纳米钠基蒙脱土复合超强机械性能双网络抗菌水凝胶
2016-12-29向双飞东为富马丕明施冬健陈明清
李 婷 向双飞 东为富 马丕明 施冬健 陈明清
(江南大学化学与材料工程学院,江苏无锡214122)
纳米钠基蒙脱土复合超强机械性能双网络抗菌水凝胶
李 婷 向双飞 东为富 马丕明 施冬健 陈明清*
(江南大学化学与材料工程学院,江苏无锡214122)
通过两步自由基聚合法成功制备了纳米蒙脱土复合双网络水凝胶。蒙脱土与阳离子单体进行离子交换后以离子键作用于凝胶网络体系成为化学交联点,同时吸附大分子链作为水凝胶的物理交联点。双网络结构进一步提高凝胶的机械性能。力学性能结果表明,随着蒙脱土含量增加,水凝胶的杨氏模量、拉伸强度、压缩模量以及压缩强度提高,同时增加第一网络单体浓度,双网络水凝胶的机械性能进一步提高。扫描电镜微观形貌分析表明,蒙脱土的加入促进水凝胶的网络结构更加紧密,并形成大量的纤维状微网络结构,由此,水凝胶的力学性能明显提高。抑菌实验表明,因含有季铵盐阳离子,水凝胶对大肠杆菌以及金黄色葡萄球菌都具有良好的抑菌效果。
纳米蒙脱土;纳米复合凝胶;双网络;机械性能;抗菌性能
1 Introduction
Polymer hydrogels are hydrophilic three-dimensional polymeric networks with large amount of water which could keep a certain shape in water.Hydrogels have been used in a variety of applications1,such as separation membranes,biosensors,artificial muscles,chemical valves2,superabsorbents3,and drug delivery devices but the network defects of polymer gels often limit the maximum strain they can achieve4.In recent years,gel scientists have created several types of robust hydrogels,such as slide-ring hydrogels5,nanocomposite(NC)hydrogels6,double-network(DN) hydrogels7-11,tetra-PEG hydrogels12,macromolecular microsphere composite hydrogels13,14,and hydrophobic association hydrogels15, which are supposed to expand applications of gels.
Double network hydrogels are regarded as one of the most robust synthetic hydrogels16.They are comprised of a rigid and brittle first network of densely-cross-linked short chains in dilute concentration and a soft and stretchable second network of looselycross-linked long chains in high concentration17.Fu et al.have combined the advantages of DN gels and NC gels to achieve silicagrafted DN gels with high mechanical properties18,in which the functional silica nanoparticles are used as macro-crosslinkers to copolymerize with the first network and subsequently swollen to host the polymerization of a second network.
Recently,nanocomposite gels are focused as new class of hydrogels and consisting of organic/inorganic network structures with superior mechanical properties19-21.In these NC hydrogels, the dispersed clay platelets just act as physical crosslinkers and a number of flexible polymer chains are linked by them.It was considered that the polymer chains were attached to clay platelets through ionic or polar interactions22.In our previous study,we have fabricated nanocomposite double-network gels with excellent mechanical properties by compositing of carbon nanotubes (CNTs)without organic modification23.
The aim of this work was to synthesize and characterize the double-network hydrogels which had the cationic structure exchanged with montmorillonite(MMT).MMT consists of positively charged platelets and charge-balanced anions in the interlayer.The positively charged platelets have a single-layer thickness of~1 nm24.In our paper,(3-acrylamidopropyl)trimethylammonium chloride hydrogels by compositing MMT was fabricated as the first nanocomposite network and swollen to balance in acrylamide solution,then polymerized to form the second network.It was believed that the participation of MMT into the hydrogels led to formation of a stretchable microcomplex structure due to positively charged quaternary ammonium groups connected to network matrix throughout the ionic band.In addition,the quaternary ammonium groups made the DN hydrogels show antibacterial ability,which may expand applications of the gel.
2 Experimental
2.1Materials
(3-Acrylamidopropyl)trimethylammonium chloride(ATC, 74%-76%in water)was purchased fromTokyo Kasei Kogyo Co., Ltd.,Shanghai,China,N,N′-methylene-bis-acrylamide(MBAA, 99.0%),acrylamide(AAm,A.R.),2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone(Irgacure 2959,98%)and ammonium persulphate(APS,A.R.)were purchased from Aladdin Chemistry Co.Ltd.,Shanghai,China,and used as received. Montmorillonite(MMT,PGW)was supplied by Nanocor Inc., America and used as received.
2.2Preparation
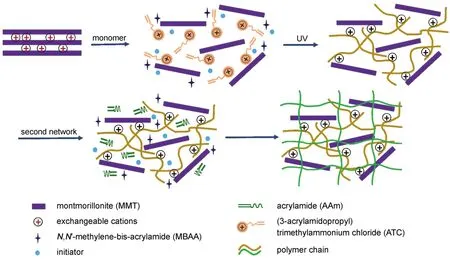
Scheme 1 Schematic illustration of the preparation of PATC/PAAm/MMT nanocomposite DN hydrogels
The DN hydrogels were prepared by a two-step free-radical polymerization in aqueous medium(Scheme 1).The nomenclature and compositions of DN hydrogels were summarized in Table 1. Firstly,an amount of MMT clay was dispersed in distilled water and allowed to stir for 24 h(speed of stirrer was 250 r·min-1).Then,the dispersed MMT solution was sonicated for 30 min.The cross-linking agent MBAA,the initiator Irgacure 2959,and the monomer ATC were added into an aqueous dispersion of MMT (varied at 0.5%,1%,and 2%(mass ratio of MMT to ATC)with respect to the mass of ATC).The mixture was injected into a rectangular frame of silicone rubber of a thickness of 1.5 mm which was sandwiched between two glass plates for platelike gel preparation.The polymerization was performed by irradiating UV light with a mercury lamp of 400 W for 1 h and the sample cell was set at the distance of 15 cm from the lamp.Next,these PATC gels were immersed and swollen in the second network precursor solution which contained 2 mol·L-1of AAm,0.001 mol·L-1of MBAA,and 0.002 mol·L-1ofAPS for 3 days.The swollen PATC gel was synthesized at 60°C for 10 h in a reaction container to yield a PATC/PAAm/MMT nanocomposite double-network hydrogel.
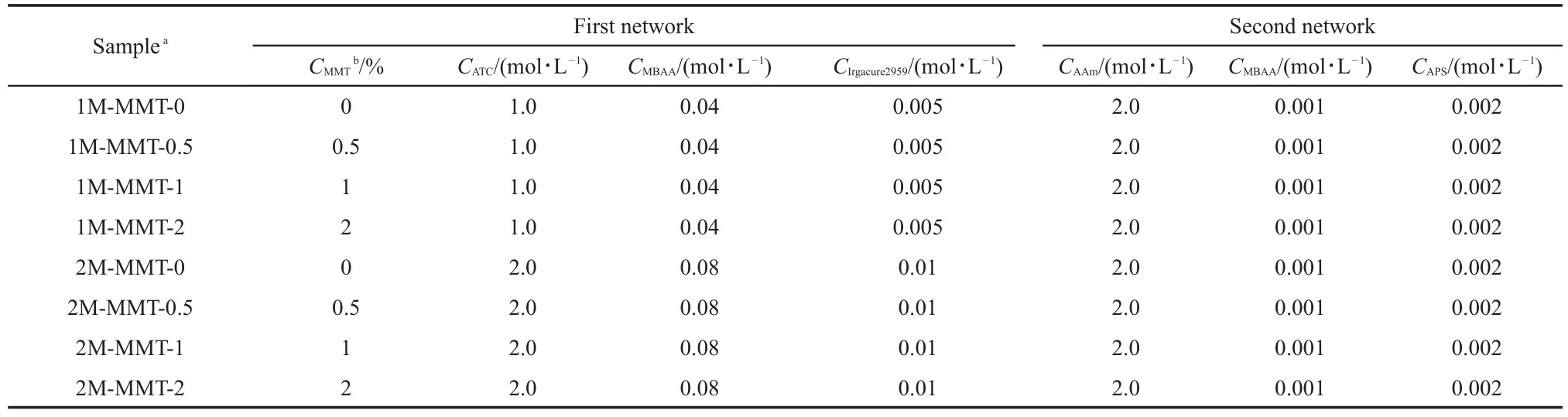
Table 1 Composition of PATC/PAAm/MMT nanocomposite DN hydrogels
2.3Measurements and characterization
2.3.1 Swelling experiments
The as-prepared three cylindrical samples with 10 mm diameter and 5 mm height were swelled in a large amount of water to reach swelling equilibrium for the swelling experiments.The swollen samples were weighed at set intervals followed by blotting off the excess water from the sample surface with a filter paper.The swelling ratios(SR)were calculated by the following equation, SR=(Ms-Md)/Md×100%,where Msand Mdwere the masses of the swollen and corresponding dried hydrogels,respectively.
2.3.2 Tensile measurements
Equilibrium swollen samples(in water)were used for tensile tests of DN hydrogels.All the tensile tests were carried out at 25°C in air environment and pre-cut dumbbell shaped gel samples standardized as the sizes(length of the thinner portion(L):10 mm, width of the thinner portion(b):4 mm,thickness(w):1.5-3.0 mm)were used.Tensile tests were measured with the tensile tester Instron 5960(Instron Co.)and an elongation speed of 20 mm· min-1was applied to determine the tensile properties of each sample.At least three specimens were tested for each hydrogel. Elastic modulus,yield stress,fracture stress,and ultimate strain were calculated from the stress-strain measurement.The fracture stress,defined as engineering stress,was determined as the stress at breaking point.The ultimate strain was determined as the strain at breaking point.The initial slope in the region of 0.5 m·m-1<strain<1 m·m-1of the first loading stress-strain curve was used to calculate the initial elastic modulus.The yield stress was determined as the point at which slope of stress-strain curves becomes zero,if such point was not observed,the yield stress was estimated as the point where the slope of the stress-strain curve changed dramatically.
2.3.3 Compressive measurements
Compressive tests were measured on cylindrical samples of 8 mm in diameter and 4-6 mm with the tensile tester Instron 5960 (Instron Co.)at 25°C.The compressive rate was 1 mm·min-1.The compressive fracture stress σfdetermined as the stress at breaking point was approximately calculated as σf=F/πR2,where R is the original radius of the specimen.The Young′s modulus(E)was determined as the slope of stress-strain curves in the region of 0 m·m-1<strain<0.1 m·m-1.The strain under compression is defined as the change in the thickness(h)relative to the original thickness(h0)of the freestanding specimen,ε=(h0-h)/h0.At least three specimens were tested for each hydrogel.
2.3.4 Scanning electron microscopy
The micromorphology of hydrogel samples was evaluated using a Hitachi S4800 scanning electron microscope(Tokyo,Japan)at an accelerating voltage of 10 kV.Firstly,the hydrogel samples were cut into 20 mm×2 mm pieces,and rapidly quenched into liquid nitrogen and freeze-dried.Then,the samples were fractured using a cold scalpel after freeze-dried.The fractured surfaces of cross-section were observed by scanning electron microscopy (SEM)after sputter-coating with a thin gold layer.
2.3.5 In vitro antibacterial effect
Two bacterial strains,namely E.coli and S.aureus(from School of Pharmaceutical Science of Jiangnan University),were subjected to this analysis.They were both cultured on nutrient broth,then subcultured monthly and subsequently stored at 4°C. The strains were incubated for 24 h at 37°C on a rotary shaker in 250 mL flasks filled with 50 mL of mediums.The effect of hydrogels on Gram-negative(E.coli)and Gram-positive(S.aureus), pathogenic bacteria was investigated according to the agar diffusion method25-28.Each gel sample was placed in the middle of sterilized Petridishes after the bacterium suspension was added. All of the gel samples were incubated at 37°C for 24 h.
3 Results and discussion
3.1Swelling behavior
The swelling behaviors of the PATC/PAAm/MMT nanocomposite DN hydrogels with different MMT content were shown in Fig.1.As could be seen from Fig.1(a),the swelling ratios of the hydrogels with 1%of MMT in water increased with time going until reaching equilibrium at about 30 h.With increasing the content of MMT,the equilibrium-swelling ratio(ESR)of the PATC/PAAm/MMT nanocomposite DN gels was much lower than that of neat DN gels,which was due to the fact that MMT was easily to form physical and chemical cross-linkers and increased the cross-linking degree of DN network.Thus,the ESR decreased with increasing the content of MMT.
According to Fig.1(b),the swelling ratios of the hydrogels with 2%of MMT reached equilibrium at about 7 h,which was much faster than the gels with 1%.It was attributed to the increasing repulsion force of ionic of the monomer,which provided more space to absorb water.The ESR of nanocomposite DN gels with 1%and 2%were similar and lower than that of DN gels without MMT.However,with the MMT content of 0.5%,the ESR reached 5.44 which was similar to the ESR of neat DN gels.
It was inferred that nano-clay could occupy the void space in gels in order to provide the space to contain more water during swelling and resulted in a more porous and higher swelling degree network23.However,2M-MMT DN hydrogels with the increasing amount of the exchange reaction between the monomer and MMT would have greater degree of crosslinking in the first network.It formed a rigid and tight skeleton so the equilibrium-swelling ratio of 2M-MMT DN hydrogels was much smaller than 1M-MMT DN hydrogels.Results indicated that the ESR of the PATC/PAAm/ MMT nanocomposite DN gels was determined to both swelling and crosslinking of MMT.
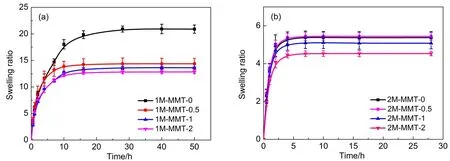
Fig.1 Swelling curves of nanocomposite DN hydrogels composited with various MMT contents
3.2Tensile properties
The data of Young′s modulus,yield stress,ultimate stress,and ultimate strain for the neat and nanocomposites DN gels with different amount of ATC were listed in Table 2.The compressive stress-strain curves of DN hydrogels(ATC-1M)were shown in Fig.2.Obviously,the addition of MMTled to an increase inYoung′s modulus and yield strength.As can be seen from Fig.2(a),the yield strength increased from 0.37 MPa for neat DN gel to 0.54 MPa for MMT-1.0.With further increase to the amount of MMT, all of Young′s modulus,yield stress,and ultimate stress decreased while the ultimate strain increased.Since the exchange reaction between cationic monomer and MMT increased,the hydrophilicity of modified MMT decreased slightly and a small part of the modified MMT may aggregate in the system during the preparation of the gel,which caused the stress concentration.So the yield strength and ultimate stress decreased when the mass ratio of MMT toATC increased from 1%to 2%.Furthermore,yielding phenomenon was observed in the DN gels,which showed necking phenomena during stretching.On tensile tests of DN gels that made from relatively sparse(fragile)first networks,narrowing zones(“necks”)appeared in the sample and grew up with further stretching,as shown in Fig.2(b).
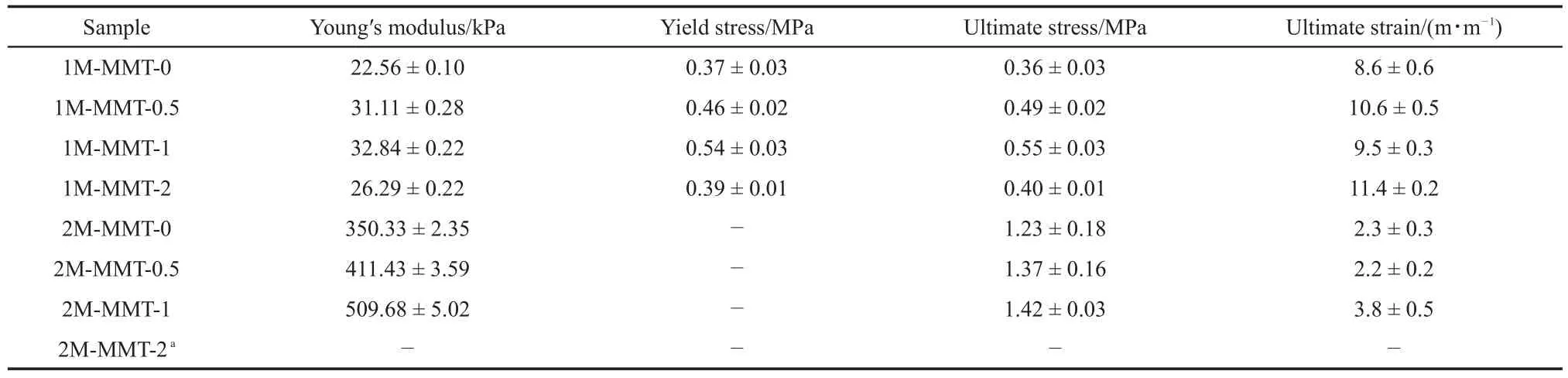
Table 2 Ttensile properties of the PATC/PAAm/MMT nanocomposite DN hydrogels
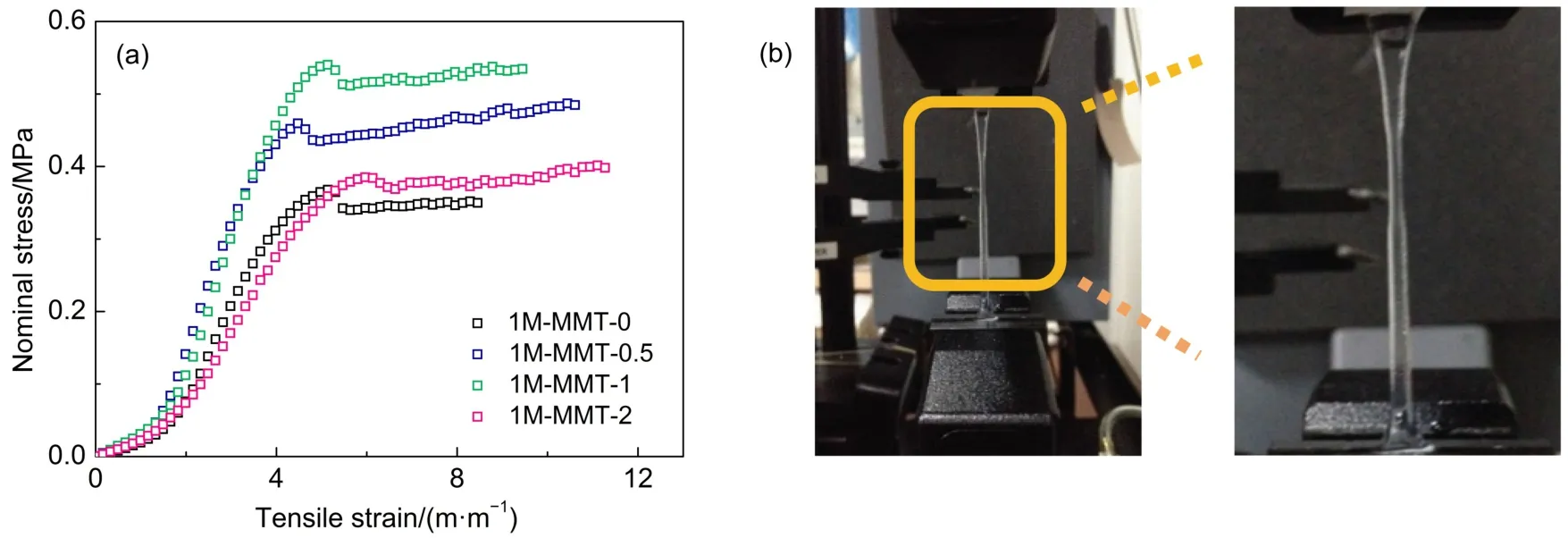
Fig.2 (a)Tensile stress-strain curves of the PATC/PAAm/MMT nanocomposite DN hydrogels; (b)pictures demonstrating the necking process
The improvement in tensile properties might be explained as the follows:(1)there were the strong physical interactions between polymer chains and MMT,and MMT could be used as physical cross-linkers;and(2)MMT clay into the hydrogels led to formation of a stretchable microcomplex structure due to positively charged quaternary ammonium groups connected to network matrix throughout the covalent bonds.So besides the crosslinker N,N′-methylene-bis-acrylamide,MMT clay also played a role as chemical cross-linkers,and reinforce the hydrogels.
Fig.3 showed effects of different MMT content on mechanical properties of hydrogels with different amount of ATC.As shown in Fig.3,the ultimate stress increased from 0.4 MPa for 1M-MMT-2.0 gel to 1.74 MPa for 2M-MMT-2.0 gel,but decreased in ultimate strain.Compared with the gel withATC-1M,the addition of ATC may enhance ultimate stress obviously,however,there was a decrease in ultimate strain.It was due to the rigid and brittle first network which comprised of ATC.The mechanical properties of DN hydrogels were determined both by the first network and the second network.Hydrogels with smaller equilibrium-swelling ratio and greater degree of crosslinking in the first network would have the higher tensile strength than others.Since the increase content of cationic monomer and the exchange reaction between the monomer and MMT,the first network formed with the greater degree of crosslinking that increased tensile strength and modulus of the nanocomposite DN hydrogels.
3.3 Compressive properties
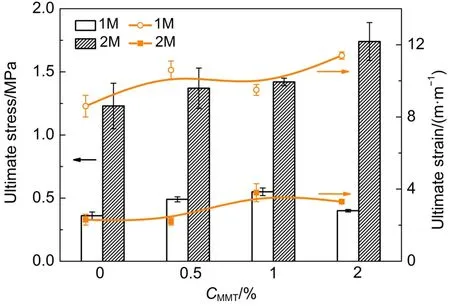
Fig.3 Ultimate stress and ultimate strain of PATC/PAAm/MMT nanocomposite DN hydrogels as a function of MMT content
The compressive stress-strain curves were shown in Fig.4,and the data of mechanical properties were summarized in Table 3.As shown in Fig.4,the compressive strengths(at 0.90 and 0.99 m·m-1strain)of the gels with MMT were higher than the neat gels. Furthermore,when the content of MMT was 1%,the compressive stress of the PATC/PAAm/MMT nanocomposite DN hydrogels significantly increased from 13.48 to 18.36 MPa at a strain of 0.99 m·m-1.However,the mechanical properties for PATC/PAAm/ MMT nanocomposite DN hydrogels decreased with further increasing the amount of MMT clay.This was probably a consequence of local concentration of stress and a similar phenomenon had been reported in previous studies32.It was clear that the PATC/PAAm/MMT nanocomposite DN hydrogels exhibited superior strength and toughness.
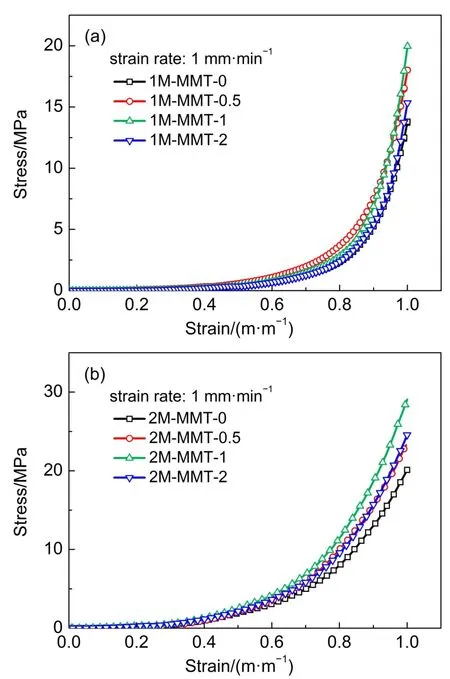
Fig.4 Compressive stress-strain curves for PATC/PAAm/MMT nanocomposite DN hydrogels
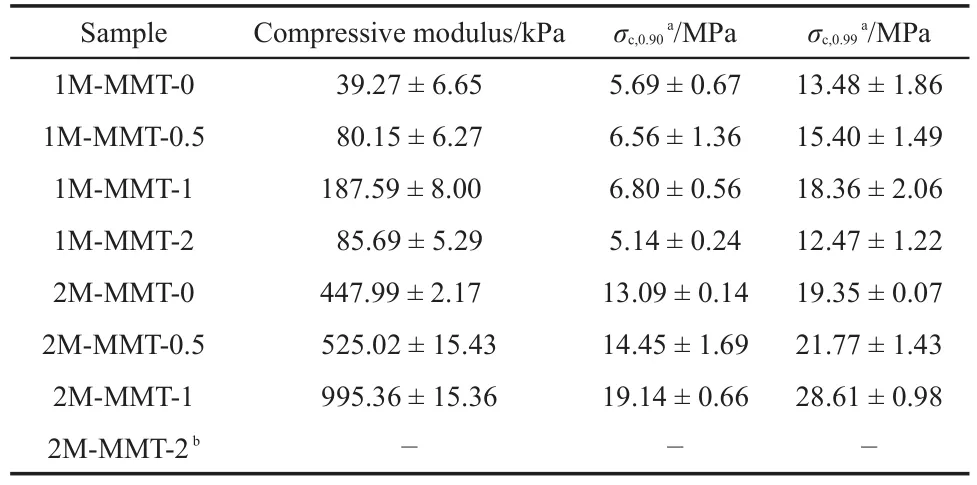
Table 3 Compressive properties of PATC/PAAm/MMT nanocomposite DN hydrogels
According to Fig.5,there was an increase in the compressive stress of the hydrogel at a strain of 0.99 m·m-1with the addition of ATC which was owing to a rigid and brittle first network of ATC.Hydrogels with smaller equilibrium-swelling ratio and greater degree of crosslinking in the first network would have the higher mechanical properties than others.The increase amount of the exchange reaction between ATC and MMT made the first network form a rigid skeleton which increased compressive properties of the hydrogels.
3.4Morphology
Fig.6 showed the SEM micrographs of freeze-dried the PATC/ PAAm/MMT nanocomposite DN gels with different MMT contents.It was quite clear that neat PATC/PAAm hydrogel exhibited somewhat porous structure with micro-network structures.With further increasing of the amount of MMT,the porous became irregular.Similarly,there were large quantities of micro-network structures located in the pores.
As can be seen from Fig.6,when the content of MMT was 1%, a large amount of micro-network structures located on the pore walls,which was more than the pores without MMT.As reported previously,the formation of embedded micro-network structures led to increase in the compressive strength and toughness23,29-31. The compressive stress of PATC/PAAm DN gels was 13.48 MPa at a fracture strain of 0.99 m·m-1,whereas that of nanocomposite DN hydrogel with 1%MMT reached 18.36 MPa at a strain of 0.99 m·m-1.It seems that the addition of MMT may improve mechanical properties of nanocomposite DN hydrogels.
Unlike the neat PATC/PAAm,which had a common large-pore structure at the micrometer scale,the present NC xerogels with 2% MMT displayed a layered pore structure with the further increasing of the amount of MMT.Inside the microporous structure, a great many micro-network structures were present(Fig.6).MMT played a role as physical and chemical cross-linkers,which immobilized part of the networks during swelling which may lead to the formation of micro-network structures.The formation of embedded micro-network structures would make a contribution to an improvement in mechanical properties of the hydrogels.
3.5Antibacterial ability of the nanocomposite DN hydrogels
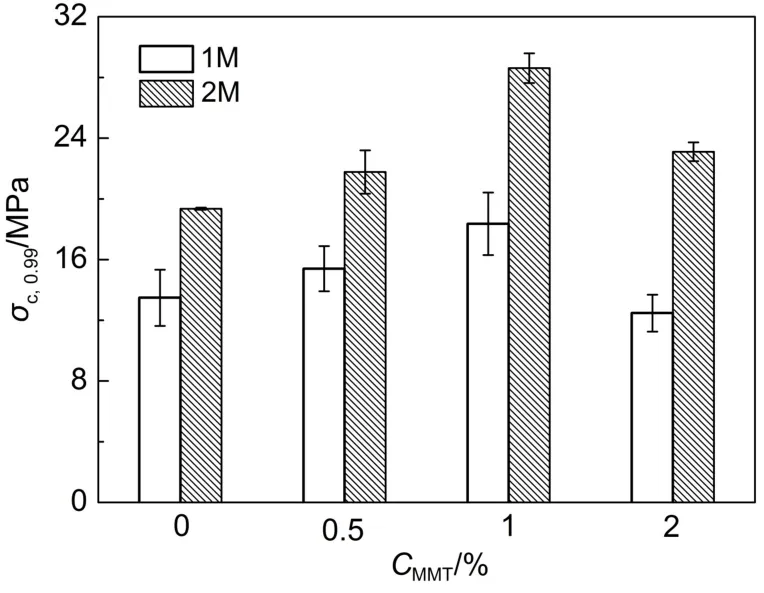
Fig.5 Compressive stress of the hydrogel at a strain of 0.99 m·m-1of PATC/PAAm/MMT nanocomposite DN hydrogels as a function of MMT content
The antibacterial test resulted for DN hydrogels were shown in Fig.7 and the data were obviously shown in Table 4.The diameter of inhibition ring(Ri)was calculated as Ri=Rt-Rh,where Rtwas the diameter of the tests and Rhwas the diameter of the DN hydrogels.As a control,the pure AAm/AAm DN hydrogel showed no inhibition ability.As can be seen from the size of inhibitionzones,similar antibacterial activity was observed for E.coli and S.aureus.It was noticed that higher inhibition zone sizes against all strains were observed with 2M-MMT-0 DN hydrogels which had more amounts ofATC than others.Hence,it can be also stated thatATC would be the effective antimicrobial components.

Fig.6 SEM micrographs of the PATC/PAAm/MMT nanocomposite DN hydrogels

Fig.7 Antibacterial test results for E.coli(A)and S.aureus(B)after 24 h incubation

Table 4 Diameter of inhibition ring ofAAm/AAm DN hydrogels and PATC/PAAm/MMT nanocomposite DN hydrogels
4 Conclusions
The nanocomposite DN hydrogels had been successfully prepared by free radical copolymerization of cationic monomer (ATC)and acrylamide in presence of nano Na-montmorillonite. It was believed that the participation of MMT into the hydrogels led to formation of a stretchable microcomplex structure due to positively charged quaternary ammonium groups connected to network matrix throughout the ionic band.Besides MMT,double networks also played an important part to improve the mechanical properties of the hydrogels.In addition,the quaternary ammonium groups made the DN hydrogels show antibacterial ability,which may expand applications of gels.
(1) Jin,S.P.;Liu,M.Z.;Chen,S.L.;Bian,F.L.;Chen,Y.;Wang, B.;Zhan,F.L.;Liu,S.X.Acta Phys.-Chim.Sin.2007,23(3), 438.[金淑萍,柳明珠,陈世兰,卞凤玲,陈 勇,王 斌,詹发禄,刘守信.物理化学学报,2007,23(3),438.]doi:10.3866/ PKU.WHXB20070330
(2) Arndt,K.F.;Kuckling,D.;Richter,A.Polym.Adv.Technol. 2000,11(8-12),496.doi:10.1002/1099-1581(200008/12)11:8/ 12<496::AID-PAT996>3.3.CO;2-Z
(3) Liu,Y.;Xie,J.;Zhu,M.;Zhang,X.Macromol.Mater.Eng. 2002,69(289),1074.doi:10.1002/mame.200400154
(4) Calvert,P.Adv.Mater.2009,21(7),743.doi:10.1002/ adma.200800534
(5) Okumura,Y.;Ito,K.Adv.Mater.2001,13(7),485.doi:10.1002/ 1521-4095(200104)13:7<485::AID-ADMA485>3.0.CO;2-T
(6) Haraguchi,K.;Takehisa,T.Adv.Mater.2002,14(16),1120. doi:10.1002/1521-4095(20020816)14:16<1120::AIDADMA1120>3.0.CO;2-9
(7) Nakajima,T.;Kurokawa,T.;Ahmed,S.;Wu,W.L.;Gong,J.P. Soft Matter 2013,9,1955.doi:10.1039/c2sm27232f
(8) Nakajima,T.;Fukuda,Y.;Kurokawa,T.;Sakai,T.;Chung,U.; Gong,J.P.ACS Macro Lett.2013,2(6),1293.doi:10.1021/ mz4002047
(9) Ronken,S.;Wirz,D.;Daniels,A.U.;Kurokawa,T.;Gong,J.P.; Arnold,M.P.Biomech.Model.Mechan.2013,12(2),243. doi:10.1007/s10237-012-0395-6
(10) Liu,H.X.;Zhang,L.H.;Tan,Y.Y.;Wei,C.;Lü,J.;Yu,C.B. Acta Polym.Sin.2013,12,1573.[刘红霞,张丽华,谭玉园,韦春,吕 建,余传柏.高分子学报,2013,12,1573.]doi: 10.3724/SP.J.1105.2013.13205
(11) Tian,S.;Shan,G.R.;Wang,L.Y.Acta Polym.Sin.2010,10, 1175.[田 帅,单国荣,王露一.高分子学报,2010,10,1175.] doi:10.3724/SP.J.1105.2010.09372
(12) Zu,G.Q.;Shen,J.;Wang,W.Q.;Zou,L.P.;Xu,W.W.;Zhang, Z.H.Acta Phys.-Chim.Sin.2015,31(2),360.[祖国庆,沈军,王文琴,邹丽萍,许维维,张志华.物理化学学报,2015,31 (2),360.]doi:10.3866/PKU.WHXB201412243
(13) Malkoch,M.;Vestberg,R.;Gupta,N.;Mespouille,L.;Dubois, P.;Mason,A.F.;Hedrick,J.L.;Liao,Q.;Frank,C.W.; Kingsbury,K.;Hawker,C.J.Chem.Commun.2006,26(26), 2774.doi:10.1039/b603438a
(14) Huang,T.;Xu,H.;Jiao,K.;Zhu,L.;Brown,H.;Wang,H.Adv. Mater.2007,19(12),1622.doi:10.1002/adma.200602533
(15) Jiang,G.;Liu,C.;Liu,X.;Zhang,G.;Yang,M.;Liu,F.Macromol.Mater.Eng.2009,294(12),815.doi:10.1002/ mame.200900160
(16) Gong,J.P.Soft Matter 2010,6,2583.doi:10.1039/b924290b
(17) Ahmed,S.;Nakajima,T.;Kurokawa,T.;Haque,M.A.;Gong,J. P.Polymer 2014,55(3),914.doi:10.1016/j. polymer.2013.12.066
(18) Wang,Q.;Hou,R.X.;Cheng,Y.J.;Fu,J.Soft Matter 2012,8, 6048.doi:10.1039/C2SM07233E
(19) Haraguchi,K.;Song,L.Macromolecules 2007,40(15),5526. doi:10.1021/ma070695p
(20) Hu,Z.;Chen,G.Adv.Mater.2014,26(34),5950.doi:10.1002/ adma.201400179
(21) Shen,M.;Li,L.;Sun,Y.;Xu,J.;Guo,X.;Prud′Homme,R.K. Langmuir 2014,30(6),1636.doi:10.1021/la4045623
(22) Haraguchi,K.;Li,H.J.;Matsuda,K.;Takehisa,T.;Elliott,E. Macromolecules 2005,38(8),33.doi:10.1021/ma047431c
(23) Dong,W.F.;Huang,C.G.;Wang,Y.;Sun,Y.J.;Ma,P.M.; Chen,M.Q.Int.J.Mol.Sci.2013,14(11),22380.doi:10.3390/ ijms141122380
(24) Dalaran,M.;Emik,S.;Güçlü,G.;İyim,T.B.;Özgümüş,S. Desalination 2011,279(s1-3),170.doi:10.1016/j. desal.2011.06.004
(25) Lalani,R.;Liu,L.Biomacromolecules 2012,13(6),1853. doi:10.1021/bm300345e
(26) Zhao,J.;Ma,L.;Millians,W.;Wu,T.;Ming,W.H.ACS Appl. Mater.Interfaces 2016,8,8737.doi:10.1021/acsami.6b00748
(27) Wang,M.Z.;Wang,T.;Yuan,K.;Du,J.Z.Chin.J.Polym.Sci. 2016,34(1),44.doi:10.1007/s10118-016-1725-4
(28) Zhou,C.C.;Wang,M.Z.;Zou,K.D.;Chen,J.;Zhu,Y.Q.;Du, J.Z.ACS Macro Lett.2013,2,1021.doi:10.1021/mz400480z
(29) Wang,Q.;Hou,R.X.;Cheng,Y.J.;Fu,J.Soft Matter 2012,8, 6048.doi:10.1039/C2SM07233E
(30) Huang,M.;Furukawa,H.;Tanaka,Y.;Nakajima,T.;Osada,Y.; Gong,J.P.Macromolecules 2007,40(18),6658.doi:10.1021/ ma062482q
(31) Nakajima,T.;Furukawa,H.;Tanaka,Y.;Nakajima,T.;Osada, Y.;Gong,J.P.Macromolecules 2009,42(6),2184.doi:10.1021/ ma802148p
(32) Li,T.;Xiang,S.F.;Ma,P.M.;Bai,H.Y.;Dong,W.F.;Chen,M. Q.J.Polym.Sci.Pol.Phys.2015,53(14),1020. doi:10.1002/polb.23732
Double-Network Hydrogel Consisting of Nano Na-Montmorillonite with Enhanced Mechanical and Antimicrobial Properties
LITing XIANG Shuang-Fei DONG Wei-Fu MAPi-Ming SHIDong-Jian CHEN Ming-Qing*
(School of Chemical and Material Engineering,Jiangnan University,Wuxi 214122,Jiangsu Province,P.R.China)
(3-Acrylamidopropyl)trimethylammonium chloride(ATC),acrylamide and montmorillonite(MMT) without organic modification were synthesized through free radical polymerization to strengthen the mechanical properties of hydrogels.MMT platelets were considered as chemical“plane”cross-linkers different from“point”cross-linkers because of the cation-exchange reaction between MMT and ATC(cationic monomer) during the synthesis of hydrogels,while a double network was used to improve the mechanical properties of the hydrogels.Investigations of compressive and tensile properties indicated that compressive modulus and stress,fracture stress,ultimate strain and Young′s modulus were significantly improved in the presence of MMT. The mechanical properties of double-network hydrogels improved with increasing monomer concentration of the first network.Scanning electron microscopy(SEM)revealed that large quantities of micro-network structures were located in the pores and the formation of embedded micro-network structures led to an increase in the compressive strength and toughness.Moreover,the gels with ATC exhibited good antibacterial effects against E.coli and S.aureus.These developments provide a new route to prepare hydrogels with high mechanical properties.
Montmorillonite;Nanocomposite hydrogel;Double-network;Mechanical property; Antibacterial property
O648
10.3866/PKU.WHXB201608261
Received:June 29,2016;Revised:August 26,2016;Published online:August 26,2016.
*Corresponding author.Email:mq-chen@jiangnan.edu.cn;Tel:+86-510-85917763.
The project was supported by the National Natural Science Foundation of China(51373070),Research Project of Chinese Ministry of Education, China(113034A),and Fundamental Research Funds for the Central Universities,China(JUSRP51624A).
国家自然科学基金(51373070),教育部科学技术研究项目(113034A)及中央高校基本科研基金(JUSRP51624A)资助
