以油酸为原料的新型生物基支链十七烷基苯磺酸钠的合成及性质
2016-12-29卞鹏程张大鹏刚洪泽刘金峰牟伯中杨世忠
卞鹏程 张大鹏 刚洪泽 刘金峰 牟伯中,2 杨世忠,*
(1华东理工大学应用化学研究所,生物反应器工程国家重点实验室,上海200237;2上海生物制造技术合作创新中心,上海200237)
以油酸为原料的新型生物基支链十七烷基苯磺酸钠的合成及性质
卞鹏程1张大鹏1刚洪泽1刘金峰1牟伯中1,2杨世忠1,*
(1华东理工大学应用化学研究所,生物反应器工程国家重点实验室,上海200237;2上海生物制造技术合作创新中心,上海200237)
生物基表面活性剂由于其可再生资源和优异的表面/界面性质吸引了越来越多的关注。本文以可再生的油酸为原料,通过四步反应,制备了新型生物基支链表面活性剂,并评价了其表/界面性质、润湿性和生物降解性能。该新型生物基支链表面活性剂为4-(1-十七烷基)苯磺酸钠(9ΦC17S),依次经过烷基化反应、脱羧反应、磺化反应和中和反应而制得。其化学结构已通过电喷雾质谱、红外光谱和核磁共振波谱得以确认。4-(1-十七烷基)苯磺酸钠展现出良好的表/界面张力,临界胶束浓度(CMC)为317.5 mg·L-1,CMC处的表面张力为32.54 mN·m-1,当水溶液中碳酸钠浓度为8.48×104mg·L-1、4-(1-十七烷基)苯磺酸钠浓度为8.36× 104mg·L-1时,油水的界面张力约为10-2mN·m-1。此外,4-(1-十七烷基)苯磺酸钠在生物降解性和润湿性方面也显示出了良好的性能,最终生物降解评分为2.99,0.500 g·L-19ΦC17S溶液的气液固接触角为63.08°。该新型生物基表面活性剂丰富了以可再生资源为原料的生物基表面活性剂的结构多样性。
生物基表面活性剂;支链十七烷基苯磺酸钠;脱羧;油酸;可再生资源
1 Introduction
Traditional alkylbenzene sulfonates are a class of petroleumbased surfactants and have been widely used in many industries such as washing1,oil recovery2-4,pesticide chemistry5,6,papermaking7,etc.Most of the petroleum-based alkylbenzene sulfonates used in industries were a mixture of linear and branched alkylbenzene sulfonates.Recent studies indicated that the branched alkylbenzene sulfonates performed more outstanding interfacial activities than that of the linear ones8-10,and a great effort has been made in synthetic methods of the branched alkylbenzene sulfonate surfactants and in performance evaluations by both the experiment and computer simulations.Yang et al.11,12investigated interfacial behaviors of the three isomers of hexadecylbenzene sulfonates with the benzene ring at different site along the alkyl chain,and the surfactant molecule with a phenyl group near the center of the alkyl chain showed the best result with a reduction of the interfacial tension to ultra-low values at low alkali concentrations.The experimental results suggested that the alkylbenzene sulfonate with a phenyl group in the middle of hydrocarbon chain contributed to the lower surface tension compared to those with a phenyl group in the end of hydrocarbon chains11,12,which was coincident with the results of computer simulation13-15.However, most of the reported synthesis routes of alkylbenzene sulfonate isomers with the phenyl group at a specific site of hydrocarbon chains were considerably complicated,which has been a block for further development and application of branched alkylbenzene sulfonate surfactants in industries.
Bio-based surfactants have received increasing attention from industrial and scientific fields,due to their renewable resource, outstanding physicochemical property,and low costs16.Bio-based surfactants were mainly using biomass as their raw materials,such as carbohydrates17,proteins18,and vegetable oils19,which inherited harvestable sustainability,high output capability,and environmental compatibility.Biomass was seen as one of the best substitutes to petroleum chemicals to be used as alternative resources of surfactants20,21.The oleic acid,obtained by hydrolyzation of vegetable oils,was one of the most common kinds in biomass and has attracted great interests as platform chemicals for surfactants. Oleic acids have been applied to produce cationic gemini surfactants,which showed better surface properties than that of similar commercial surfactants22.However,the knowledge about oleic acid-based and branched alkylbenzene sulfonates and their application are still limited.
In the present study,the 4-(1-heptadecyl)benzene sodium sulfonate(9ΦC17S),a novel bio-based branched heptadecylbenzene sulfonate surfactant was synthesized using renewable oleic acids as starting material by a strategy of a facile four-step route.The chemical structure of the 4-(1-heptadecyl)benzene sodium sulfonate was determined by Fourier transform infrared(FT-IR) spectra,electrospray ionization high resolution mass spectrometry (ESI HRMS),and1H nuclear magnetic resonance(1H NMR) spectra,and its surface behavior,wettability,and biodegradibility were evaluated in this paper.
2 Materials and methods
2.1Materials
The oleic acid(AR)was purchased from Tokyo Chemical Industry Co.,Ltd,Tokyo,Japan.AlCl3(99%)was purchased from Aladdin,Shanghai,China.NiCl2·6H2O(AR),methanol(AR),and ethanol(AR)were purchased from Sinopharm Chemical Reagent Co.,Ltd,Shanghai,China.Benzene(AR),MgCl2·6H2O(AR), AlCl3·6H2O(AR),carbamide(AR),n-hexane(AR),sulfuric acid (AR),fuming sulfuric acid(AR),phosphorus pentoxide(AR)and sodium hydroxide(AR)were purchased from Shanghai Lingfeng Chemical Reagent Co.,Ltd,Shanghai,China.All the materials were used without further purification.
2.2Chemical analyses
Gas chromatography mass spectrometry(GC-MS)was recorded on Agilent 6890N Network GC system and 5978 inter Mass Selective Detector.Infrared spectra were recorded on a Nicolet iS10 FT-IR spectrometer.Electrospray ionization high resolution mass spectrometry was recorded on the Waters LCT Premier XE Mass Spectrometers.1H nuclear magnetic resonance spectra were recorded on a Bruker Advance 400 spectrometer(400 MHz)in D2O at room temperature.Tetramethylsilane(TMS)was used as reference.
2.3Synthetic methods
The branched heptadecylbenzene sulfonate was synthesized from oleic acid through four steps including the alkylation of benzene,catalyzed decarboxylation,sulfonation and neutralization.
2.3.1 Alkylation
The phenyl stearic acid was prepared in the presence of anhydrous AlCl3by the Friedel-Crafts reaction of oleic acids with benzene in a flask,in which the molar ratio of oleic acid,benzene and anhydrous AlCl3was 1:5:1.The mixture was heated to 65°C and maintained for 6 h with stirring.The resulted mixtures were then cooled to 0°C,and equal volume of 6 mol·L-1HCl was added slowly into the flask.The organic layer was seperated andevaporated under reduced pressure to obtain phenyl stearic acid23.
2.3.2 Decarboxylation
The catalyst,MgO-Al2O3-NiO,was prepared by MgCl2·6H2O, AlCl3·6H2O,NiCl2·6H2O,and carbamide,in which the molar ratio of MgO-Al2O3-NiO was 16:5:424.The catalyst was prepared by homogeneous precipitation method25.The prepared catalyst was calcined at 550°C for 3 h and then cooled down to room temperature.
The decarboxylation reaction was carried out in a batch mode operating autoclave reactor(70 mL),which was designed for operation up to 40 MPa and 350°C.The reactor was equipped with a multi-blade impeller to mix liquid reactants and solid catalyst.In a typical batch experiment,20.0 g of phenyl stearic acid and a series of given quality of catalyst(reactant/catalyst mass ratio:40/1,20/1,10/1)was placed in the reactor.The reactor was then flushed with nitrogen to remove oxygen.Next,the reactions were carried out at a high temperature(150,180,210°C).The reactor was subsequently cooled down to room temperature.The liquid products were collected after filtering solid catalysts.
2.3.3 Sulphonation and neutralization
The heptadecylbenzene was sulfonated with SO3provided by the reaction of fuming sulfuric acid and phosphorus pentoxide and then neutralized with sodium hydroxide to obtain the final compounds26.The mixture was deoiled with normal hexane and desalted in anhydrous ethyl alcohol respectively.The final products were purified by recrystallization in three times with ethanol solution(ethanol/water volume ratio:1/1).
2.4Measurements of surface tensions
Surface tensions(SFT)were measured by a DCAT 21 surface tensiometer using the plate method at(25.0±0.1)°C.Aqueous solutions of samples were prepared with double distilled water. Each surface tension value was evaluated as the average of at least three replicate measurements.The critical micelle concentration (CMC)was obtained from the break point of the curve of surface tension versus concentration.
Maximum surface excess at CMC(Γmax)was derived from the Gibbs adsorption isotherm equation27:

where R=8.314 J·mol-1·K-1,Tis the absolute temperature,(∂SFT/∂lgC)Tis the slope of the surface tension-log concentration curve at temperature T,η(the number of species at the interface)is taken as 1 for the alkyl benzene sulfonate.The minimum area per molecule occupied at CMC,Aminwas calculated from the relationship with maximum surface excess Γmax27.

where NAis theAvogadro constant.
2.5Measurements of interfacial tensions
Surfactant solutions were prepared at different concentrations from 0.10 to 0.80 g·L-1with Daqing oil field simulated formation water.Interfacial tensions(IFT)were measured by the spinningdrop method using SVT 20 tensiometer at(50.0±0.1)°C.Daqing crude oil was used as oil phase.
2.6Measurement of the Krafft point temperature
The conductivities of 1.0%surfactant solution at different temperatures were measured with a DDSJ-308A conductivity meter,and the Krafft point temperature was obtained at the temperature of the break point of the curve of conductivity versus temperature28.
2.7Measurement of the HLB value
The hydrophilic-lipophilic balance(HLB)value of the surfactant was computed by a formula where A=1.961 and B=16.235 for the sodium sulfonate surfactants29.

2.8Measurement of contact angles
The contact angles were measured by sessile drop technique for 0.500 g·L-1surfactant solution at 25.0°C30.The measurements were repeated 5 times and the average value was calculated.
2.9Prediction of ultimate biodegradation
The ultimate biodegradation of surfactant was performed using the EPI Suite,BIOWIN3 biodegradation model,which is frequently applied for the degradation estimate of organic chemicals31.
3 Results and discussion
3.1Yields and structural analyses
The strategy route for synthesis of the bio-based branched alkylbenzene sulfonate was shown in Scheme 1.The branched heptadecylbenzene sulfonate was synthesized from oleic acids and intermediate products were detected by GC-MS after esterification.The GC spectrograms of methyl oleate,phenyl methyl stearate,heptadecylbenzene were shown in Fig.1,in which peaks A,B,C,and D indicated the methyl oleate,methyl stearate,phenyl methyl stearate,and heptadecylbenzene,respectively.Line 1 showed the products of esterification of oleic acid samples.There was little stearic acid in this sample,which could be used as the internal standard for the calculation of yields of Friedel-Crafts reaction.Line 2 indicated the result of Friedel-Crafts reaction of oleic acids.It showed that the oleic acid was completely expendedfrom the comparison with line 1,and it yielded 97.7%in the Friedel-Crafts reaction.The GC spectrogram of decarboxylation of phenyl stearic acid was shown in line 3.The yield of decarboxylation reaction was 93.2%,as shown for the number 6 in Table 1.The reactions of sulfonation and neutralization of heptadecylbenzene carried out with SO3yielded about 77.9%by weighing method.
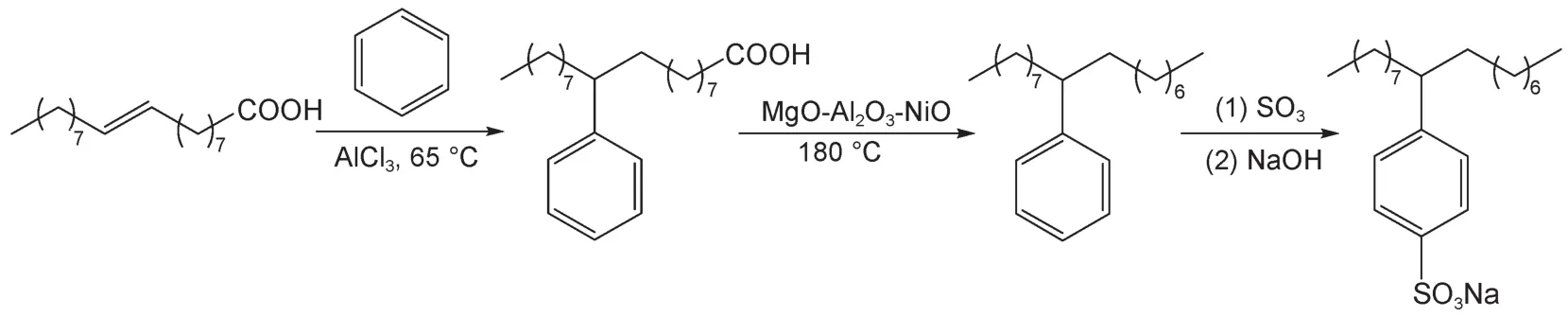
Scheme 1 Strategy route for synthesis of the bio-based branched alkylbenzene sulfonate

Fig.1 GC spectrogram of the methyl oleate, phenyl methyl stearate,and heptadecylbenzene
The molecular weight(MW)of 9ΦC17S was determined by ESI HRMS and the ionization way of ESI HRMS was negative ion mode without Na+.The mass-to-charge ratio(m/z)of the main peak was 395.3,as shown in Fig.2.For a further structral analysis the FT-IR and1H NMR were applied.It indicated in1H NMR spectral pattern(as Fig.S1 shown in the Supporting Information) that the hydrogen atoms in the surfactant were identified according to their chemical environment using1H NMR.The signals at 0.79 and 1.16 were attributed to the hydrogen atoms on the end methyl and methylene of the long carbon chains,respectively.Peaks at 1.49 indicated the hydrogen atoms in―CH2―CH―C6H4―.And the resonance of the hydrogen atom neighboring the benzene rings was 2.39.The signals at 7.16-7.65 were assigned to the resonances of the benzene ring.In the FT-IR spectrogram(Fig.S2 shown in the Supporting Information),the peaks at 2961.76 and 2858.59 cm-1suggested the absorbance of methylene and methyl groups in the carbon chain.The absorbance wavelengths of the benzene ring were 1650.62 and 1467.25 cm-1.And the peaks at 1186.30,1129.80,1043.35 cm-1indicated the absorbance of S=O double bond.

Table 1 Design and optimum conditions of decarboxylation of phenyl stearic acid

Fig.2 ESI HRMS spectrum of 9ΦC17S
In general routes for synthesis of the branched alkylbenzene sulfonates there included at least 8 steps11,12.However,in this study a novel bio-based branched alkylbenzene sulfonate was synthesized by a strategy of only four-step route as shown in Sheme 1, which was more simple and convenient.In addition,the starting material used in this synthetic method was oleic acids,a class of renewable resources from oils,or even waste cooking oils.
3.2Optimization of decarboxylation
The yields in different conditions for the optimization were shown in Table 1.The numbers 1,2,6 were carried out at 150,210,and 180°C,respectively,in which the yields were 3.9%, 93.5%,and 93.2%,respectively.Numbers 3-11 were performed at 180°C using an optimization design method with various conditions,including reaction time,the stirring speed and the ratio of reactant to catalyst.And the yields were also listed.Number 6 suggested the optimized result for the decarboxylation reaction. Besides,the mass spectrum of the product was shown in Fig.S3 (Supporting Information).
The Friedel-Crafts reaction and sulfonation have been extremely studied in the synthesis of alkylbenzene sulfonate,compared to the decarboxylation.Herein,the decarboxylation was researched with the catalyst MgO-Al2O3-NiO by optimization method.In the recent research about decarboxylation,the reaction temperature was 200°C for fatty acids with Pd/C catalyst32,the temperature was 400°C for microalgal oil with MG6333,and the temperature of decarboxylation of oleic acid with Ni/MgO-Al2O3was 300°C24. With the MG63 or Ni/MgO-Al2O3catalyst,the temperature for decarboxylation was higher,which implied that the reaction would need more energy consumption.However,in our study,with the catalyst MgO-Al2O3-NiO,the reaction of decarboxylation was carried out completely under the condition of 180°C,the number 6 as shown in Table 1.This temperature was much lower,while the yield with the catalyst MgO-Al2O3-NiO was 93.2%,as high as that in reaction with Pd/C catalyst.
However,when the temperature of decarboxylation reaction was below 180°C,for example,150°C,the productivity was very low (only 3.9%).On the other hand,when the temperature was above 180°C,such as 210°C,there was not obvious improvement for the productivity(yield 93.5%).Consequently,an optimization design method was proposed with various conditions as numbers 3-11, and the optimum conditions for the decarboxylation reaction was 150 r·min-1of stirring speed,20:1 for reactant/catalyst and 6 h.
3.3Surface properties

Fig.3 Variation of the surface tensions with the concentrations of 9ΦC17S at 25.0°C
The surface tension as a function of 9ΦC17S concentrations at 25.0°C was illustrated in Fig.3.The CMC and the surface tension at CMC(SFTCMC)were obtained from the breakpoint of the plot in Fig.3.The surface excess(Γmax)and the area occupied per surfactant molecule(Amin)at CMC on air/water interface were computed by Eqs.(1)and(2),respectively.It showed in Fig.3 that 9ΦC17S presented a sharp break corresponding to CMC of 317.5 mg·L-1with SFTCMC=32.54 mN·m-1at 25°C,and the calculated minimum surface area per molecule for 9ΦC17S was 0.39 nm2,as summarized in Table 2.The CMC by electrical conductivity was 304.5 mg·L-1from Fig.S4(Supporting Information),similar to that by a surface tension method.The relative deviation between them was 4.3%.
3.4Interfacial properties
Fig.4 showed the dynamic interfacial tensions(DIFT)between Daqing crude oil and simulated formation water at different concentrations of 9ΦC17S solutions measured at 50.0°C.Fig.4(a) displayed that the equilibrium IFT values were:2.44,1.65,0.78, and 1.16 mN·m-1at the concentrations of 9ΦC17S solutions of: 0.10,0.20,0.50,and 0.80 g·L-1,respectively.The minimum value of 0.78 mN·m-1appeared at 0.50 g·L-1,and the equilibrium IFT value between Daqing crude oil and simulated formation water without surfactants was 9.47 mN·m-1.Fig.4(b),(c),and(d)indicated the dynamic interfacial tensions between Daqing crude oil and 0.10,0.20,and 0.50 g·L-19ΦC17S solutions with adding different concentrations of extra Na2CO3,respectively.All the equilibrium values of DIFT between crude oil and 9ΦC17S solutions decreased with the concentration of Na2CO3increased.The interfacial tension of~10-2mN·m-1was obtained at 0.2 mol·L-1with 0.8 mol·L-1Na2CO3.Dynamic interfacial tensions between Daqing crude oil and different concentrations Na2CO3solutions in simulated formation water were measured at 50.0°C,displayed in Fig.S5(Supporting Information),which suggested that only Na2CO3could not decrease IFT to ultralow value in our experiments.
One of the most important applications of alkylbenzene sulfonates was to enhance oil recovery34,35.The IFT in 10-3mN·m-1order of magnitude was critically required for availably displacement of residual crude oil from the capillaries and pores of petroleum reservoirs36.To achieved ultra-low IFT value,alkali were widely used accompanying with alkyl benzene sulfonates in oil recovery37.The alkali solution could react with acidic components of petroleum to produce in situ surfactants,which may interact with alkyl benzene sulfonates and reduce the IFT value between crude oil and water observably38-40.As the IFT value between Daqing crude oil with the 9ΦC17S solution were far away from ultra-low IFT value in Fig.4(a),different concentration of Na2CO3solution was added into different concentration of 9ΦC17S solution(Fig.4(b),(c)and(d))to achieve lower IFT values.All the minimum values of DIFT were in 10-3mN·m-1order of magnitude combined with 0.80 mol·L-1Na2CO3,which indicated that 9ΦC17S could be a class of candidate for oil re-covery.Although all the equilibrium values of DIFT between crude oil and 9ΦC17S solutions decreased with the concentration of Na2CO3increased,these decrement degrees of DIFT equilibrium values were different.The DIFT equilibrium values decreased from 0.460 to 0.026 mN·m-1in Fig.4(b),and reduced from 0.170 to 0.016 mN·m-1in Fig.4(c)with the Na2CO3concentrations increased from 0.10 to 0.80 mol·L-1.While for the higher 9ΦC17S concentration as shown in Fig.4(d),this value reduced to 0.026 mN·m-1from 0.13 mN·m-1with the same Na2CO3concentration recruitment.It illustrated that the absorbance of surfactant on the oil/water interface did not change coincident with the surfactant concentration in solution,as the dynamic equilibrium value depends on the adsorption of surfactant molecules.

Table 2 Surface properties,HLB value,contact angle,and Krafft point of 9ΦC17S

Fig.4 Interfacial properties of 9ΦC17S solutions in simulated formation water at 50.0°C
3.5Contact angles,HLB values,and the Krafft pointTable 2 suggested the contact angle θaverageof air/water/solid was 63.08°at 0.500 g·L-19ΦC17S solutions and the HLB value was 10.12 calculated by Eq.(3).The Krafft point was 58.15°C from Fig.S6(Supporting Information),obtained by the conductivities curve of 1%surfactant solution under different temperatures,as shown in Table 2.The average contact angle of double distilled water on the hydrophobic acrylic substrate was 92.04°at 25.0°C19.The contact angle results suggested a great wettability of 9ΦC17S.The HLB value indicated that 9ΦC17S was almost balanced in hydrophile-lipophile property and could be used as emulsifier.The Krafft points of C16H33SO3Na and C18H37SO3Na were 55.3 and 64.8°C,respectively,in the previous research41.The Krafft point for 9ΦC17S in this study was coincident with the previous report due to the relation between Krafft point and molecular structure.
3.6Biodegradability prediction
The ultimate biodegradation score of 9ΦC17S was 2.99.The score above 2.8 was the biodegradation order of“weeks”,and scores between 2.0 and 2.8 were in order of“months”.This result meant the expected total degradation time was between“weeks”, and suggested that 9ΦC17S was biodegradable.The ultimate biodegradation scores of normal alkyl benzene sulfonate and alkyl naphthalene sulfonate were 2.59-2.84 and 2.48-2.73,respectively19.The predicted result of 9ΦC17S was higher than that of other surfactants,indicated that the degradation period of 9ΦC17S was the shortest,which meant that 9ΦC17S was friendly allowable in surfactant′s industrial applications.
4 Conclusions
In summary the novel bio-based branched surfactant 4-(1-heptadecyl)benzene sodium sulfonate(9ΦC17S)was synthesizedfrom renewable oleic acids using a strategy of a four-step route of the alkylation,decarboxylation,sulfonation and neutralization. The novel bio-based branched alkylbenzene sulfonate showed a CMC as low as 317.5 mg·L-1and the higher interfacial activities in aqueous solutions with Na2CO3.The interfacial tension between crude oil and simulated formation water was greatly reduced to a ultra-low order(≤10-2mN·m-1)at 8.36×10-4mg·L-19ΦC17S in solutions,and the prediction of the ultimate biodegradation score(2.99)showed the biodegradation order of“weeks”,which suggested that the novel bio-based branched surfactant would be a very competitive candidate in surfactant flooding in oil recovery, and friendly allowable in oil spill treatment and many other industrial applications.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1) Cohen,L.;Moreno,A.;Berna,J.J.Am.Oil Chem.Soc.1993, 70,79.doi:10.1007/BF02641010
(2) Llenado,R.A.Enhanced Oil Recovery.Google Patents: US4565647A,1986-01-21.
(3) Shupe,R.D.Surfactant Oil Recovery Process Usable in High Temperature Formations.Google Patents:US4018278A,1977-04-19.
(4) Dai,C.;Wang,K.;Liu,Y.;Li,H.;Wei,Z.;Zhao,M.Energy Fuels 2015,29,2304.doi:10.1021/acs.energyfuels.5b00507
(5) Holmstrup,M.;Krogh,P.H.Environ.Toxicol.Chem.1996,15, 1745.doi:10.1002/etc.5620151013
(6) Nimer,M.;Ballesteros,O.;Navalon,A.;Crovetto,G.;Verge, C.;López,I.;Berna,J.;Vílchez,J.Anal.Bioanal.Chem.2007, 387,2175.doi:10.1007/s00216-006-1069-y
(7) Schultz,W.S.;Beyer,U.Invert Size for the Internal and Surface Sizing of Paper.Google Patents:US4983257A,1991-01-08.
(8) Cao,Y.;Zhao,R.H.;Zhang,L.;Xu,Z.C.;Jin,Z.Q.;Luo,L.; Zhang,L.;Zhao,S.Energy Fuels 2012,26,2175.doi:10.1021/ ef201982s
(9) Zhang,L.;Wang,X.C.;Gong,Q.T.;Zhang,L.;Luo,L.;Zhao, S.;Yu,J.Y.J.Colloid Interface Sci.2008,327,451. doi:10.1016/j.jcis.2008.08.019
(10) Zhao,R.H.;Huang,H.Y.;Wang,H.Y.;Zhang,J.C.;Zhang,L.; Zhang,L.;Zhao,S.J.Dispersion Sci.Technol.2013,34,623. doi:10.1080/01932691.2012.685844
(11) Zhao,Y.;Li,P.;Li,Z.;Qiao,W.;Cheng,L.;Yang,J.Pet.Sci. Technol.2007,25,1429.doi:10.1056/NEJMoa1014618
(12) Yang,J.;Qiao,W.;Li,Z.;Cheng,L.Fuel 2005,84,1607. doi:10.1016/j.fuel.2005.01.014
(13) He,X.;Guvench,O.;MacKerell,A.D.,Jr.;Klein,M.L. J.Phys.Chem.B 2010,114,9787.doi:10.1021/jp101860v
(14) Jang,S.S.;Lin,S.T.;Maiti,P.K.;Blanco,M.;Goddard,W.A.; Shuler,P.;Tang,Y.J.Phys.Chem.B 2004,108,12130. doi:10.1021/jp048773n
(15) Zhao,T.;Xu,G.;Yuan,S.;Chen,Y.;Yan,H.J.Phys.Chem.B 2010,114,5025.doi:10.1021/jp907438x
(16) Foley,P.;Beach,E.S.;Zimmerman,J.B.Chem.Soc.Rev.2012, 41,1499.doi:10.1039/C1CS15217C
(17) Rajabi,F.;Luque,R.RSC Adv.2014,4,5152.doi:10.1039/ C3RA45757E
(18) Sreenu,M.;Rao,B.V.;Prasad,R.B.N.;Sujitha,P.;Chityala, G.K.Eur.J.Lipid Sci.Technol.2014,116,193.doi:10.1002/ ejlt.201300189
(19) Zhang,Q.Q.;Cai,B.X.;Xu,W.J.;Gang,H.Z.;Liu,J.F.; Yang,S.Z.;Mu,B.Z.Colloids Surf.A 2015,483,87. doi:10.1016/j.colsurfa.2015.05.060
(20) Melero,J.A.;Iglesias,J.;Garcia,A.Energy Environ.Sci.2012, 5,7393.doi:10.1039/C2EE21231E
(21) Climent,M.J.;Corma,A.;Iborra,S.Green Chem.2014,16, 516.doi:10.1039/C3GC41492B
(22) Sakai,K.;Saito,Y.;Uka,A.;Matsuda,W.;Takamatsu,Y.; Kitiyanan,B.;Endo,T.;Sakai,H.;Abe,M.J.Oleo Sci.2013, 62,489.doi:10.5650/jos.62.489
(23) Zhang,Q.Q.;Cai,B.X.;Xu,W.J.;Gang,H.Z.;Liu,J.F.; Yang,S.Z.;Mu,B.Z.Sci.Rep.2015,5,9971.doi:10.1038/ srep09971
(24) Roh,H.S.;Eum,I.H.;Jeong,D.W.;Yi,B.E.;Na,J.G.;Ko,C. H.Catal.Today 2011,164,457.doi:10.1016/j. cattod.2010.10.048
(25) Ogawa,M.;Kaiho,H.Langmuir 2002,18,4240.doi:10.1021/ la0117045
(26) Ortega,J.A.T.;Aldana,L.A.D.;Castellanos,F.J.S.Ing. Investig.2009,29,48.
(27) Du,X.;Lu,Y.;Li,L.;Wang,J.;Yang,Z.Colloids Surf.A 2006, 290,132.doi:10.1016/j.colsurfa.2006.05.013
(28) Guo,Y.J.;Liu,J.X.;Zhang,X.M.;Feng,R.S.;Li,H.B.; Zhang,J.;Lv,X.;Luo,P.Y.Energy Fuels 2012,26,2116. doi:10.1021/ef202005p
(29) Jiahua,Z.;Yingde,C.Special Petrochem.2001,2,004.
(30) Nedyalkov,M.;Alexandrova,L.;Platikanov,D.;Levecke,B.; Tadros,T.F.Colloid.Polym.Sci.2008,286,713.doi:10.1007/ s00396-007-1823-5
(31) Song,S.;Song,M.;Zeng,L.;Wang,T.;Liu,R.;Ruan,T.;Jiang, G.Environ.Pollut.2014,186,14.doi:10.1016/j. envpol.2013.11.023
(32) Santillan-Jimenez,E.;Morgan,T.;Lacny,J.;Mohapatra,S.; Crocker,M.Fuel 2013,103,1010.doi:10.1016/j. fuel.2012.08.035
(33) Na,J.G.;Han,J.K.;Oh,Y.K.;Park,J.H.;Jung,T.S.;Han,S. S.;Yoon,H.C.;Chung,S.H.;Kim,J.N.;Ko,C.H.Catal. Today 2012,185,313.doi:10.1016/j.cattod.2011.08.009
(34) Li,Y.;He,X.;Cao,X.;Zhao,G.;Tian,X.;Cui,X.J.Colloid Interface Sci.2007,307,215.doi:10.1016/j.jcis.2006.11.026
(35) Li,Y.;Zhang,P.;Dong,F.L.;Cao,X.L.;Song,X.W.;Cui,X.H.J.Colloid Interface Sci.2005,290,275.doi:10.1016/j. jcis.2005.04.035
(36) Qiao,W.;Li,J.;Zhu,Y.;Cai,H.Fuel 2012,96,220. doi:10.1016/j.fuel.2012.01.014
(37) Dai,X.;Suo,J.;Duan,X.;Bai,Z.;Zhang,L.J.Surfactants Deterg.2008,11,111.doi:10.1007/s11743-008-1061-y
(38) Chen,L.;Zhang,G.;Ge,J.;Jiang,P.;Tang,J.;Liu,Y.Colloids Surf.A 2013,434,63.doi:10.1016/j.colsurfa.2013.05.035
(39) Tang,M.;Zhang,G.;Ge,J.;Jiang,P.;Liu,Q.;Pei,H.;Chen,L. Colloids Surf.A 2013,421,91.doi:10.1016/j. colsurfa.2012.12.055
(40) Zhao,X.;Bai,Y.;Wang,Z.;Shang,X.;Qiu,G.;Chen,L. J.Dispersion Sci.Technol.2013,34,756.doi:10.1080/ 01932691.2012.686252
(41) Fekarcha,L.;Tazerouti,A.J.Surfactants Deterg.2012,15,419. doi:10.1007/s11743-012-1335-2
Synthesis and Properties of a Novel Bio-Based Branched Heptadecylbenzene Sulfonate Derived from Oleic Acid
BIAN Peng-Cheng1ZHANG Da-Peng1GANG Hong-Ze1LIU Jin-Feng1MU Bo-Zhong1,2YANG Shi-Zhong1,*
(1State Key Laboratory of Bioreactor Engineering and Institute of Applied Chemistry,East China University of Science and Technology,Shanghai 200237,P.R.China;2Shanghai Collaborative Innovation Center for Biomanufacturing Technology,Shanghai 200237,P.R.China)
Bio-based surfactants have attracted increasing attention because they are made from renewable resources and have excellent surface/interfacial properties.In this study we prepared a novel bio-based branched alkylbenzene sulfonate surfactant by a four-step route using renewable oleic acids as starting materials.We evaluated the surface behavior,wettability,and biodegradability of our surfactant.The surfactant, 4-(1-heptadecyl)benzene sodium sulfonate(9ΦC17S),was synthesized using a facile four-step route involving alkylation,decarboxylation,sulfonation and neutralization,respectively.The chemical structure of 4-(1-heptadecyl)benzene sodium sulfonate was confirmed by infrared(IR)spectroscopy,electrospray ionization high resolution mass spectrometry(ESIHRMS)and1H nuclear magnetic resonance(1H NMR)spectroscopy. The surfactant demonstrated an excellent surface tension of 32.54 mN·m-1at the critical micelle concentration (CMC)of 317.5 mg·L-1and outstanding interfacial tension of~10-2mN·m-1at 8.36×104mg·L-1with 8.48×104mg·L-1Na2CO3.The surfactant also showed good biodegradability with an ultimate biodegradation score of 2.99.The surfactant had good wettability with an air/water/solid contact angle(θaverage)of 63.08°for a 0.500 g·L-19ΦC17S solution.This novel bio-based branched surfactant contributes to the structural diversity of biobased surfactants from renewable feedstock.
Bio-based surfactant;Branched heptadecylbenzene sulfonate;Decarboxylation;Oleic acid; Renewable feedstock
O647
10.3866/PKU.WHXB201608231
Received:May 23,2016;Revised:August 17,2016;Published online:August 23,2016.
*Corresponding author.Email:meor@ecust.edu.cn;Tel:+86-21-64252063.
The project was supported by the National Natural Science Foundation of China(51574125,21203063),National High Technology Research and Development Program of China(2013AA064403),and Foundation of the Ministry of Education of China for Outstanding Young Teachers in University(WJ1514313).
国家自然科学基金(51574125,21203063),国家高技术研究发展计划项目(863)(2013AA064403)及国家教育部高等学校优秀青年教师研究基金(WJ1514313)资助
