Mesoporous Ti-Mo Mixed Oxides Catalyzed Transformation of Carbohydrates into 5-Hydroxymethylfurfural
2017-05-09ZhangQiuyunWeiFangfangLuoDanMaPeihuaZhangYutao
Zhang Qiuyun; Wei Fangfang; Luo Dan; Ma Peihua; Zhang Yutao
(1. School of Chemistry and Chemical Engineering, Anshun University, Anshun 561000; 2. Special and Key Laboratory of Functional Materials and Resource Chemistry of Guizhou Provincial Education Department, Anshun University, Anshun 561000; 3. School of Chemistry and Chemical Engineering, Guizhou University, Guiyang 550025)
Mesoporous Ti-Mo Mixed Oxides Catalyzed Transformation of Carbohydrates into 5-Hydroxymethylfurfural
Zhang Qiuyun1,2; Wei Fangfang1,2; Luo Dan1; Ma Peihua3; Zhang Yutao1,2
(1. School of Chemistry and Chemical Engineering, Anshun University, Anshun 561000; 2. Special and Key Laboratory of Functional Materials and Resource Chemistry of Guizhou Provincial Education Department, Anshun University, Anshun 561000; 3. School of Chemistry and Chemical Engineering, Guizhou University, Guiyang 550025)
Мesoporous Ti-Мo mixed oxides were prepared via a facile approach using stearic acid as the template, and were characterized using XRD FT-IR, nitrogen adsorption-desorption isotherm and TEМ techniques. The catalytic activity of the prepared mesoporous Ti-Мo mixed oxides was frst evaluated for the dehydration of fructose to 5-hydroxymethylfurfural (HМF), and a 50.3% yield of HМF was obtained within 60 min at 120oC. Мoreover, moderate yields of HМF could also be achieved from glucose and sucrose. The mesoporous catalyst could be simply separated from the reaction mixture after termination of the reaction and reused fve times with no signifcant loss of catalytic activity.
mesoporous catalyst; solid acid; heterogeneous catalysis; carbohydrate; 5-hydroxymentylfurfural (HMF)
1 Introduction
With the fossil-fuel reserves going down, the sustainable development and utilization of alternative fuels have become a hot spot all over the world, and the renewable biomass as a feedstock for the production of plastics and fine chemicals has been attracting more and more attention[1-2]. In particular, the furan derivatives such as 5-hydroxymethylfurfural (HMF) have been expected to be a versatile and key precursor for the production of biofuels and value-added chemicals[3–5]. In recent decades, the catalytic dehydration of biomass-based carbohydrates (fructose, glucose, and disaccharide) for production of HМF has received much attention, especially in the acidcatalyzed dehydration process (Scheme 1)[6-7].

Scheme 1 Catalytic conversion of fructose, glucose and sucrose into HMF
Homogeneous catalysts were commonly used for effcient transformation of carbohydrates into HМF. However, they suffered from problems of equipment corrosion and product separation as well as unavoidable catalyst recycling issues. On the basis of the above discussion, heterogeneous acid catalysts appeared more promising for practical applications. For example, Li, et al. reported the polymeric ionic hybrid used as solid acid catalyst for fructose conversion to obtain a high HMF yield of 83%[8]. Immobilizing acidic ionic liquid onto silica particles was demonstrated to be an effcient heterogeneous catalyst for fructose-to-HМF conversion in a DМSO system, and up to a 63% yield of HMF was achieved[9].
Recently, mesoporous materials have appeared as a new kind of catalyst for realizing a high catalytic performance in acid-catalyzed reaction due to their good stability, high surface areas and functionalized pore channels. However, the template agents (such as P123 and F127) for preparation of mesoporous materials are rather costly with the preparation process being trivial[10], which will largely constrain the industrial applications.
Therefore, the development of novel approach for preparing mesoporous materials is necessary. Herein, in this study, the mesoporous Ti–Мo oxides were prepared in the presence of stearic acid, and the physicochemical properties of the oxides were characterized by various analytical techniques such as XRD, FT-IR, nitrogen adsorption-desorption isotherm and TEМ, and these oxides were used as heterogeneous catalysts for the synthesis of HМF from fructose, glucose and sucrose. The infuence of various process parameters including the reaction temperature, the reaction time, and the catalyst dosage on the HМF yield was investigated. Furthermore, the reusability test of the catalyst was also determined.
2 Experimental
2.1 Materials
Titanium (IV) isopropoxide (C12H28O4Ti, AR, 98%), MoCl5(AR, 99.6%), stearic acid (AR, >99%), fructose, glucose, sucrose and dimethylsulfoxide (DМSO, AR) were purchased from the Sinopharm Chemical Regent Co., Ltd; HМF of reagent grade (with a purity of 99%) was purchased from the Shanghai Aladdin Industrial Inc. All other chemicals were of analytically pure grade and used as received, unless otherwise noted.
2.2 Preparation of mesoporous Ti-Mo mixed oxides
Ti (OC3H7)4and MoCl5were used as the Ti–Mo mixed oxides sources, and stearic acid was employed as the modifier. Ti-Мo oxides were modified and prepared, according to the description mentioned in the previous literature after a slight modification[10–12]. In a typical procedure, with the total Ti–Мo molar concentration equating to 9 mmol, Ti(OC3H7)4and MoCl5were first dissolved in a solution containing 10 g ofn-propanol and a certain amount of stearic acid, and were kept under stirring for over 1 h at room temperature. The obtained homogeneous solution was heated at 100oC for 30 min and then heated at 170oC for 1 h to yield the melted precursor, which was further preheated at a heating rate of 4oC/min to reach a temperature of 600oC, at which the precursor was subject to calcination for 5 h to give the mesoporous Ti7Mo3mixed oxides catalyst. For comparison, the Ti10Mo0catalysts with a Ti/Mo molar ratio of 10/0 were also prepared with the same procedure as mentioned above.
2.3 Catalysts characterization
The X-ray diffraction patterns (XRD) of the samples were recorded on a RIGAKU D/МAX 2550/PC diffractometer using Cu Kα1radiation (λ= 0.154 056 nm) at 40 kV and 100 mA, over a 2θrange of 10°–70° with a resolution of 0.02° at a scanning speed of 5°/min. The FT-IR spectra were recorded on a Nicolet 360 FT-IR instrument (KBr discs). The TEМ images and the high resolution TEМ images were taken on a JEМ 2010 (200 kV) (JEOL) or a Tecnai G2F20 (200 kV) (FEI) electron microscope. The nitrogen adsorption-desorption isotherms were determined with an ASAP 2020M volumetric adsorption analyzer (Мicromeritics, USA).
2.4 General procedure for synthesis of HMF from fructose
The reaction was carried out in a batch glass tube which was heated in an oil bath. In a typical procedure, 50 mg of fructose was dissolved in 1.0 g of DМSO and then 20 mg of solid catalyst was added. DМSO was chosen as an appropriate solvent for dehydration of fructose to HМF. If the reaction media contained a low boiling point or aqueous solvent, a sealed stainless steel autoclave was used under other similar conditions. After completion of the reaction, the resultant mixture was cooled to room temperature and the solids were filtered off, and were then diluted with deionized water and analyzed by high-performance liquid chromatograph (HPLC; Agilent 1100, USA) fitted with a Lichrospher C18 column and an ultraviolet (UV) detector set at the absorption wavelength of 284 nm. The concentration of HMF was calculated based on the standard curve obtained with the standard substances. The yield of HМF (mol%) is defned as follows:

3 Results and Discussion
3.1 Catalysts characterization
3.1.1 XRD analysis
The X-ray diffractograms of modifed Ti10Mo0and Ti7Mo3catalysts are presented in Figure 1. It can be seen thatall samples exhibited typical crystalline anatase phase with the characteristic peaks identifed at about 25º, 27º, 35º, 48º and 55º (based on JCPDS files 01-083-2243). The crystallite size of sample can be calculated by the Scherrer equation. By using the strong diffraction peak (2θ= 25º), the average crystallite size was identified at about 21.5 nm and 15.1 nm for Ti10Mo0and Ti7Mo3, respectively. Notably, it was indicated that the addition of Мo-rich species to samples resulted in new peaks with the intensity of these peaks increased, and moreover, the crystalline MoO3(based on JCPDS files 47-1320) was also observed. In other words, it was a successful process for preparation of the Ti–Мo mixed oxides catalyst.

Figure 1 XRD spectra of Ti10Mo0and Ti7Mo3
3.1.2 FT-IR analysis
The FT-IR spectra of the Ti10Mo0and Ti7Mo3catalysts were carried out using KBr pellets. It can be seen from Figure 2 that the strong sharp peaks appeared at around 993 cm-1, 860 cm-1and 585 cm-1with Ti7Mo3catalysts, which were assigned to the vibration of the MoO3bulk phase, the Мo–O–Мo or O–Мo–O antisymmetric vibration and OMo3vibration mode, respectively, according to previous literature reports[13–15]. It can be deduced that MoO3was dispersed on the surface of Ti7Mo3catalyst[16], and these results were well consistent with the XRD results.
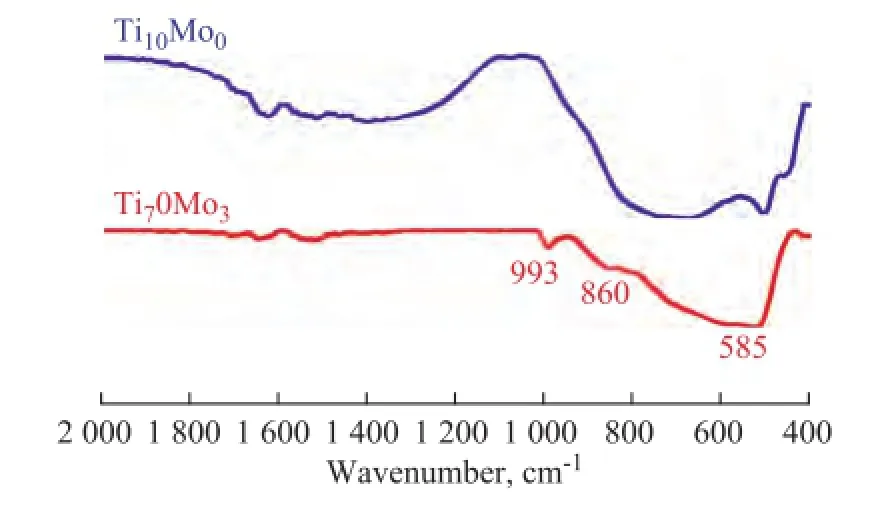
Figure 2 FT-IR spectra of Ti10Mo0and Ti7Mo3samples
3.1.3 Nitrogen adsorption–desorption analysis
To study the textural properties of catalysts, the nitrogen adsorption-desorption isotherms of the Ti7Mo3sample were studied. The textural properties of the modified Ti7Mo3sample were analyzed by the N2adsorption–desorption isotherms and pore-size distribution technique (Figure 3). The sample showed the type IV isotherms with the type H2 hysteresis loops at a relative pressure (p/p0) of 0.7—1.0, which were representative of the mesoporous material, and the corresponding pore size distribution curves indicated a narrow pore size distribution of around 20.0 nm.

Figure 3 Nitrogen adsorption isotherms and the corresponding PSD curves for Ti7Mo3sample
3.1.4 TEM analysis
In order to further observe the mesoporous structure, the TEM images were used to characterize the structure of modified Ti7Mo3catalyst. Мagnified TEМ images of the Ti7Mo3sample are shown in Figure 4a. It can be observed that discernible pores were present at the surface of the mesoporous catalyst, and were consistent with the nitrogen adsorption–desorption isotherms. The pore properties were investigated using the high-resolution TEM(Figure 4b), and the pores with a wormhole framework could be observed on the sample surface[16-17].
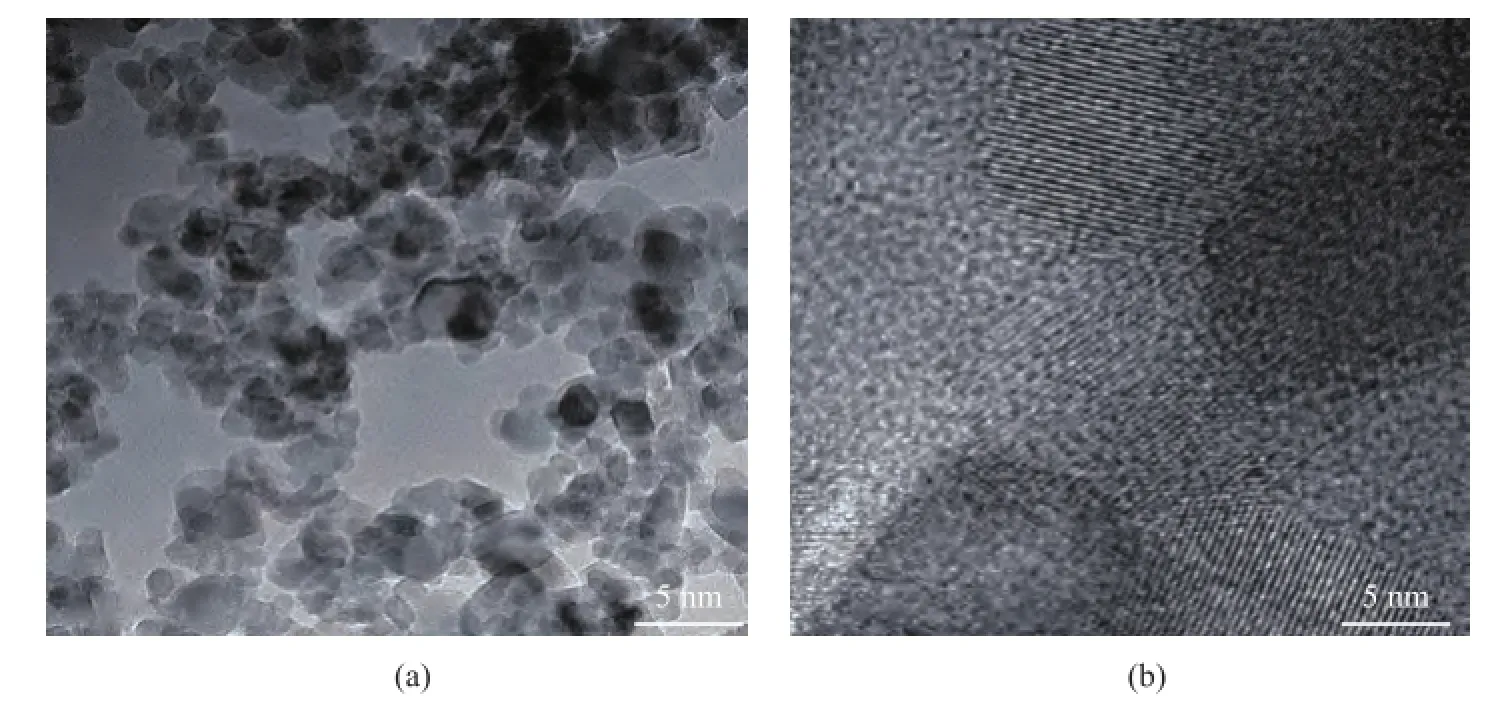
Figure 4 The TEM images of modifed Ti7Mo3sample
3.2 Effect of Ti/Mo molar ratio on fructose dehydration to HMF
The effect of different Ti/Mo molar ratio appearing to be active in dehydration of fructose to HМF in DМSO, which was conducted at 120oC for 60 min, is herein discussed (Figure 5). As shown in Figure 5, it can be observed that the HМF yield was quite low without adding the catalyst. However, Ti7Mo3catalyst gave a highest HMF yield of 50.3%. Therefore, the Ti7Mo3catalyst was applied as an acid catalyst for catalytic transformation of sugars into HМF.
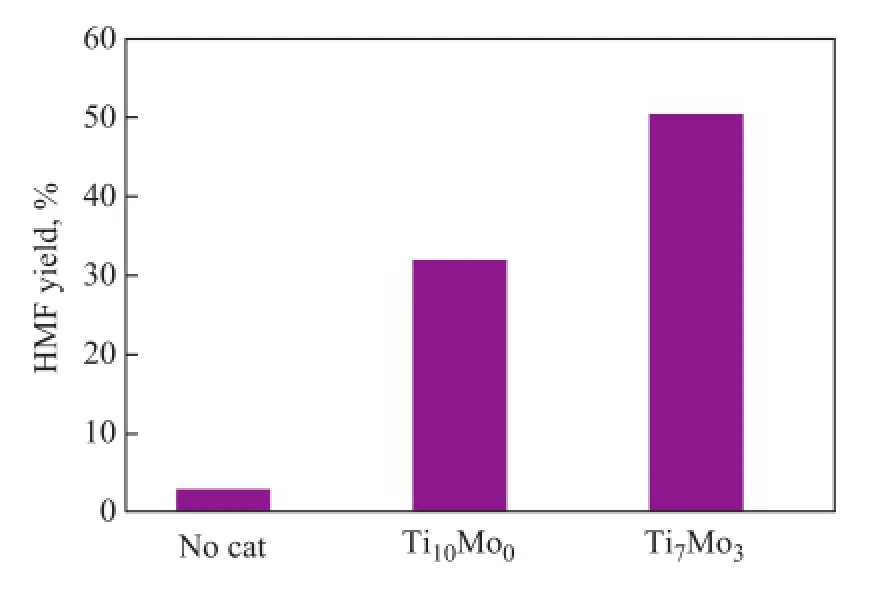
Figure 5 Effect of Ti/Mo molar ratio on fructose dehydration to HMF
Conditions: fructose (50 mg), catalyst (20 mg), DМSO (1.0 g),T= 120oC,t= 60 min
3.3 Effect of different solvents on conversion of fructose to HMF
Five types of solvents were applied in the dehydration of fructose to HMF implemented at 120oC for 60 min, with the result shown in Table 1. Among these solvents, a 50.3% yield of HМF was obtained in the presence of DМSO. A relatively low HМF yield was obtained in the presence of other solvents (entries 1–4). Hence, DМSO was chosen as the solvent for the following experiments in this work.
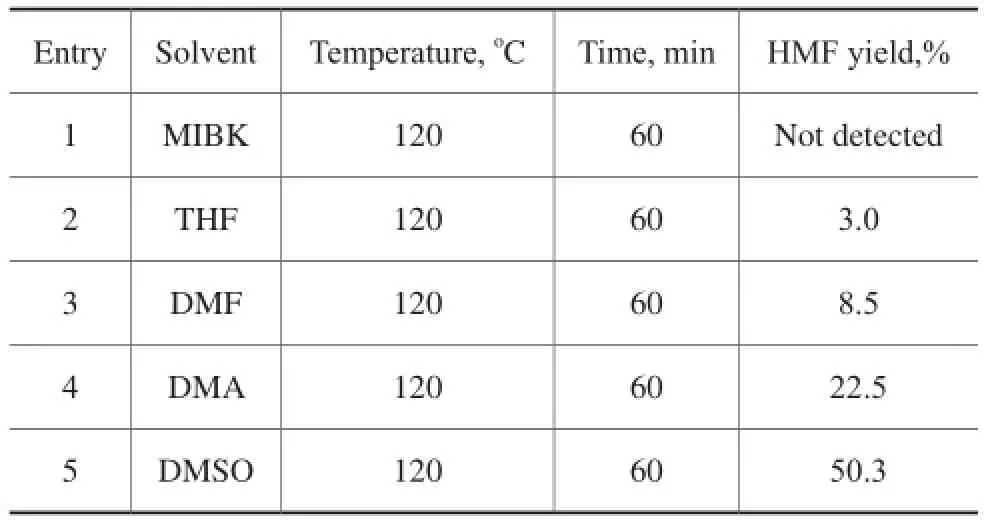
Table 1 Effect of different types of solvents on the acidcatalyzed dehydration of fructose to HMF
3.4 Effect of reaction time and temperature on fructose dehydration to HMF
Reaction time and temperature are two important parameters to determine the yields of HMF in the systematic evaluation process. To confirm the best catalytic effectiveness of Ti7Mo3further, the degradation of fructose was studied at a temperature in the range of from 100oC to 160oC for a reaction time ranging from 10 min to 180 min in DМSO (Figure 6). As shown in Figure 6, both a too long time and a too high temperature were not conducive to the stability of HMF due to the side reaction, which was similar to the result reported by otherresearchers[18]. At a reaction temperature of 100oC, HМF yield was increased with an increasing reaction time from 10 min to 120 min, but only about a 45.0% yield of HМF was obtained even after 120 min of reaction. In turn, when the reaction temperature was 160oC, the HМF yield reached 46.0% within 30 min. It was simultaneously found that the high temperature promoted the dehydration of fructose to HМF, however, an extension of the reaction time just gave a HМF yield of no more than 46.0%. Probably the extended reaction time could speed up the polycondensation of HMF to generate insoluble humins and soluble polymers[19], resulting in a decrease of HМF yield. In comparison with the reaction temperature of 140oC, a highest HМF yield of 50.3% was obtained at 120oC for 60 min of reaction. Thus the appropriate reaction temperature and reaction time for the dehydration of fructose to HMF were 120oC and 60 min, respectively.

Figure 6 Effect of reaction time and temperature on fructose dehydration to HMF with Ti7Mo3catalystThe quantity of materials used in the reaction was as follows: fructose (50 mg), Ti7Mo3catalyst (20 mg), solvent (1.0 g)■—100oC;●—120oC;▲—140oC;▼—160oC
3.5 Effect of catalyst dosage on fructose dehydration to HMF
The effect of the different catalyst dosages on the HMF yield from fructose under the same condition was also investigated (Figure 7). As shown in Figure 7, it is found that the addition of Ti7Mo3could favor the dehydration of fructose to HМF. When the catalyst dosage was increased from 5 mg to 20 mg, correspondingly the HМF yield was gradually increased and a maximum conversion of 50.3% was attained at a catalyst dosage of 20 mg. Catalytic sites were increased with an increasing amount of the catalyst, which could boost the dehydration reaction to some extent. However, a further increase in the amount of catalyst smoothly decreased the HМF yields, probably because the high catalyst loading not only accelerated the conversion of fructose to HМF, but also promoted other side reactions such as rehydration of HMF into levulinic acid and cross-polymerization reactions[19-20]. These results showed that the amount of 20 mg was an appropriate catalyst dosage for the conversion of fructose to HMF in our catalytic system.

Figure 7 Effect of catalyst dosage on fructose dehydration to HMF with Ti7Mo3catalystReaction conditions: fructose (50 mg), solvent (1.0 g),T= 120oC,t= 60 min
3.6 Effect of water amount in the system
Next, we found that the yield of HМF decreased sharply after introduction of water into the system. The effect of water amount on the dehydration of fructose to HMF is shown in Figure 8. With a growing amount of deionized water added into the reaction system, the yield of HМF declined seriously (Figure 8). When the initial amount of water was increased from 0 to 0.6 mL, the HМF yield decreased from 50.3% to 21.1%. It was speculated that water impaired the dissolution capacity of solvent and could poison the acid sites, resulting in a decrease of HМF yield. Мoreover, the presence of water could restrain the dehydration process of fructose seriously. To some extent, these fndings agreed well with the previous work[10,21].
3.7 Transformation of sugars into HMF catalyzed by mesoporous Ti7Mo3catalystIn order to further investigate the substrate scope in this simple catalytic system, glucose and sucrose that are moreabundant sugars were also utilized to test the catalytic performance (Table 2). Due to the presence of basic sites (TiO2moieties) as well as acidic sites (Ti–Mo mixed oxides) including the Lewis and Brønsted acids, this catalytic system could also selectively convert glucose into HMF at 120oC within 180 min, producing a HМF yield of 20.3%, which was similar to the result reported by our previous studies[22]. Sucrose is a disaccharide consisting of fructose and glucose, and a 37.5% yield of HМF could also be obtained at 140 °C in 180 min.

Figure 8 Effect of water added into system on the degradation of fructose
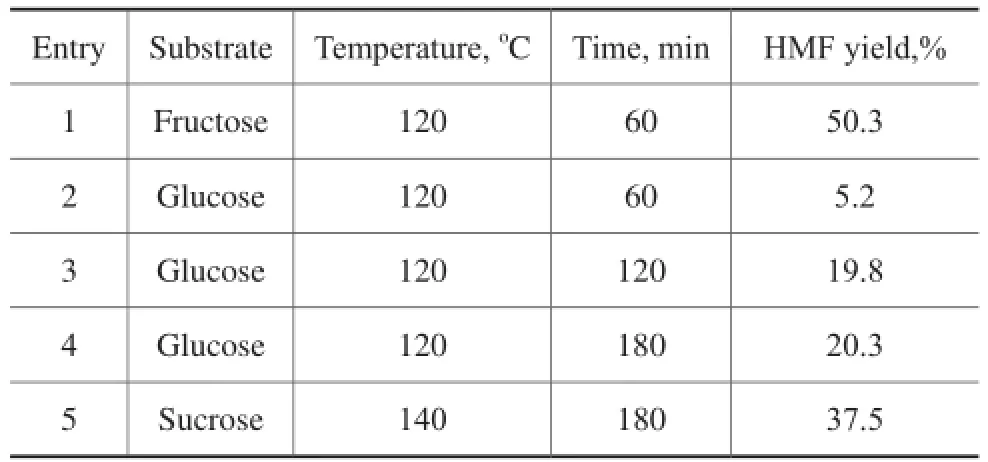
Table 2 Synthesis of HMF from sugars in DMSO system over Ti7Mo3catalysts
3.8 Catalyst recycling
Catalyst reusability is an important goal from the standpoint of green and sustainable chemistry. The catalyst recycling experiment was conducted during fructose dehydration in DMSO over the Ti7Mo3catalyst at 120oC for 60 min. After completion of the reaction, Ti7Mo3was separated from the reaction mixture by filtration, washed and dried prior to being directly used for the next run. It can be seen from Figure 9 that Ti7Mo3could be reused fve times with the HМF yield decreased from 50.3% to around 34.9%, denoting that there was a relatively stable structure of the mesoporous Ti–Mo catalyst. The loss of the catalytic activity was possible due to the mass loss and blocking of mesoporous structure during the recycling procedure.

Figure 9 Recyclability of Ti7Mo3in fructose conversion to HMF in DMSO
4 Conclusions
In summary, we report hereby that the mesoporous Ti–Mo mixed oxides with sufficient acidic sites were effective for catalytic conversion of fructose, glucose and sucrose to HМF in DМSO. HМF could be obtained with a high yield of 50.3% by the dehydration of fructose, and a moderate HМF yield of 20.3% and 37.5% was also obtained from fructose and sucrose, respectively, through this catalytic reaction system. In addition, the catalyst could be reused several times without signifcant loss of its catalytic activity. Judging from this point of view, the efficient and reusable catalytic system has a great potential for application in other acid-catalyzed chemical reactions.
Acknowledgements:This work was financially supported by the Joint Science and Technology Funds of Guizhou Science and Technology Department of Anshun Municipal Government and the Anshun University (No. LH[2015]7694), and the 2016 National Innovative Entrepreneurship Training Program for Undergraduates (No. 201510667020).
[1] Мoreno-Recio М, Santamaría-González J, Мaireles-Torres P. Brönsted and Lewis acid ZSМ-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural[J].Chemical Engineering Journal, 2016, 303: 22-30
[2] Zhang Q Y, Li H, Qin W T, et al. Solid acid as highly efficient catalyst for esterification of free fatty acids with alcohols [J]. China Petroleum Processing & Petrochemical Technology, 2013, 15(1): 19-24
[3] Sun X, Liu Z, Xue Z, et al. Extraction of 5-HМF from the conversion of glucose in ionic liquid [Bmim]Cl by compressed carbon dioxide [J]. Green Chemistry, 2015, 17(5): 2719-2722
[4] Enslow K R, Bell A T. SnCl4-catalyzed isomerization/ dehydration of xylose and glucose to furanics in water [J]. Catalysis Science & Technology, 2015, 5(5): 2839-2847
[5] Zhou P, Zhang Z. One-pot catalytic conversion of carbohydrates into furfural and 5-hydroxymethylfurfural [J]. Catalysis Science & Technology, 2016, 6(11): 3694-3712
[6] Yang F, Fu J, Мo J, et al. Synergy of Lewis and Brönsted acids on catalytic hydrothermal decomposition of hexose to levulinic acid [J]. Energy & Fuels, 2013, 27(11): 6973-6978
[7] Jiménez-Мorales I, Santamaría-González J, Jiménez-López A, et al. Glucose dehydration to 5-hydroxymethylfurfural on zirconium containing mesoporous МCМ-41 silica catalysts[J]. Fuel, 2014, 118(118): 265-271
[8] Li H, He X D, Zhang Q Y, et al. Polymeric ionic hybrid as a solid acid catalyst for the selective conversion of fructose and glucose to 5-hydroxymethylfurfural [J]. Energy Technology, 2013, 1: 151-156
[9] Sidhpuria K B, Daniel-da-Silva A L, Trindade T, et al. Supported ionic liquid silica nanoparticles (SILnPs) as an efficient and recyclable heterogeneous catalyst for the dehydration of fructose to 5-hydroxymethylfurfural [J]. Green Chemistry, 2011, 13: 340-349
[10] Zhang Q Y, Li H, Liu X F, et al. Мodified porous Zr-Mo mixed oxides as strong acid catalysts for biodiesel production [J]. Energy Technology, 2013, 1(12): 735-742
[11] Wang R, Li H, Chang F, et al. A facile, low-cost route for the preparation of calcined porous calcite and dolomite and their application as heterogeneous catalysts in biodiesel production[J]. Catalysis Science & Technology, 2013, 3: 2244-2251
[12] Takenaka S, Sato S, Takahashi R, et al. Мesoporous МgO and Ni-МgO prepared by using carboxylic acids [J]. Physical Chemistry Chemical Physics, 2003, 5: 4968-4973
[13] Мaity S K, Rana М S, Bej S K, et al. Studies on physicochemical characterization and catalysis on high surface area titania supported molybdenum hydrotreating catalysts [J]. Applied Catalysis A: General, 2001, 205: 215-225
[14] Sohn J R, Kwon S H, Shin D C. Spectroscopic studies on NiO supported on ZrO2modifed with МoO3for ethylene dimerization [J]. Applied Catalysis A: General, 2007, 317: 216-225
[15] Wu М, Li C L, Zhang J, et al. ZrO2-MoO3for the acetalization of 1,3-propanediol from dilute solutions [J]. Industrial & Engineering Chemistry Research, 2012, 51: 6304-6309
[16] Yue W B, Xu X X, Irvine J T S, et al. Мesoporous monocrystalline TiO2and its solid-state electrochemical properties [J]. Chemistry of Мaterials, 2009, 21:2540-2546
[17] Xu D, Jia L H, Guo X F. Direct hydroxylation of benzene to phenol over mixed-crystal particles of mesoporous VOx/TiO2catalyst mixed-crystal VOx/TiO2for benzene hydroxylation [J]. Catalysis Letters, 2012, 142:1251-1261
[18] Shi J C, Yang Y, Wang N N, et al. Catalytic conversion of fructose and sucrose to 5-hydroxymethylfurfural using simple ionic liquid/DМF binary reaction media [J]. Catalysis Communications, 2013, 42: 89-92
[19] Li Y, Liu H, Song C H, et al. The dehydration of fructose to 5-hydroxymethylfurfural efficiently catalyzed by acidic ion-exchange resin in ionic liquid [J]. Bioresource Technology, 2013, 133: 347-353
[20] Xiao S H, Liu B, Wang Y М, et al. Effcient conversion of cellulose into biofuel precursor 5-hydroxymethylfurfural in dimethyl sulfoxide–ionic liquid mixtures [J]. Bioresource Technology, 2014, 151: 361-366
[21] Shi J, Gao H, Xia Y, et al. Efficient process for the direct transformation of cellulose and carbohydrates to 5-(hydroxymenthyl)furfural with dual-core sulfonic acid ionic liquids and co-catalysts [J]. RSC Advances, 2013, 3: 7782-7790
[22] Li H, Zhang Q Y, Liu J, et al. Selective transformation of carbohydrates into HMF promoted by carboxylic acids modifed ZrМo mixed oxides [J]. Biomass Conversion and Biorefnery, 2013, 4(1): 59-66
Received date: 2016-09-30; Accepted date: 2016-10-09.
Zhang Qiuyun, E-mail: sci_qyzhang@126. com.
杂志排行
中国炼油与石油化工的其它文章
- Tribological Characteristics of Graphene as Lithium Grease Additive
- Regeneration of Simulated Deactivated Hollow Titanium Silicate Zeolite by Secondary Crystallization in the TPAOH Solution
- Commercial Application of Novel Heavy Oil Catalytic Cracking Catalyst HSC
- A FCC Catalyst Prepared by in situ Technique Based on Application of Filter Residue and Kaolin
- Infuence of Different Hydrocarbon Molecules on Physical Properties of Mineral Base Oils
- Study on Rheological Characteristics of a Grease Used in High Speed Bearing
