this polymer system can alleviate the corrosion of Li metal caused by airand H2O.Subsequently,in order to improve themechanical strength and ionic conductivity of SSE,Yiand Zhou77proposed a plausible solution by designing ahybrid quasisolid-state electrolyte combing a polymer electrolyte of poly (methylmethacrylate-styrene)w ith a ceram ic amorphous LiNbO3electrolyte.Thishybrid electrolyteoffersa stable interface toward Lianode and cathode electrode.The enhanced cycling stability shown in Fig.7c and 7d can be attributed to the stable interfacial resistancebetween hybrid quasi-solid-stateelectrolyte(HQSSE) and electrodes.

Fig.6 Discharge and charge profiles of Li-O2cells in(a)1m ol·L-1EM ITFSI-LiTFSI-0.1%PVDF,(b)1m ol·L-1EM ITFSI-LiTFSI-0.1% PEO68,(c)1mol·L-1LiPF6(PEO 0.5%and Al2O31%,mass fraction),(d)1mol·L-1LiPF6w ithoutany additives in NMP69color online
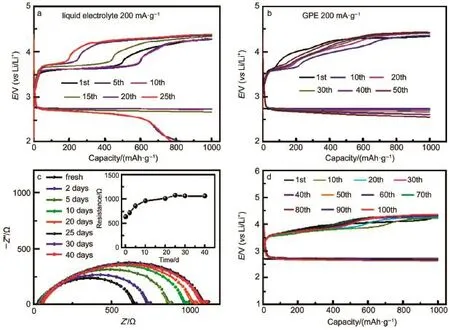
Fig.7 Voltage profilesof L i-O2cells at different cyclesusing(a)liquid electrolyte,(b)as-synthesized polym er electrolyte76; (c)the time-dependent EISof the Li/HQSSE/Licellatopen circuit potential, (d)the discharge-chargeprofilesof Li-O2cellassembled w ith HQSSE atdifferentcycles77color online
A lthough the liquid or IL electrolyte own high ionic conduc-tivity,many safety issuesstill limited their application.Herein,the CSE,SPE or theirm ixture systems display satisfied advantages. Nevertheless,problems arising from all of electrolyte systems associated with the interphase stability will be exposed when permeated into O2,H2O,CO2used in lithium-air batteries.Somuch effortsshould be paid to exploreand developmore compatibleand durable electrolyte system s.
3.2 Com posite p ro tec t layer and separator
Except for the in situ SEI,someartificial SEI layers,such as some kinds of polymermembranes or separatorswhich can store electrolyte and provide pathway for ion transportation,havebeen synthesized via skillfulm ixture of ceram ic fillers and polymer additives.Forexample,a polymermembrane consisting of PVDF, LiTFSIand ZrO2exhibitsobviousadvantages to prevent the direct reaction between the LixSn-C anode and the liquid electrolyte (Fig.8aand 8b)78.Fig.8b also verifies the instability of the Li-Sn-C anodewith the permeated O2in theelectrolyte,whichwe have discussed above.
Lee etal.79reported a composite protective layer comprising A l2O3and PVDF-HFP coated on the Limetal.The CPL closely contactswith Limetaland lowers the resistanceof the cell,which makesagreatcontribution to the cyclic performance(Fig.8c and 8d).The shiny silverand smooth surface of the disassembled Li foil in Fig.8f demonstrates that the CPL can effectively suppress the dendrite formation and separate the liquid electrolyte corrosion.Besides,they also demonstrated a high-performance nonaqueous Li-O2battery through synergistic combination of 2,2,6,6-tetramethylpiperidinyl-1-oxyl(TEMPO)as redoxmediator(RM) with theabovementioned CPL80.As reported81,the RM reactswith the Limetal in theelectrolyte,which isdescribed asself-discharge process.Their experimental results explain the stability of Li interface with CPL when it is in contact with the TEMPO-con-

Fig.8 Com parison of the third cycle discharge-charge behavior(a)and time-dependent voltage evolution curves(b)of LixSn-C/O2cellsw ith and w ithout the gelpolymermembrane78;discharge-charge profilesof Li-O2cells fabricated using Lielectrode(c)w ithout CPL, (d)w ith CPL,and SEM and optical im ages of Lielectrode(e)w ithout CPL,(f)w ith CPL after 80 cycles79co lor online
taining electrolyte with increasing storage time as depicted in Fig.9a-9d.
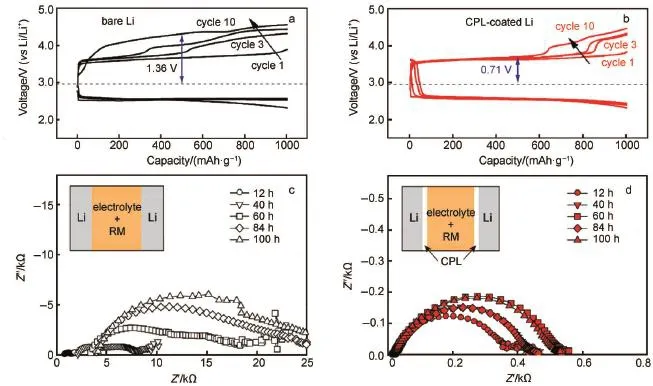
Fig.9 Charge/discharge p rofiles of Li-O2cells(a)w ithout CPL and(b)w ith CPL at 250m A·g-1(based on them assof carbon),Nyquist plotsof the Li/Lisymmetric cell(c)w ithout CPL and(d)w ith CPL in 1mol·L-1LiClO4/TEGDME containing 0.05mol·L-1TEMPO80color online
To our know ledge,thewidely-used separatorsareusually porous such as polyethylene(PE).K im et al.82synthesized the porelesspolyurethane(PU)separator resulting from thehigh chain packing density.The poreless PU can preventwater and oxygen from penetrating to Limetalwhile Liion can diffuse freely.They also use redox mediator LiI to test the protective ability in comparisonwith commercialporouspolyethylene(PE)for Li-O2cell (Fig.10aand 10b).Theabsenceof LiOH and uncorroded Limetal indicate that the PU separator can block the diffusion of the LiI and alleviate the side reaction.Besides,the smooth surfaceof Li metal was obtained by using a two-dimensionally ordered nanoporous aluminium oxide separator after 15 cycles in Li-O2cell (Fig.10c and 10d)83.The flatand smooth surface can be attributed to theuniform distribution Li+through porousseparator during the repeated charge and discharge processes.
The abovementioned artificial SEI(CPL and separators), synthesized via the integrating the liquid electrolytewith ceramic solid or solid polymer fillers,can be w idely broadened.The properties e.g.sizeof the pore,elastic ormechanicalmodulus,ion conductivity,can be tuned or tailored via optimizing the composition and content.Although,the CPLorseparatorscan suppress the volume change of the Limetaland weaken the diffusion ofO2, waterand some other parasitic substances,their durability especially the electrochem icalstability against the Limetaland oxygen reduction species(e.g.O2-)isnotclearand need to be improved because of the lack of long-term cyclic performance in the reported studiesof Li-O2batteries.
4 Experim en tal techniques
In order tofully understand and solve the problemsarising from Lianode,the investigations on thenatureof Lianode interphase should rely on the rapid development of modern advanced characterization techniques.At present,the spectroscopic investigationsof SEIon negativeand positiveelectrodes for lithium-ion batterieshavew idely developed with Fourier transform infrared (FTIR)spectroscopy,X-ray photoelectron spectroscopy(XPS),X-ray diffraction(XRD),Raman and surface enhanced Raman scattering(SERS),scanning electronmicroscopy(SEM)and highresolution transm ission electron microscopy(HRTEM)etc.84,85These techniques can be used to study the structure,composition and evolution of thebulk,surfaceand interphaseof SEI.However, ex situ analysisof the SEI(or Limetal)requires to be separated and isolated,this disassemble processmay change the surface morphology,chem ical content and even the structure of SEI. Herein,in situ space-resolution,time-resolution and energy-resolution technologies are favored to providemore reliable and accurate information.
Liu and his collaborators86reported an operando,spatially resolved phaseand structural investigation on the lithium anode in an operating Li-O2cellusing am icro-focused synchrotron X-ray diffraction(μ-XRD)technique.The experimental set-up and cell design are shown in Fig.11aand 11b.The specialpolyimide tube of the design cell can pass through the high-energy X rays.The results of this time and space-resolutionμ-XRD reveal the conversion of metallic lithium into LiOH in 1mol·L-1LiCF3SO3/ TEGDMEelectrolyte.The thicknessof the LiOH layer increased as the cycle progressed,possibly resulting from the reaction of Liwith H2O formed through the TEGDME decomposition.It prelim inary discloses the reason for the failureof Li-O2cell.The in situ XRD characterization can directly observe phase transition during the electrochem ical reaction.
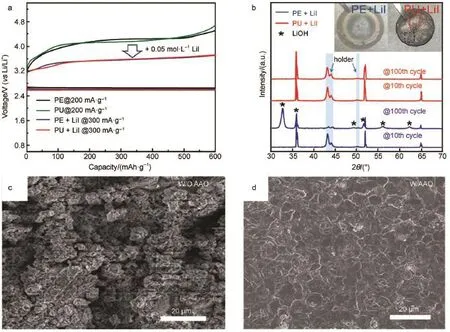
Fig.10(a)Voltagep rofiles in the first cycles for PE,PU,PE+LiIand PU+LiIcells in 1mol·L-1LiClO4/TEGDME,(b)XRD patternsand digital photograph im ages of the cyclic L im etal82;SEM im ages of the Lim etalafter 15 cycles(c)w ithout AAO and (d)w ith AAO of Li-O2cell in 1mol·L-1LiNO3/DMAc electrolyte cycled at0.5m A for 1000 s chargeor discharge83coloronline

Fig.11 Schematic of operandom icro-focused synchrotron X-ray diffraction(μ-XRD)technique(a)and themodedesign of Li-O2cell(b)86
Scanning probemicroscopy(SPM)in different variations,for example,theatomic forcem icroscopy(AFM)iswellknown for its high-resolution topographic imaging capabilities of characterizing thin film and quantifying 3D roughnessand texture,has been used to study evolution of SEIin Li-ion batteries87-90.Above all,the characterization of SEIon Limetal isa challengebecause of the variety of chemically similar components,enclosed electrolyte species and the sensitive interphase.Herein,the advanced in situ/operando combination techniques,e.g.in situ SERS-DEMS etc.are powerful tools to understand thenatureand quantitatively assess the relationship between Limetaland O2upon forming SEI. Furtherworksareurgent to be conducted.
5 Conc lusions and ou tlook
In this review,we summarize the current development on protecting the Limetalanode in aprotic Li-O2battery.Although parasitic reaction and dendrite can be avoided as long as the Li metal is replaced by the alternative counter electrodes,this sacrifices the high energy of Li anode.The integration of organic liquid,polymer,ceram ic and gelelectrolytesw ith robust additives shows promising to constructive electrochem icalandmechanical stable SEIon the Limetal.While the interface stability between the Limetal and the electrolytes,O2etc.still lack definite explanation.Besides,the thermodynamicsand kinetics properties, such as thickness,ion conductivity,mechanicalmodulus of durable SEIhave not be concluded.Fundamental understand the mechanisms of SEI formation are critical to develop safe,longterm rechargeable Li-O2batteries.
(2)Girishkumar,G.;McCloskey,B.;Luntz,A.C.;Swanson,S.; W ilcke,W.J.Phys.Chem.Lett.2010,1(14),2193. doi:10.1021/jz1005384
(3)Lu,J.;Li,L.;Park,J.B.;Sun,Y.K.;Wu,F.;Amine,K.Chem. Rev.2014,114(11),5611.doi:10.1021/cr400573b
(4)Luntz,A.C.;M cCloskey,B.D.Chem.Rev.2014,114(23), 11721.doi:10.1021/cr500054y
(5)Balaish,M.;K raytsberg,A.;Ein-Eli,Y.Phys.Chem.Chem. Phys.2014,16,2801.doi:10.1039/C3CP54165G
(6)Chang,Z.W.;Xu,J.J.;Liu,Q.C.;Li,L.;Zhang,X.B.Adv. EnergyMater.2015,5(21),1500633.doi:10.1002/ aenm.201500633
(7)Feng,N.N.;He,P.;Zhou,H.S.Adv.Energy Mater.2016,6(9), 1502303.doi:10.1002/aenm.201502303
(8)Geng,D.S.;Ding,N.;Andy Hor,T.S.;Chien,S.W.;Liu,Z.L.; Wuu,D.;Sun,X.L.;Zong,Y.Adv.EnergyMater.2016,6(9), 1502164.doi:10.1002/aenm.201502164
(9)Abraham,K.M.;Jiang,Z.J.Electrochem.Soc.1996,143(1),1. doi:10.1149/1.1836378
(10)Oh,S.H.;Black,R.;Pomerantseva,E.;Lee,J.H.;Nazar,L.F. Nat.Chem.2012,4,1004.doi:10.1038/nchem.1499
(11)Thapa,A.K.;Hidaka,Y.;Hagiwara,H.;Ida,S.;Ishihara,T. J.Electrochem.Soc.2011,158(12),A1483.doi:10.1149/ 2.090112jes
(12)M cCloskey,B.D.;Scheffler,R.;Speidel,A.;Bethune,D.S.; Shelby,R.M.;Luntz,A.C.J.Am.Chem.Soc.2011,133(45), 18038.doi:10.1021/ja207229n
(13)Lu,J.;Lei,Y.K.;Lau,C.;Luo,X.;Du,P.;Wen,J.;Assary,R. S.;Das,U.;M iller,D.J.;Elam,J.W.;A lbishri,H.M.;El-Hady, D.A.;Sun,Y.K.;Curtiss,L.A.;Am ine,K.Nat.Commun.2013, 4,2383.doi:10.1038/ncomms3383
(14)Liu,B.;Yan,P.;Xu,W.;Zheng,J.;He,Y.;Luo,L.Bow den,M. E.;Wang,C.M.;Zhang,J.G.Nano.Lett.2016,16(8),4932. doi:10.1021/acs.nanolett.6b01556
(15)Wang,Z.D.;You,Y.;Yuan,J.;Yin,Y.X.;Li,Y.T.;Xin,S.; Zhang,D.W.ACSAppl.Mater.Interfaces2016,8(10),6520. doi:10.1021/acsami.6b00296
(16)Zhang,T.;Liao,K.;He,P.;Zhou,H.S.Energy Environ.Sci. 2016,9,1024.doi:10.1039/C5EE02803E
(17)Elia,G.A.;Hassoun,J.;Kwak,W.J.;Sun,Y.K.;Scrosati,B.; Mueller,F.;Bresser,D.;Passerini,S.;Oberhumer,P.; Tsiouvaras,N.;Reiter,J.Nano Lett.2014,14(11),6572. doi:10.1021/nl5031985
(18)Liu,B.;Xu,W.;Yan,P.F.;Sun,X.L.;Bowden,M.E.;Read,J.; Qian,J.F.;Mei,D.H.;Wang,C.M.;Zhang,J.G.Adv.Funct. Mater.2016,26(4),605.doi:10.1002/adfm.201503697
(19)Le,H.T.T.;Kalubarme,R.S.;Ngo.D.T.;Jadhav,H.S.;Park, C.J.J.Mater.Chem.A 2015,3,22421.doi:10.1039/ C5TA06374D
(20)M archini,F.;Herrera.S.;Torres,W.;Tesio,A.Y.;W illiams,F. J.;Calvo,E.J.Langmuir2015,319(33),9236.doi:10.1021/acs. langmuir.5b02130
(21)Giordani,V.;Tozier,D.;Tan,H.J.;Burke,C.M.;Gallant,B. M.;Uddin,J.;Greer,J.R.;M cCloskey,B.D.;Chase,G.V., Addison,D.J.Am.Chem.Soc.2016,138(8),2656. doi:10.1021/jacs.5b11744
(22)Kim,D.W.;Ahn,S.M.;Kang,J.W.;Suk,J.D.;Kim,H.K.; Kang,Y.K.J.Mater.Chem.A 2016,4,6332.doi:10.1039/ C6TA00371K
(23)Black,R.;Oh,S.H.;Lee,J.H.;Yim,T.;Adams,B.;Nazar,L.F. J.Am.Chem.Soc.2012,134(6),2902.doi:10.1021/ja2111543
(24)Nasybulin,E.;Xu,W.;Engelhard,M.H.;Nie,Z.;Li,X.S.; Zhang,J.G.J.Power Sources2013,243,899.doi:10.1016/j. jpow sour.2013.06.097
(25)Scheers,J.;Lidberg,D.;Sodeyama,K.;Futera,Z.;Tateyama,Y. Phys.Chem.Chem.Phys.2016,18,9961.doi:10.1039/ C5CP08056H
(26)Qiao,Y.;Ye,S.J.Phys.Chem.C 2016,120(15),8033. doi:10.1021/acs.jpcc.5b11692
(27)Wang,J.W.;Zhang,Y.L.;Guo,L.M.;Wang,E.K.;Peng,Z.Q. Angew.Chem.Int.Ed.2016,55,1.doi:10.1002/anie.201600793
(28)David,G.K.;M ichał,T.;Nir,P.;Daniil,M.I.;Carl,V.T.;Yang, S.H.J.Phys.Chem.Lett.2016,7(7),1204.doi:10.1021/acs. jpclett.6b00323
(29)Giordani,V.;Walker,W.;Bryantsev,V.S.;Uddin,J.;Chase,G. V.;Addison,D.J.Electrochem.Soc.2013,160(9),A 1544. doi:10.1149/2.097309jes
(30)Roberts,M.;Younesi,R.;Richardson,W.;Liu,J.;Gustafsson, T.;Zhu,J.F.;Edström,K.ECSElectrochem.Lett.2014,3(6), A62.doi:10.1149/2.007406eel
(31)Zhang,Y.T.;M a,L.P.;Zhang,L.Q.;Peng,Z.Q. J.Electrochem.Soc.2016,163(7),A1270.doi:10.1149/ 2.0871607jes
(32)Bruce,P.G.;Freunberger,S.A.;Hardw ick,L.J.;Tarascon,J. M.;Nat.Mater.2012,11,19.doi:10.1038/nmat3191
(33)Kim,H.;Jeong,G.;Kim,Y.U.;Kim,J.H.;Park,C.M.;Sohn, H.J.Chem.Soc.Rev.2013,42,9011.doi:10.1039/C3CS60177C
(34)Cheng,X.B.;Zhang,R.;Zhao,C.Z.;Wei,F.;Zhang,J.G.; Zhang,Q.Adv.Sci.2015,3(3),1500213.doi:10.1002/ advs.201500213
(35)Choi,N.S.;Chen,Z.;Freunberger,S.A.;Ji,X.;Sun,Y.K.; Am ine,K.;Yushin,G.;Nazar,L.F.;Cho,J.;Bruce,P.G.Angew. Chem.Int.Ed.2012,51(40),9994.doi:10.1002/ anie.201201429
(36)Scrosati,B.;Garche,J.J.Power Sources2010,195(9),2419. doi:10.1016/j.jpowsour.2009.11.048
(37)Zhang,R.;Cheng,X.B.;Zhao,C.Z.;Peng,H.J.;Shi,J.L.; Huang,J.Q.;Wang,J.;Wei,F.;Zhang,Q.Adv.Mater.2016,28 (11),2155.doi:10.1002/adma.201504117
(38)Elia,G.A.;Bresser,D.;Reiter,J.;Oberhumer,P.;Sun,Y.K.; Scrosati,B.;Passerini,S.;Hassoun,J.ACSAppl.Mater. Interfaces2015,7(40),22638.doi:10.1021/acsami.5b07414
(39)Assary,R.S.;Lu,J.;Du,P.;Luo,X.;Zhang,X.;Ren,Y.; Curtiss,L.A.;Am ine,K.ChemSusChem 2013,6(1),51. doi:10.1002/cssc.201200810
(40)Chen,Y.H.;Freunberger,S.A.;Peng,Z.Q.;Bardé,F.;Bruce,P. G.J.Am.Chem.Soc.2012,134(18),7952.doi:10.1021/ ja302178w
(41)M cCloskey,B.D.;Bethune,D.S.;Shelby,R.M.;M ori,T.; Scheffler,R.;Speidel,A.;Sherwood,M.;Luntz,A.C.J.Phys. Chem.Lett.2012,3(20),3043.doi:10.1021/jz301359t
(42)Chen,Y.H.;Freunberger,S.A.;Peng,Z.Q.;Fontaine,O.; Bruce,P.G.Nat.Chem.2013,5,489.doi:10.1038/nchem.1646
(43)Peng,Z.Q.;Freunberger,S.A.;Chen,Y.H.;Bruce,P.G. Science2012,337(6094),563.doi:10.1126/science.1223985
(44)M a,S.C.;Wu,Y.;Wang,J.W.;Zhang,Y.L.;Zhang,Y.T.;Yan, X.X.;Wei,Y.;Liu,P.;Wang,J.P.;Jiang,K.L.;Fan,S.S.;Xu, Y.;Peng,Z.Q.Nano Lett.2015,15(12),8084.doi:10.1021/acs. nanolett.5b03510
(45)Chun,J.Y.;Kim,H.;Jo,C.;Lim,E.;Lee,J.;Kim,Y. ChemPlusChem 2015,80(2),349.doi:10.1002/cplu.201402035
(46)Hassoun,J.;Jung,H.G.;Lee,D.J.;Park,J.B.;Amine,K.;Sun, Y.K.;Scrosati,B.Nano Lett.2012,12(11),5775.doi:10.1021/ nl303087j
(47)Guo,Z.Y.;Dong,X.L.;Wang,Y.G.;Xia,Y.Y.Chem. Commun.2015,51,676.doi:10.1039/C4CC07315K
(48)Aurbach,D.;Zinigrad,E.;Cohen,Y.;Teller,H.Solid State Ionics 2002,148(3),405.doi:10.1016/S0167-2738(02)00080-2
(49)Peled,E.J.Electrochem.Soc.1979,126(12),2047. doi:10.1149/1.2128859
(50)Aurbach,D.;Pollak,E.;Elazari,R.;Salitra,G.;Kelley,C.; Affintio,J.J.Electrochem.Soc.2009,156(8),A694. doi:10.1149/1.3148721
(51)Liang,X.;Wen,Z.;Liu,Y.;Wu,M.;Jin,J.;Zhang,H.;Wu,X. J.PowerSources2011,196(22),9839.doi:10.1016/j. jpow sour.2011.08.027
(52)Zhang,S.S.J.Power Source 2016,322,99.doi:10.1016/j. jpow sour.2016.05.009
(53)Walker,W.;Giordani,V.;Uddin,J.;Bryantsev,V.S.;Chase,G. V.;Addison,D.J.Am.Chem.Soc.2013,135(6),2076. doi:10.1021/ja311518s
(54)Aurbach,D.;Daroux,M.;Faguy,P.;Yeager,E.J.Electroanal. Chem.1991,297(12),225.doi:10.1016/0022-0728(91)85370-5
(55)Younesi,R.;Hahlin,M.;Roberts,M.;Edstrom,K.J.Power Sources2013,225(2),40.doi:10.1016/j.jpow sour.2012.10.011
(56)Bryantsev,V.S.;Giordani,V.;Walker,W.;Uddin,J.;Lee,I.; Duin,A.C.T.;Chase,G.V.;Addison,D.J.Phys.Chem.C 2013,117(23),11977.doi:10.1021/jp402844r
(57)Liu,Q.C.;Xu,J.J.;Yuan,S.;Chang,Z.W.;Xu,D.;Yin,Y.B.; Li,L.;Zhong,H.X.;Jiang,Y.S.;Yan,J.M.;Zhang,X.B.Adv. Mater.2015,27(35),5241.doi:10.1002/adma.201501490
(58)Ding,F.;Xu,W.;Graff,G.L.;Zhang,J.;Sushko,M.L.;Chen, X.L.;Shao,Y.Y.;Engelhard,M.H.;Nie,Z.M.;Xiao,J.;Liu, X.J.;Sushko,P.V.;Liu,J.;Zhang,J.G.J.Am.Chem.Soc. 2013,135(11),4450.doi:10.1021/ja312241y
(59)Lee,C.K.;Park,Y.J.ACSAppl.Mater.Interfaces2016,8(13), 8561.doi:10.1021/acsam i.5b11709
(60)Ishikawa,M.;Kawasaki,H.;Yoshimoto,N.;Morita,M. J.PowerSources2005,146(1),199.doi:10.1016/j. jpow sour.2005.03.007
(61)Kumar,J.;Kumar,B.J.Power Sources2009,194(2),1113. doi:10.1016/j.jpow sour.2009.06.020
(62)Jadhav,H.S.;Kalubarme,R.S.;Jadhav,A.H.;Seo,J.G. Electrochim.Acta 2016,199,126.doi:10.1016/j. electacta.2016.03.143
(63)Hasegawa,S.;Imanishi,N.;Zhang,T.;Xie,J.;Hirano,A.; Takeda,Y.;Yamamoto,O.J.Power Sources 2009,189(1),371. doi:10.1016/j.jpowsour.2008.08.009
(64)Imanishi,N.;Hasegawa,S.;Zhang,T.Hirano,A.;Takeda,Y.; Yamamoto,O.J.Power Sources2008,185(185),1392. doi:10.1016/j.jpowsour.2008.07.080
(65)Kumar,B.;Kumar,J.;Leese,R.;Fellner,J.P.;Rodrigues,S.J.; Abraham,K.M.J.Electrochem.Soc.2010,c157(1),A50. doi:10.1149/1.3256129
(66)Wu,S.;Yi,J.;Zhu,K.;Bai,S.;Liu,Y.;Qiao,Y.;Ishida,M.; Zhou,H.Adv.EnergyMater.2016,1601759.doi:10.1002/ aenm.201601759
(67)Hassoun,J.;Croce,F.;Armand,M.;Scrosati,B.Angew.Chem. Int.Ed.2011,50(13),2999.doi:10.1002/anie.201006264
(68)Tokur,M.;Algul,H.;Ozcan,S.;Cetinkaya,T.;Uysal,M.; Guler,M.O.;Akbulut,H.Solid State Ionics 2016,286,51. doi:10.1016/j.ssi.2015.12.017
(69)Tokur,M.;Algul,H.;Cetinkaya,T.;Uysal,M.;Akbulut,H. J.Electrochem.Soc.2016,163(7),A 1326.doi:10.1149/ 2.0961607jes
(70)Rahman,M.A.;Wang,X.;Wen,C.A.J.Appl.Electrochem. 2014,44(1),5.doi:10.1007/s10800-013-0620-8
(71)Croce,F.;Sacchetti,S.;Scrosati,B.J.Power Sources2006,161 (1),560.doi:10.1016/j.jpow sour.2006.03.069
(72)Sarnowska,A.;Polska,I.;Niedzicki,L.;Marcinek,M.; Zalewska,A.Electrochim.Acta 2011,57,180.doi:10.1016/j. electacta.2011.04.079
(73)Mazor,H.;Golodnitsky,D.;Peled,E.;Wieczorek,W.;Scrosati, B.A.J.Power Sources 2008,178(2),736.doi:10.1016/j. jpowsour.2007.09.056
(74)Panero,S.;Scrosati,B.;Sumathipala,H.H.;W ieczorek,W. J.PowerSources2007,167(2),510.doi:10.1016/j. jpowsour.2007.02.030
(75)Zhang,Y.;Wang,L.;Guo,Z.Y.;Xu,Y.F.;Wang,Y.G.;Peng, H.S.Angew.Chem.Int.Ed.2016,55,4487.doi:10.1002/ anie.201511832
(76)Yi,J.;Liu,X.;Guo,S.;Zhu,K.;Xue,H.;Zhou,H.ACSAppl. Mater.Interfaces2015,7(42),23798.doi:10.1021/ acsami.5b08462
(77)Yi,J.;Zhou,H.ChemSusChem 2016,9(17),2391.doi:10.1002/ cssc.201600536
(78)Elia,G.A.;Hassoun,J.Solid State Ionics2016,287,22. doi:10.1016/j.ssi.2016.01.043
(79)Lee,D.J.;Lee,H.;Song,J.;Ryou,M.H.;Lee,Y.M.;Kim,H. T.;Park,J.K.Electrochem.Commun.2014,40,45. doi:10.1016/j.elecom.2013.12.022
(80)Lee,D.J.;Lee,H.;Kim,Y.J.;Park,J.K.;Kim,H.T.Adv. Mater.2016,28,857.doi:10.1002/adma.201503169
(81)Wang,Y.;Xia,Y.Nat.Chem.2013,5,445.doi:10.1038/ nchem.1658
(82)Kim,B.G.;Kim,J.S.;M in,J.;Lee,Y.H.;Choi,J.H.;Jang,M. C.;Freunberger,S.A.;Choi,J.W.Adv.Funct.Mater.2016,26 (11),1747.doi:10.1002/adfm.201504437
(83)Kang,S.J.;Mori,T.;Suk,J.;Kim,D.W.;Kang,Y.;Wilcke,W.; Kim,H.C.J.Mater.Chem.A 2014,2,9970.doi:10.1039/ C4TA 01314J
(84)Wu,C.H.;Weatherup,R.S.;Salmeron,M.B.Phys.Chem. Chem.Phys.2015,17,30229.doi:10.1039/C5CP04058B
(85)Balbuena,P.B.;Wang,Y.X.Lithium-Ion Batteries:Solid Electrolyte Interphase;ImperialCollege Press:London,2004; pp 140-189.doi:10.1142/p291
(86)Shui,J.L.;Okasinaki,J.S.;Kenesei,P.;Dobbs,H.A.;Zhao, D.;A lmer,J.D.;Liu,D.J.Nat.Commun.2013,4,2255. doi:10.1038/ncomms3255
(87)Shen,C.;Wang,S.W.;Jin,Y.;Han,W.Q.ACSAppl.Mater. Interfaces2015,7,25441.doi:10.1021/acsami.5b08238
(88)Koltypin,M.;Cohen,Y.S.;Markovsky,B.;Cohen,Y.;Aurbach, D.Electrochem.Commun.2002,4,17.doi:10.1016/S1388-2481 (01)00264-8
(89)M ogi,R.;Inaba,M.;Jeong,S.K.;Iriyama,Y.;Abe,T.;Ogum i, Z.J.Electrochem.Soc.2002,149,A1578.doi:10.1149/ 1.1516770
(90)Cohen,Y.S.;Cohen,Y.;Aurbach,D.J.Phys.Chem.B 2000, 104,12282.doi:10.1021/jp002526b
Recent Advances in Li Anode for Aprotic Li-O2Batteries
ZHANG Yan-Tao1,2LIU Zhen-Jie1,2WANG Jia-Wei1WANG Liang1,2PENG Zhang-Quan1,*
(1State Key Laboratory ofElectroanalytical Chemistry,Changchun Institute ofApplied Chem istry,Chinese Academy ofSciences, Changchun 130022,P.R.China;2University ofChinese Academy ofSciences,Beijing 100049,P.R.China)
The ap rotic Li-O2battery has attracted considerab le interest in recent years because of its high theoretica lspecific energy that is fargreater than thatachievable with state-of-the-art Li-ion technologies.To date,most Li-O2studies,based on a cellconfiguration w ith a Limetalanode,apro tic Li+electrolyte and po rous O2cathode,have focused on O2reactions at the cathode.However,these reactionsm ightbe com plicated by the use of Limetalanode.This is because both the electrolyte and O2(from cathode)can reactwith the Limetal and some parasitic products could cross over to the cathode and interferewith the O2reactions occurring therein. In addition,the possibility ofdendrite formation on the Lianode,during itsmultiple plating/stripping cycles,raises serious safety concerns tha t im pede the realization of practical Li-O2cells.Therefore,solutions to these issues are urgently needed to achieve a reversible and sa fety Lianode.This review summarizes recentadvances in this field and strategies forachieving high performance Lianode foruse in aprotic Li-O2batteries.Topics include alternative counter/reference electrodes,electrolytes and additives,composite protection layers and separators, and advanced experimental techniques for studying the Lianode|electrolyte interface.Future developments in rela tion to Lianode foraprotic Li-O2ba tteries a re also d iscussed.
O646;TM911.41
rmand,M.;Tarascon,J.M.Nature2008,451,652.
10.1038/451652a
doi:10.3866/PKU.WHXB201611181
www.whxb.pku.edu.cn
Received:September29,2016;Revised:November18,2016;Published online:November18,2016.
*Corresponding author.Email:zqpeng@ciac.ac.cn;Tel:+86-431-85262660.
The projectwas supported by the National Natural Science Foundation of China(21605136,91545129,21575135),“Strategic Priority Research
Program”of the CAS(XDA09010401),“RecruitmentProgram of GlobalYouth Experts”of China,National Key Research and Development
Program of China(2016YFB0100100),and Science and Technology Development Program of Jilin Province,China(20150623002TC,
20160414034GH).
国家自然科学基金(21605136,91545129,21575135),中国科学院战略性先导科技专项(A类)(XDA09010401),国家青年千人计划,国家重点研发计划(2016YFB 0100100),吉林省科学技术厅科技发展项目(20150623002TC,20160414034GH)资助©Editorialoffice of Acta Physico-Chim ica Sinica
