Clinical Observation of Bevacizumab Combined with S-1 in the Treatment of Pretreated Advanced Esophageal Carcinoma△
2017-01-13KekeNieChuanxinGengLingZhangShichaoLiuZhongfaZhangRongWangXiaoZouandYouxinJi
Ke-ke Nie, Chuan-xin Geng, Ling Zhang, Shi-chao Liu, Zhong-fa Zhang, Rong Wang, Xiao Zou, and You-xin Ji*
Clinical Observation of Bevacizumab Combined with S-1 in the Treatment of Pretreated Advanced Esophageal Carcinoma△
Ke-ke Nie1, Chuan-xin Geng1, Ling Zhang1, Shi-chao Liu1, Zhong-fa Zhang1, Rong Wang2, Xiao Zou2, and You-xin Ji2*
1Department of Oncology, Qingdao Cancer Hospital, Qingdao, Shandong 266042, China2Department of Oncology, Qingdao Central Hospital, 2ndAffiliated Hospital of Qingdao University, Qingdao,Shandong 266042, China
anti-angiogenesis; bevacizumab; chemoradiation; S-1; esophageal carcinoma
Objective To investigate the clinical effects and safety of bevacizumab combined with S-1 as the second-line treatment of recurrent and/or metastatic esophageal cancer after chemoradiation.
Methods Patients with recurrent or metastatic esophageal cancer after chemoradiation were treated with bevacizumab and S-1. Bevacizumab was used by intravenous infusion, 7.5mg/kg body weight on day 1; S-1 was used by oral at 80mg/m2·d on day 1-14, 21 days as a cycle of treatment and repeated until either pro- gressive disease or intolerable toxicity occurred. Chest CT were performed and RECIST 1.1 was used for response evaluation. Kaplan-Meier method was used for survival analysis. Side effects were recorded and analyzed.
Results Totally 78 patients were enrolled in the study, including 67 squamous cell carcinoma and 11 adenocarcinoma histologically. The overall response (CR+PR) rate was 22.4% (17/76) and disease control (CR+PR+SD) rate was 61.8% (47/76) respectively. The median follow-up time was 20 months (range from 9 to 44 months). The median progression-free survival (PFS) was 4.9 months (95%4.4-5.5) and the median overall survival (OS) was 8.1 months (95%7.6-9.2). The median PFS and OS of patients with metastasis diseases were 6.2 months (95%3.3 to 6.3) and 8.5 months (95%5.8 to 11.2), where PFS was longer than that of patients with local regional recurrence (median 5.0 months, 95%3.0 to 5.5,=0.017) and OS was longer than that of patients with regional disease and metastasis (median 8.0 months, 95%4.6 to 9.5,=0.010). The common adverse effects were mild to moderate neutropenia (84.2%), grade I-II hand and foot syndrome (51.3%), grade I-II nausea (48.7%), mild epistaxis (30.1%) and mild vomiting (14.5%). Esophageal bleeding occurred in 7.9% of patients. One patient (1.3%) died from massive bleeding which was caused by esophageal perforation.
Conclusion Bevacizumab combined with S-1 was effective and safe for esophageal cancer patients who had recurrent or metastatic diseases after chemoradiation.
Chin Med Sci J 2016; 31(4):221-227
SOPHAGEAL cancer is one of the leading causes of cancer death globally. More than half of newly diagnosed esophageal cancer and death from esophageal cancer happened in China.1-3Most of the esophageal cancer patients were over 40 years old and diagnosed at advanced stages. The incidence of esophageal cancer is closely related to tobacco use and/or alcohol consumption, which are also the etiologies of pulmonary and heart diseases.1-4Complicated chronic heart and lung diseases combined with dysfunction, hypertension, diabetes etc, further reduce patients’ comp- liance to the treatment and increase risks of complications related to treatment or disease.5Concurrent chemotherapy and radiotherapy improve patient survival, which is considered to be the standard therapy in patients who is inoperable for unsuitable for surgery.6Most of the patients treated by chemoradiation would develop relapse or metastasis in the first two year. These patients are usually unsuitable for surgical resection and/or re-chemoradiationbecause of their poor physical compliance or the advanced stages.
Bevacizumab, a vascular endothelial growth factor A (VEGFA) monoclonal antibody, proved to be a tumor angiogenesis inhibitor, is wildly used in the colorectal cancer and lung adenocarcinoma, for it increase ORR or survival of both diseased. Bevacizumab combined with VEGFA could blockades VEGFA dependent tumor blood vessels formation, attenuates tumor nutrition, prompts tumor cell apoptosis and finally shrinks tumor. Esophageal carcinoma, which is abundant with blood supply, has a character of early metastasis.7-9VEGF over expression is correlated with lymph node metastases and poor prog- nosis.10-11
S-1, a novel oral dihydropyrimidine dehydrogenase (DPD) inhibitory fluoropyrimidine (DIF) based on a biochemical modulation of 5-fluorouracil (5-FU), was developed in 1990s for the treatment of gastric cancer. It contains tegafur (FF) and two types of enzyme inhibitor, 5-chloro-2,4-dihydroxypyridine (CDHP) and potassium oxonate (Oxo), in a molar ratio of 1:0.4:1. In pharma- cokinetic studies, S-1 showed high 5-FU concentration in blood for long periods of time.12S-1 achieved a 46% response rate for single drug therapy in advanced gastric cancer. When combined with nedaplatin or cis-platin, the response rate reached 83.3%-90.9%, but these results need to be confirmed by further phase Ⅲ studies.13-15
Doublets drug regimes have been found to have promising efficacy for esophageal cancer. But there is no standard treatment for patients with recurrence or metastatic disease after chemoradiation. Outcome is extremely poor. It is important to find an effective way with low toxicities to ensure treatment compliance. Combined use of anti-angiogenic antibody with chemotherapy may play an important role in the management of pretreated advanced esophageal cancer and may improve survival of patients with heavily treated advance esophageal cancer. This study was performed in purpose of evaluating efficacy and safety of the combined treatment in advanced and pretreated esophageal cancers.
PATIENTS AND METHODS
Study design and patients enrollment
This study was designed as a two-center open-labeled PhaseⅡ-Ⅲ clinical trial to exam the response rates, toxicities, andoutcomes of bevacizumab combined with S-1 in the treatment of pretreated advanced esophageal carcinoma. It was app- roved by the ethics committee of Qingdao Central Hospital and the 2nd Affiliated Hospital of Qingdao University, and complied with the provisions of Good Clinical Practice Guideline and the Declaration of Helsinki and local laws.
Study patients inclusion criteria were: 1) pathological or cytological confirmed esophageal squamous cell carcinoma or adenocarcinoma; 2) local regional relapse or metastasis after esophageal chemoradiation; 3) having one or more measureable target lesions other than previously treated primary lesions; 4) ineligible to surgery or re-irradiation because of advanced stages; 5) estimated life time was 3 months or longer according to patients performance status; 6) radiation therapy completed at least 4 weeks prior to the enrollment; 7) ECOG (Eastern Cooperative Oncology Group)16performance states were 2 or less. Patients were excluded if they were pre-treated by any anti-angiogenesis therapies, or had uncontrolled hypertension or uncontrolled bleeding. Adequate cardiovascular function, liver function and renal function were also required. Informed consents were obtained before enrollment.
Treatments and follow-up
All eligible patients received bevacizumab (Avastin, Roche) 7.5 mg/kg intravenous infusion on day 1 every 21 days and S-1 (Taiho, Japan) 80 mg/m2·d, day 1 to day 14, 21 days a cycle. Treatment was discon- tinued if: 1) the disease progression defined by RECIST 1.1;172) intolerable side effects occurred; 3) ECOG performance state changed to 3; or 4) requested by patients or physicians. One dose reduction (60 mg/m2·d for 14 days, every 21 days a cycle) of S-1 was acceptable. If further reduction had to be performed, patients would be withdrawn from the study. Allpatients were followed up by telephone call.
Assessment
Tumor measurements were performed on CT images. CT scans were performed before treatment start as the base- line and every 4 weeks thereafter. Patients’ compliance, treatment safety, side effects were accessed at each check point.
Responses to the treatment were classified into 4 categories according to RECIST 1.1: complete response (CR, disappearance of tumor lesions), partial response (PR, a decrease of at least 30% in the sum of maximum diameter of target lesions), stable disease (SD, steady state of disease), or progressive disease (PD, an increase of 20% or over in the sum of maximum diameter of target lesions). The overall response rate (ORR) was defined as (CR+PR)/patients number×100%, and disease control rate (DCR) was defined as (CR+PR+SD) /patients number×100%. The survival outcomes were assessed by progression-free survival (PFS), which was defined as the time from treat- ment to disease progression or death, and overall survival (OS), which was defined as the time from treatment to death or the last follow-up. Treatment-related adverse events were assessed with WHO adverse reaction criteria. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria version 3.0.18
Statistical analysis
Data were statistically analyzed using Sigma Plot 11.0 (Systat, USA). Median PFS and OS curves were analyzed by Kaplan-Meiers log Rank test. Covariates analyses were used to assess survival differences in patients with local regional disease, metastatic diseases, or both, as well as between squamous cell carcinoma and adenocar- cinoma. Avalue of <0.05 was considered statistically significant.
RESULTS
Patients and characteristics
From November 2011 to November 2014, totally 76 patients were enrolled the study. All patients were Chinese from the mainland China. The clinical characteristics of enrolled patients were listed in Table 1. All enrolled patients had recurrent or metastatic diseases with at least one site measurable lesion and had a history of concurrent chemotherapy (TP: docetaxel with cis-platin or nedaplatin; FP: 5-FU with cis-platin or nedaplatin) and radiotherapy (conformal or intensity-modulated radiotherapy 60-66 Gy/30-33 f in 6-7 weeks period). The median follow-up time was 20 months (Range from 9 to 44 months). One patient missed follow-up at 5th month.
Clinical efficacy
Totally 17 patients reached PR and 30 patients maintained SD. There was no CR. The ORR was 22.4% (17/76) and DCR was 61.8% (47/76) respectively. Patients with squamous cell carcinoma had ORR of 22.4% (15/67) and DCR of 61.2% (41/67) respectively, which was comparable to patients with adenocarcinoma, where ORR and DCR were 22.2% (2/9) and 66.7% (6/9) respectively (Table 2). It had to be noted that 12 patients received only 2 cycles of the treatment because of non medical reasons.

Table 1. Characteristics of 76 enrolled patients
ECOG: Eastern Cooperative Oncology Group score standard

Table 2. Comparisons of clinical efficacy between squamous cell carcinoma and adenocinoma(n=76)
CR: complete response; PR: partial response; SD: stable disease; ORR: overall response rate; DCR: disease control rate.
The median PFS was 4.9 months (95%4.4 to 5.5) (Fig. 1) and the median OS was 8.1 months (95%7.6 to 9.2) (Fig. 2).Stratified by cancer characteristics, patients with metastatic disease had a longer PFS (6.2 months, 95%3.3-6.3) than those with local regional recurrence (5.0 months 95%3.0-5.5,=0.017) or local regional recurrence concurrent with metastasis (=0.025) (Fig. 3). OS was also favorable to metastasis only patients. Patients with metastasis had a longer OS (8.5 months, 95%5.8-11.2) than patients with local regional recurrence concurrence with metastasis (8.0 months, 95%9.0-9.5,=0.010) (Fig. 4). PFS and OS of patients with squamous cell carcinoma (PFS 5.5 months, OS 8.0 months) were superior to those of adenocarcinoma patients (PFS 4.7 months, OS 7.5 months), but there were no significant difference (both>0.05) (Figs. 5, 6).

Figure 1. Kaplan-Meier plot of progression-free survival (PFS).

Figure 2. Kaplan-Meier plot of overall survival (OS).

Figure 3. Kaplan-Meier analysis of the PFS stratified by chara- cteristics of the disease.A. B,=0.017; A. C,=0.025; B. C,=0.50.

Figure 4. Kaplan-Meier analysis of the OS stratified by chara- cteristics of the disease.A. C,=0.0101; A. B,=0.0819; B. C,=0.419.
Side effects
The main hematological adverse effects were mild to moderate neutropenia (84.2%), thrombocytopenia (18.4%), and anemia (14.5%). The Grade 3-4 neutropenia and/or thrombocytopenia happened rarely. The main non-hema- tological adverse effects were Grade 1-2 hand and foot syndrome (51.3%), nausea (46.1%), and epistaxis (30.1%). Six patients had mild esophageal bleeding after 4-5 cycles bevacizumab treatment, among them two discontinued the treatment and bleeding stopped afterwards, the rest four bleeding patients continued bevacizumab without severe bleeding event. One patient died from massive esophageal bleeding, which caused by esophageal perfo- ration. There was one patients who encountered esophageal perforation too, but was alleviated after gas- trointestinal decompression (Table 3).
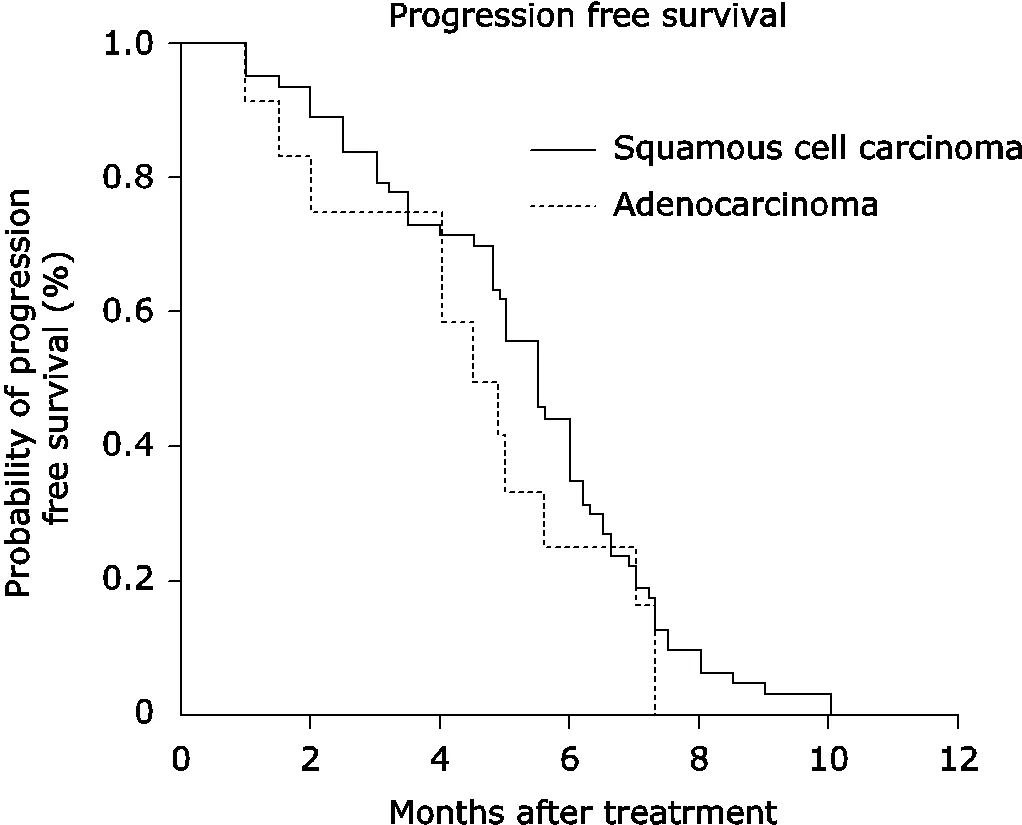
Figure 5. Kaplan-Meier analysis of the PFS stratified by his- tology of the disease.Squamous cell carcinoma. adenocarcinoma, PFS median 5.5 months4.7 months,=0.2709.

Figure 6. Kaplan-Meier analysis of the OS stratified by histology of the disease.Squamous cell carcinoma. adenocarcinoma, OS median 8.0 months. 7.5months,=0.9381.

Table 3. Side effects of bevacizumab with S-1 treatment in recurrent and/or metastatic esophageal cancer patients n(%)
*Massive bleeding caused by perforation and died.
DISCUSSION
Esophageal cancer is rampant in China. Most of patients are diagnosed in late stage and its prognosis is extremely poor. Its etiologies include family history, pre-oncology lesion, tobacco use and alcoholism. Although new chemotherapy agents including taxanes, oxaliplatin, oral fluoropyrimidines and irinotean have been demonstrated for their effectiveness, the overall survival still has been stable with no significant increase. For recurrence or metastatic esophageal cancer after chemoradiation, there is usually no standard chemotherapy regimen. Since esophageal cancer is a molecular heterogeneous disease, strategies of chemotherapy combined targeted therapy are proposed to be effective.19-20
VEGF, the most potent angiogenic molecule, partici- pate in the tumor angiogenesis and is responsible for metastasis and prognosis in esophageal cancer.21-22P53 mutation is involved in VEGF expression of esophageal cancer. VEGF gene induces neovascularization in and around tumor, and VEGF augments metastatic potential by accelerating proliferative activity after reaching the target organ.21In mouse xenograft models of human ovarian and esophageal cancer (SKOV-3 and OE19), bevacizumab treatment decreased tumor mean vessel density (MVD), permeability, and vessel normalization compared with baseline in the tumor models relative to controls.22-23Cancer angiogenesis becomes pervasive with its proliferation and metastasis, and VEGF expression is higher in late stage than early stage. Base on this, bevacizumab may work in advance stage patients of esophageal cancer even through it did not improve the resection rate or OS when it added to cis-platin and 5-FU neoadjuvant chemotherapy compared with prior regimens.20
S-1 (TS-1), a novel oral formation of 5-FU, is wildly used to treat advanced gastric cancer in Japan and recently has been studied in patients with esophageal cancer.24-28It’s convenient to take with less toxicity, and is well tolerated in pre-heavily treated esophageal cancer patient.
Patients with recurrent or metastatic esophageal cancer after chemoradiotherapy usually have a poor survival, most patients died in 8 months.29In this study, the ORR was 22.4% and DCR was 61.8%, the median PFS was 4.9 months and the median OS was 8.1 months respectively, which showed effectiveness of the combined with chemotherapy and anti-VEGF therapy. Patients with metastatic disease had a longer PFS and OS than patients with local regional recurrence or local regional recurrence concurrent with metastasis. As patients in this study patients were all irradiation treated, local regional recurrence lesions might be lack of blood supply because of treatment related fibrosis. Although bevacizumab normalized tumor vessel and reduced permeability, hypoxia cancer cells may promote VEGF expression, and resist to chemo drug and anti-angiogenesis.
ORR and DCR in patients with squamous cell carcinoma were comparable to adenocarcinoma; PFS and OS were favorable to squamous cell carcinoma, but there were no significant difference (>0.05). Because there was only a small number of adenocarcinoma enrolled in this study, the effects of bevacizumab and S-1 on esophageal adenocarcinoma need to be further investigated in the future.
The main hematological adverse effects were mild to moderate neutropenia, thrombocytopenia, and anemia. Grade 3-4 neutropenia and/or thrombocytopenia were low. Nearly half patients suffered Grade 1-2 hand and foot syndrome, nausea. This might be the contribution of united uses of S-1. Mild epistaxis happened in nearly one-third patients after 4th cycles of bevacizumab, and lasted for 2-3 days, with no need of medical intervention. Two patients suffered from esophageal perforation and one patient died of massive esophageal bleeding. So endoscopic evaluation before bevacizumab may help to exclude patient who has blood vessels invasion or is complicated by esophageal ulcers.
In conclusion, this study showed combined use of bevacizumab and S-1 in the treatment of pre-treated advanced esophageal cancer was effective. Toxicities were mild and patients could tolerate well. Further study with more patients is necessary in the future.
1. Jiménez F, Elias RE, Osella FJ, et al. Digestive tract cancer: after ten years in Santa Fe. Acta Gastroenterol Latinoam 2009; 39: 242-9.
2. Tian J, Chen JS. Time trends of incidence of digestive system cancers in changle of China during 1988-2002. World J Gastroenterol 2006; 12: 4569-71.
3. Tao Z, Cha Y, Sun Q. Cancer mortality in high background radiation area of Yangjiang, China, 1979-1995. Zhong Hua Yi Xue Za Zhi 1999; 79: 487-92.
4. Al Dulaimi D. Recent advances in esophageal diseases. Gastroenterol Hepatol Bed Bench 2014; 7: 186-9.
5. Lin D, Leichman L. The current status of neoadjuvant therapy for esophageal cancer. Semin Thorac Cardiovasc Surg 2014; 26: 102-9.
6. Shridhar R, Imani-Shikhabadi R, Davis B, et al. Curative treatment of esophageal cancer: an evidenced based review. J Gastrointest Cancer 2013; 44: 375-84.
7. Zondor SD, Medina PJ. Bevacizumab: an angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann Pharmacother 2004; 38: 1258-64.
8. Chen HX. Expanding the clinical development of bevaci- zumab. Oncologist 2004; 9 Suppl 1: 27-35.
9. Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3: 391-400.
10. Xia H, Shen J, Chen S, et al. Overexpression of VEGF-C correlates with a poor prognosis in esophageal cancer patients. Cancer Biomark 2016; 17: 165-70.
11. Möbius C, Freire J, Becker I, et al. VEGF-C expression in squamous cell carcinoma and adenocarcinoma of the esophagus. World J Surg 2007; 31: 1768-72.
12. Sakata Y, Ohtsu A, Horikoshi N, et al. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 1998; 34: 1715-20.
13. Iwase H, Shimada M, Nakamura M, et al. A pilot study of TS-1 combined with cisplatin in patients with advanced gastric cancer. Gan To Kagaku Ryoho 2002; 29:1575-82.
14. Lee SR, Kim HO, Yoo CH. Clinical outcomes of TS-1 chemotherapy for advanced and recurrent gastric cancer. J Korean Surg Soc 2011; 81:163-8.
15. Tanaka Y, Yoshida K, Tanahashi T, et al. Phase II trial of neoadjuvant chemotherapy with docetaxel, nedaplatin, and S1 for advanced esophageal squamous cell carcinoma. Cancer Sci 2016; 107: 764-72.
16. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982: 5: 649-56.
17. Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan To Kagaku Ryoho 2009; 36: 2495-501.
18. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13: 176-81.
19. Kordes S, Cats A, Meijer SL, et al. Targeted therapy for advanced esophagogastric adenocarcinoma. Crit Rev Oncol Hematol 2014; 90: 68-76.
20. Idelevich E, Kashtan H, Klein Y, et al. Prospective phase II study of neoadjuvant therapy with cisplatin, 5-fluorouracil,and bevacizumab for locally advanced resectable esophageal cancer. Onkologie 2012; 35: 427-31.
21. Arii S, Mori A, Uchida S, et al. Implication of vascular endothelial growth factor in the development and metastasis of human cancers. Hum Cell 1999; 12: 25-30.
22. Kleespies A, Guba M, Jauch KW, et al. Vascular endothelial growth factor in esophageal cancer. J Surg Oncol 2004; 87: 95-104.
23. Arjaans M, Oude Munnink TH, Oosting SF, et al. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res 2013; 73: 3347-55.
24. Park I, Ryu MH, Choi YH, et al. A phase Ⅱ study of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy followed by surgery and adjuvant S-1 chemotherapy in potentially resectable gastric or gastro- esophageal junction adenocarcinoma. Cancer Chemother. Pharmacol 2013; 72: 815-23.
25. Ohashi M, Arai K, Iwasaki Y, et al. Two cases of advanced gastric cancer responding to TS-1: a novel oral formation of 5-fluorouracil. Gan To Kagaku Ryoho 2000; 27: 1437- 41.
26. Sasaki T. Current topics of S-1 at the 74th Japanese Gastric Cancer Congress. Gastric Cancer 2003; 6 Suppl 19-12.
27. Koizumi W, Yamaguchi K, Hosaka H, et al. Randomised phase Ⅱ study of S-1/cisplatin plus TSU-68S-1/ cisplatin in patients with advanced gastric cancer. Br J Cancer 2013; 109: 2079-86.
28. Hisashige A, Sasako M, Nakajima T. Cost-effectiveness of adjuvant chemotherapy for curatively resected gastric cancer with S-1. BMC Cancer 2013; 13: 443.
29. Piessen G, Briez N, Triboulet JP, et al. Patients with locally advanced esophageal carcinoma nonresponder to radio- chemotherapy: who will benefit from surgery? Ann Surg Oncol 2007; 14: 2036-44.
for publication March 29, 2016.
Tel: 86-532-68665078,Fax: 86-532-84852500, E-mail: 123456789ji@gmail.com,niekekeqd@163.com
△Supported byMedical Technology Research Center for Health Development Grant[W2012FZ007(YJ)].
杂志排行
Chinese Medical Sciences Journal的其它文章
- Early Enteral Combined with Parenteral Nutrition Treatment for Severe Traumatic Brain Injury: Effects on Immune Function, Nutritional Status and Outcomes△
- Frontal Absence Seizures: Clinical and EEG Analysis of Four Cases
- Expression of miRNA-140 in Chondrocytes and Synovial Fluid of Knee Joints in Patients with Osteoarthritis△
- Effects of Lianhua Qingwen on Pulmonary Oxidative Lesions Induced by Fine Particulates (PM2.5) in Rats
- The Effect of Sleep Deprivation on Coronary Heart Disease△
- Uterine Artery Embolization for Management of Primary Postpartum Hemorrhage Associated with Placenta Accreta
