理想与还原Fe2O3[001]表面上汞吸附协同催化CO分解作用
2016-12-29李继红林常枫肖显斌
李继红 林常枫 覃 吴,* 肖显斌 魏 利
(1华北电力大学可再生能源学院,生物质发电成套设备国家工程实验室,北京102206;2哈尔滨工业大学,城市水资源与水环境国家重点实验室,哈尔滨150090)
理想与还原Fe2O3[001]表面上汞吸附协同催化CO分解作用
李继红1林常枫1覃 吴1,*肖显斌1魏 利2,*
(1华北电力大学可再生能源学院,生物质发电成套设备国家工程实验室,北京102206;2哈尔滨工业大学,城市水资源与水环境国家重点实验室,哈尔滨150090)
煤化学链燃烧必然释放汞,汞与载氧体表面相互作用,影响表界面的氧化还原反应。本文采用密度泛函理论计算,研究汞(Hg0)在理想表面(Fe2O3[001])和一系列被还原表面(Fe2O2.75、Fe2O2.5、Fe2O2.25、Fe2O1.625、Fe2O0.875、Fe2O0.375和Fe)的吸附,以及Hg0对Fe2O1.625、Fe2O0.875、Fe2O0.375和Fe等表面催化CO分解反应的协同作用机理。Hg0物理吸附在理想Fe2O3[001]表面。随着Fe2O3[001]表面不断被还原,Hg0发生化学吸附。Hg0吸附降低了CO与Fe2O3、Fe2O2.75、Fe2O2.5和Fe2O2.25等表面之间的相互作用,抑制O传递氧化CO为CO2的反应;载氧体进一步还原过程中,Hg0吸附促进了CO与Fe2O1.625、Fe2O0.875、Fe2O0.375及Fe等表面之间的相互作用,进而促进了表面对CO的催化分解反应,加速了载氧体表面的积碳,降低了化学链燃烧效率。因此,合理控制载氧体的还原程度既可以减弱Hg0的吸附,也可以抑制积碳的形成,这对化学链燃烧的操作优化至关重要。
化学链燃烧;载氧体;汞;CO2捕集;密度泛函理论
Key Words:Chemical looping combustion;Oxygen carrier;Mercury;CO2capture;Density functional theory
1 Introduction
In comparison with conventional combustion techniques and carbon dioxide removal techniques,chemical looping combustion (CLC)has received great attention because of its distinctive merit of efficient use of energy with in-situ CO2capture1-3.CLC produces heat and energy using oxygen carriers(OCs),usually the oxides of Fe,Ni,Co,Cu,Mn,and Cd,to supply oxygen instead of air for the combustion of fuel.CLC of fuel results in generation of CO2and H2O that is not diluted with N2or flue gas,and reduces NOxemissions4-10.These properties render CLC highly desirable as possible candidate for low-cost coal combustion and carbon capture11-13.Fe2O3is the most promising OC used in CLC process due to its low cost and environmental compatibility14,15.Fig.1 depicts the schematic process of coal CLC using Fe2O3as OC, where Fe2O3is transferred from the air reactor(AR)to the fuel reactor(FR)to realize the full conversion of coal into CO2and steam.After condensation,the pure CO2can be obtained without extra energy consumption.The reduced Fe2O3-xis transported back to the AR and regenerated therein for initiation of the next coal combustion cycle.
Nevertheless,coal CLC researches are at their very beginning, many problems and challenges lie ahead before their commercial adoption.For example,it is known that mercury exists in coal at the range of 0.02 to 1.0 mg·kg-116,which usually assumes three forms in flue gas:elemental mercury(Hg0),oxidized mercury (Hg2+),and particulate mercury(Hgp).Mercury is released into fuel gas as Hg0during coal combustion17,emitted into the atmosphere, and then bioaccumulated through the food chain,which affects human health and generates long-lasting effects18.Mendiara detected the mercury released from CLC of coal and then quantitatively tested the emissions from the fuel reactor and air reactor19. However,whether there are certain interactions happening between mercury and OC during CLC processes,and if they have certain interactions,and how these interactions affect the related reactions of CLC process,remain unknown.Usually,OC could be generally reduced into lower oxidation state during deep CLC processes,and interfacial interactions would happen to these reduced OCsurfaces. Therefore,it is necessary to detect the adsorption of mercury on the perfect and reduced OC surface at different reduction states,and the effect of mercury adsorption on the further reactions between fuel molecule and the reduced OC surface,which are fundamental understandings of the coal CLC system.
Considering that mercury firstly releases into fuel gas as Hg0during coal combustion17,and Fe2O3[001]is one of the dominant growth faces of the natural α-Fe2O318,we herein elucidated the detailed Hg0adsorption on the gradually reduced Fe2O3[001] surface and its effect on carbon deposition during CLC by using density functional theory(DFT)calculations.Results will provide insights into the mechanism of mercury adsorption and its effect on catalytic decomposition of CO during deep reduction of Fe2O3. The correlation between adsorption,decomposition,and reduction degree provides fundamental understanding to such complex CLC combustion processes.This study can serve as a reference for CLC optimization.
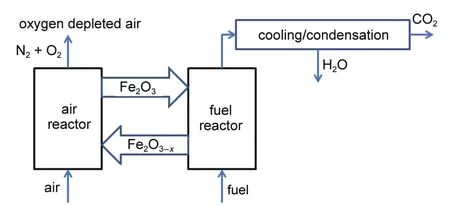
Fig.1 Schematic diagram for CLC of coal using Fe2O3as OC
2 Computational models and method
2.1Theoretical models
The pure Fe2O3[001]-p(2×2)surface with 32 Fe atoms and 48 O atoms was modeled.To eliminate the spurious interaction between periodic images in the z direction,the vacuum space is set no less than 1.5 nm,and dipole correction is used as well.Aseries of reduced Fe2O3[001]-p(2×2)surfaces was obtained by gradually removing the O atoms on the outer surface layer to do annealing under constant pressure and temperature(NPT)until their equilibrium states were reached,where the initial temperature was 300 K and the mid-cycle temperature was 1073 K(the temperature for real CLC).Further geometric optimizations were performed using DFT calculations.After geometric optimization,the stable configurations of Fe2O3,Fe2O2.75,Fe2O2.5,Fe2O2.25,Fe2O1.625,Fe2O0.875, Fe2O0.375,and Fe are shown in Fig.2.Then the Hg0adsorption,CO adsorption,and catalytic decomposition of CO on these surfaces were investigated.
2.2Parameters setting
We used the CASTEP program package for all calculations performed with a plane-wave pseudo potential method based on dispersion-corrected DFT19,which was combined with the generalized gradient approximation(GGA)parameterized by Perdew, Burke,and Ernzerhof(PBE)20,21.Ultrasoft vanderbilt pseudopotentials22were used to describe core orbitals.To correct the strong correlation between 3d-electrons of Fe atom,a Hubbard U was adopted to provide an on-site Coulomb repulsion to the DFT Hamiltonian.Previous investigations suggested that DFT+U method with U=5 eV could accurately reproduce the experimental value of bandgap(2.2 eV)and band structure of hematite23-27.We have therefore adopted U=5 eV to conduct our calculations in this work.Previous studies have confirmed the cut-off energy of 350 eV and k meshes of 4×4×1 in α-Fe2O3surface reaction field can obtain satisfied results28.Therefore,a cutoff of 350 eV was employed with smearing width of 0.1 eV,while the Brillouin zone integration of the surfaces was calculated using4×4×1 Monkhorst-Pack k-point meshes.The magnetic configuration(+--+)was set for Fe atoms in the rhombohedral unit cell of Fe2O3,which makes the optimized cell at the lowest total energy29-32,+and-designated up-spin and down-spin directions with respect to the z-axis.The convergence criteria for the structure optimization and energy calculation were set to(a)an energy tolerance of 2.0×10-5eV·atom-1,(b)a SCF tolerance of 2.0×10-6eV·atom-1,(c)a maximum force tolerance of 0.5 eV· atom-1,and(d)a maximum displacement tolerance of 0.0002 nm. A linear synchronous transit or quadratic synchronous transit approach(LST/QST)33is used to get closer to the quadratic region of the transition state and then quasi-newton or eigenvector following methods are used to complete the optimization to ensure one and only one imaginary vibrational frequency.
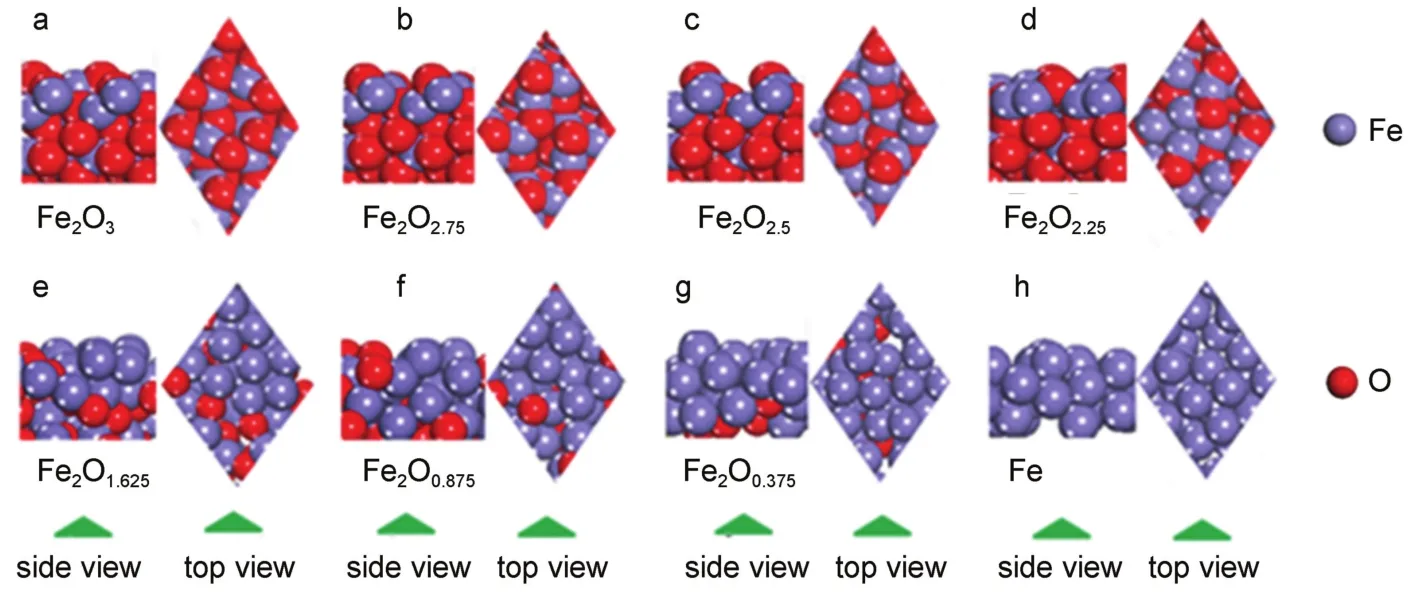
Fig.2 Top and side views of the perfect four-layer Fe2O3[001]-p(2×2)surface model(a)and the reduced surfaces: (b)Fe2O2.636,(c)Fe2O2,(d)Fe2O1.364,(e)Fe2O0.909,(f)Fe2O0.182,and(g)Fe
The adsorption energy for the studied systems was calculated by using the following equation:

where Ecompound,Eslab,and Exare the total energy for the substrate with adsorbate,the substrate,and the adsorbate,respectively. More negative adsorption energy represents stronger interaction.
3 Results and discussion
3.1Hg0adsorption on perfect and reduced Fe2O3[001] surfaces
Since Fe2O3[001]will be gradually reduced in the fuel reactor during CLC,the adsorptions of Hg0on the perfect and the reduced Fe2O3[001]surfaces were investigated.Previous work showed that Hg atom is more inclined to bind to Fe atom than O atom on oxide surface34.We approached Hg0to different Fe atomic sites of these surfaces.Fig.3 illustrated the most stable configurations for the Hg0-Fe2O3,Hg0-Fe2O2.75,Hg0-Fe2O2.5,Hg0-Fe2O2.25,Hg0-Fe2O1.625,Hg0-Fe2O0.875,Hg0-Fe2O0.375,and Hg0-Fe adsorption systems,respectively.
The corresponding calculated adsorption energies are presented in Fig.4.For Hg0-Fe2O3adsorption system,Hg0is physically adsorbed on Fe-top site of Fe2O3[001]surface with the minimum distance(Lmin)of 0.3012 nm and Eadsof-0.36 eV close to the reported value of-0.39 eV34.Then,the interaction between Hg0and reduced surface becomes stronger.For example,in the Hg0-Fe2O2.75system,weak chemisorption happens between Hg0and Fe atom of Fe2O2.75,with the Hg0―Fe bond length of 0.2846 nm and Eadsof-0.65 eV,while the Hg0-Fe2O2.5with the bond length of 0.283 nm and Eadsof-0.76 eV.Further reduction of Fe2O2.5into Fe2O2.25,Fe2O1.625,Fe2O0.875,Fe2O0.375,and Fe promotes the adsorption of Hg0on these surfaces,resulting in forming multi-coordinated Hg―Fe bonds on these surfaces.In the Hg0-Fe2O2.25configuration, the Eadsis-1.04 eV and two Hg―Fe bonds(0.275 and 0.286 nm) formed on the surface,giving the average valueLˉ2fof 0.280 nm.In the Hg0-Fe2O1.625configuration,the Eadsis-1.44 eV and five Hg―Fe bonds(0.269,0.304,0.315,0.274,and 0.287 nm)formed on the surface,giving the average valueLˉ5fof 0.290 nm.The Hg0-Fe2O0.875,Hg0-Fe2O0.375,and Hg0-Fe configurations show the average bond lengthsLˉ6fof 0.289 nm,Lˉ6fof 0.279 nm,Lˉ5fof 0.283 nm, respectively,which result in the corresponding Eadsof-1.72,-2.20,and-2.02 eV.After Fe2O3was reduced into the oxidation state that lower than Fe2O2(such as Fe2O1.625,Fe2O0.875,Fe2O0.375,and Fe),stable chemisorption appears,where Hg0binds firmly to the Fe-accumulated area on the reduced surface.
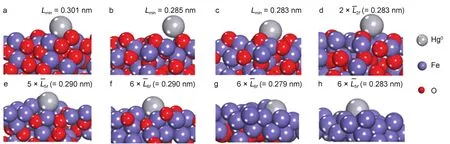
Fig.3 Stable configurations for Hg0on(a)Fe2O3[001],(b)Fe2O2.75,(c)Fe2O2.5,(d)Fe2O2.25,(e)Fe2O1.625,(f)Fe2O0.875,(g)Fe2O0.375,and(h)Fe
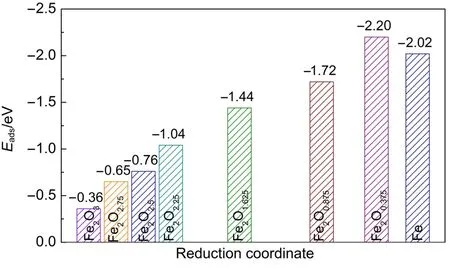
Fig.4 Eadsfor Hg0adsorption on the perfect and the reduced Fe2O3[001]surfaces
To further understand the interaction between Hg0and the surfaces,we analyzed the partial density of state(PDOS)of the adsorbedHg0,whichisplottedinFig.5.AsobservedinFig.5,4s-DOS curve for the Hg0adsorbed on Fe2O3[001]overlaps to that for the pureHg035,while4s-DOSforFe2O2.75andFe2O2.5splitsaroundFermi level(Ef=0eV)suggestingobvious electronic interactionbetween Hg0and surface.Therefore,Hg0-Fe2O2.75and Hg0-Fe2O2.5can be assigned to chemisorption.With further reduction of Fe2O3into Fe2O1.625,Fe2O0.875,Fe2O0.375,andFe,4s-DOSofHg0splitintobonding orbital and anti-bonding orbital,implying that stronger interaction happens between Hg0and surface.These results further verify that the interactions between Hg0and the deeply reduced surfaces (Fe2O1.625,Fe2O0.875,Fe2O0.375,andFe)arestronger thanthosebetween Hg0and the surfaces at relatively higher oxidation state(Fe2O3, Fe2O2.75,Fe2O2.5,andFe2O2.25).Ourresultsfirstrevealtherelationship betweentheadsorptionofHg0andtheironoxidesurfaceatdifferent reduction degrees.While iron oxide is used as oxygen carrier for coal combustion,deeper reduction of iron oxide into lowoxidation statecanresultinhigheradsorptionquantityofHg0.
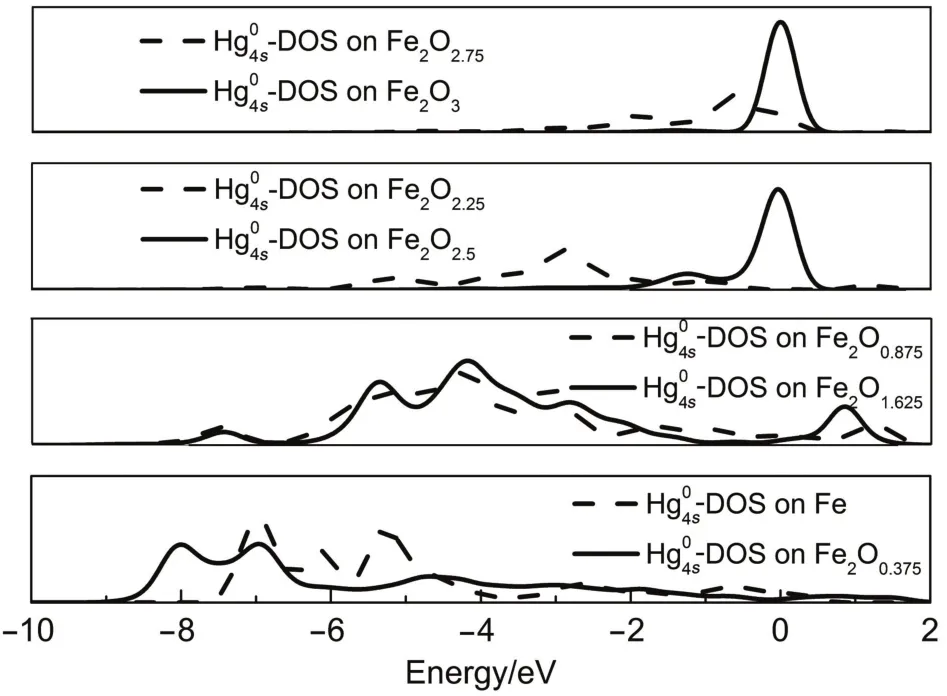
Fig.5 4s-DOS for the adsorbed Hg0on the perfect and the reduced Fe2O3[001]surfaces
Stable Hg0adsorption alters the electronic property of the surfaces,which can hence affect the adsorption and decomposition processes of CO.In our simulations,the stable CO chemisorption occurs through hybrid between C atom and Fe atom of surface, corresponding to that CO prefers to bind to transition metal site of the surface36,37.Fig.6 compares the adsorption energies for the stable configurations of CO adsorption on Fe2O3,Fe2O2.75,Fe2O2.5, Fe2O2.25,Fe2O1.625,Fe2O0.875,Fe2O0.375,and Fe with and without the adsorption of Hg0,respectively.It can be observed that Eadsshows an interesting trend for CO adsorption on Fe2O3[001],Fe2O2.75, Fe2O2.5,Fe2O2.25,Fe2O1.625,Fe2O0.875,Fe2O0.375,and Fe,as the trend for Hg0adsorption on these surfaces shown in Fig.4.
According to Fig.6,the reduced surfaces favor the adsorption of CO while compared to the perfect surface.With reduction of Fe2O3into Fe,Eads(-1.58 eV)for CO adsorption on Fe is close to the reported value of-1.56 eV for CO adsorption on Fe[110]38, but lower than the value(-2.54 eV)for CO adsorption on Fe[100]39.
However,the adsorption of Hg0decreases the interaction between CO and Fe2O3[Fe2O2.75,Fe2O2.5,and Fe2O2.25],while promotes the interaction between CO and Fe2O1.625[Fe2O0.875,Fe2O0.375,and Fe].The distinct effect of Hg0on CO adsorption can be attributed to the relativistic effect of Hg0.The band structure of the reduced surfaces shows that the band near and closed to the Fermi level between the M and K points in the brillouin zone is almost completely flat,indicating extremely high electron effective mass. While Hg0chemically bound to Fe atoms of these reduced surfaces at relatively higher oxidation state,the bands near and closed to the Fermi level become less dispersive(for example,the band structures for Fe2O2.5and Hg0-Fe2O2.5in Fig.1Sa(Supporting Information)),which depress electron and hole transport through the interface,hence decreasing the interaction between CO and the surface.During the first reduction stage(from Fe2O3to Fe2O1.625), Hg0acts as electron donor decreasing the oxidizability of thesurfaces before Fe2O1.625.Then during the last reduction state(from Fe2O1.625to Fe),Hg0chemically bound to Fe on the surfaces,the bands near and closed to the Fermi level become more dispersive (for example,the band structures for Fe2O0.375andHg0-Fe2O0.375in Fig.1Sb(in Supporting Information)),promoting electron and hole transport through the interface,where Hg0acts as electron accepter increasing the oxidizability of the reduced surfaces favoring the hybridization between C and Fe,hence promoting the interaction between CO and the reduced surface.
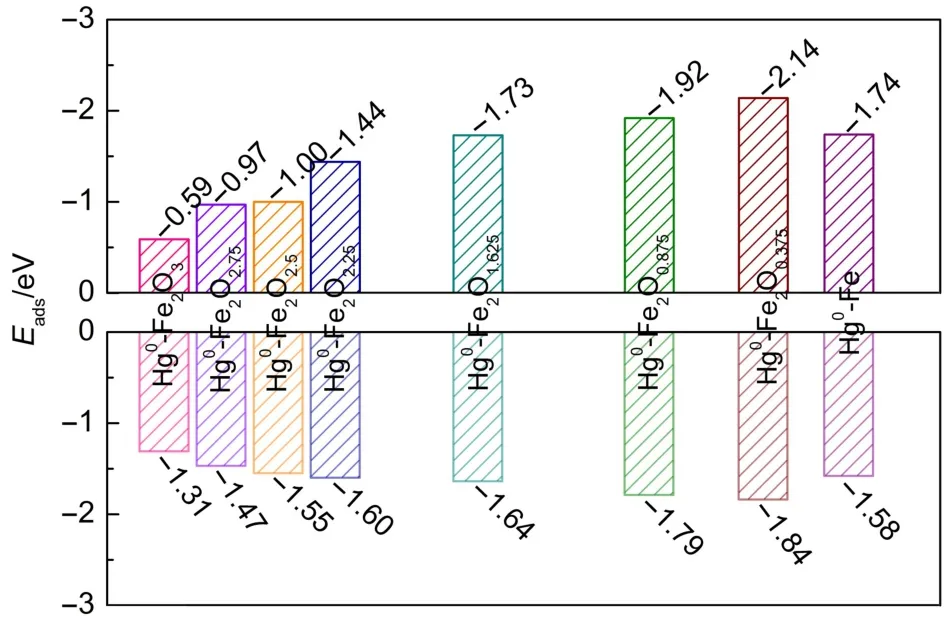
Fig.6 Eadsfor CO adsorption on the perfect and reduced Fe2O3surfaces,and the perfect and reduced Hg0-Fe2O3surfaces
Fig.7 describes the relationship between the reduction degree, charge population(on C atom and O atom of the adsorbed CO molecule,and the total population on the adsorbed CO molecule), and bond length(of the adsorbed CO molecule and the new formed Fe―C bond).Generally,it is accepted that strong interaction corresponds to high value of Eads,lengthening of the C―O bond,and obvious charge transport cross the interface.According to Fig.7a,little charge transfer happens on the perfect Fe2O3and the Hg0-Fe2O3surfaces,corresponding to small Eadsvalue above, which verified the physisorption.Then charge transfer becomes more obvious with the reduction of the surface.The representative interaction district appears after Fe2O2.25,where the Eads,charge density transfers from surface to the adsorbed CO molecule,and the lengthening of C―O bond is more obvious than the cases before Fe2O2.25.The strong interaction and activation of CO will favor the catalytic decomposition of CO and result in carbon deposit on surfaces of the reduced OC,corresponding to an obvious weight increase according to the thermogravimetric analysis of CO-Fe2O328.According to Fig.7b,interaction between CO and the reduced Hg0-doped surface also lengthens the CO bond after Hg0-Fe2O2.25with obvious charge transfer across the interface and charge repopulation during this reduction period,which hence activates the CO bond for further decomposition.
3.2Effect of mercury on catalytic CO decomposition
It is generally agreed that,with the reduction of iron oxide,CO decomposes on the reduced surface and acts as the key step for carbon deposit during CLC40,41.Therefore,we considered the following reaction mechanism on the reduced Fe2O3[001]surface
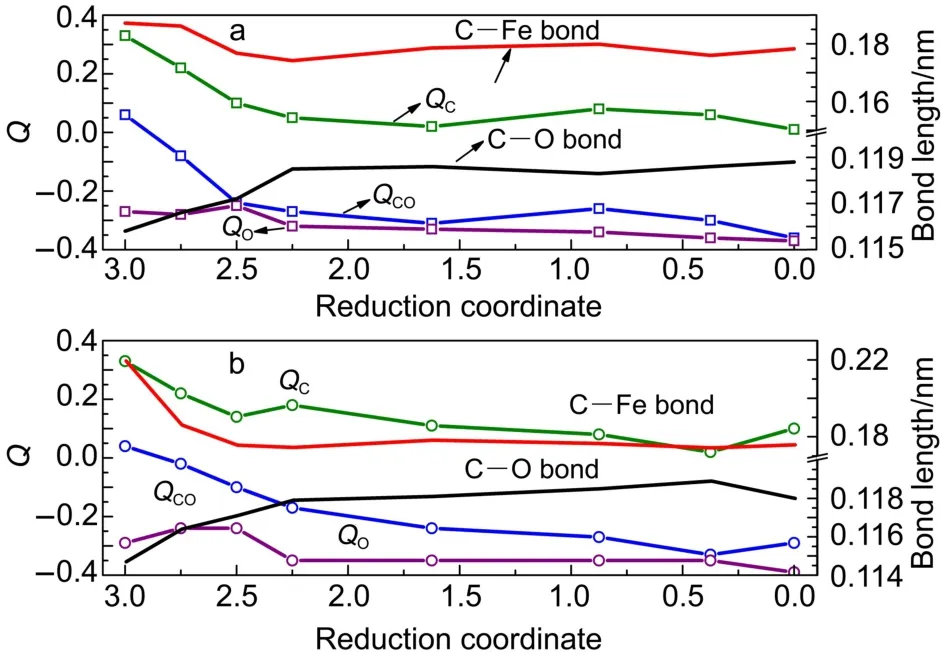
Fig.7 Charge population(Q)for the C atom,O atom,and CO molecule,and the lengths of C―Fe bond and C―O bond
CO+*⇌CO*
CO*+*⇌C*+O*
where*denotes a free step site.Catalytic decomposition of CO may result in a wide range of products.We have neglected the further interaction between C*,O*,and CO that forms CO2or carbonate species on the reduced surface.
Since carbon deposit hardly generates on the iron oxide at high oxidation state28,and stable Hg0adsorption happens on the reduced surfaces Fe2O1.625,Fe2O0.875,Fe2O0.375,and Fe(seen in Fig.3),we discussed the catalytic decomposition of CO on these reduced surfaces.The reaction initiates from the most stable CO adsorption models,and the product corresponds to the decomposition of CO* into C*and O*at the most stable state.Then transition state(TS) search calculations were performed to detect the energy profiles for the CO*decomposition reaction on these reduced surfaces with and without the adsorption of Hg0.The barrier energy(Ea) and reaction enthalpy(Er)for the two reaction cases related to catalytic decomposition of CO on Hg0-doped surfaces and the undoped surfaces are listed in Fig.8.It is clear that the adsorption of Hg0decreases the Eafor catalytic decomposition of CO on Fe2O1.625,Fe2O0.875,Fe2O0.375,and Fe.For example,Eafor catalytic CO decomposition on Fe2O1.625,Fe2O0.875,Fe2O0.375,and Fe are 3.01, 2.76,1.43,and 1.51 eV,while the Eafor Hg0-Fe2O1.625,Hg0-Fe2O0.875, Hg0-Fe2O0.375,and Hg0-Fe are 2.60,2.39,1.07,and 1.23 eV,respectively.Eafor CO decomposition on Fe is higher than that for CO dissociation on Fe(100)(1.14 eV reported by,381.07 eV reported by Sorescu et al.42,and 1.14 eV reported by Moon et al.43). The general decrease of Eaon the Hg0-doped reduce surfaces could be attributed to the obvious charge transfer and charge repopulation on the adsorbed CO that activates the C―O bond for easier decomposition(as shown above in Fig.7b).Except for CO decomposition on Fe surface,the reaction energy is in proportion to the barrier energy for CO decomposition on the reduced iron oxide surfaces.CO decompositions on Fe2O1.625and Fe2O0.875are endothermic processes,which need high temperature acting as driving force for the reactions.However,the adsorption of Hg0reduces the barrier energy for CO decomposition making the reaction exo-thermic.Both kinetics and energetics analysis imply that carbon deposit is more energetically realized on the Hg0-doped reduced iron oxide surface.
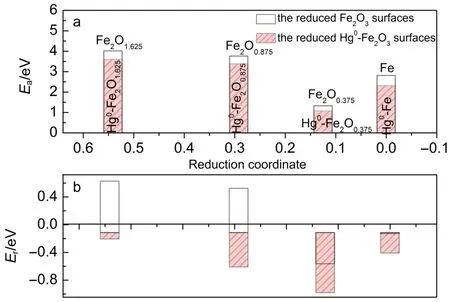
Fig.8 Eaand Erfor CO adsorption on(a)the perfect and reduced Fe2O3surfaces,and(b)the perfect and reduced Hg0-Fe2O3surfaces
Because of the low Ea,obvious carbon deposit will happen after the reduction period around Fe2O1.625,which corresponds to the conversion rate(χ)around 46%(calculated by100%,according to our previous works28,44,45).Hg0promotes catalytic decomposition of CO for carbon deposit on the surfaces and depresses the efficiency of CLC.
To address the trends in CO*decomposition more accurately, a kinetic model is required in order to treat the different time scales associated with the reduction of Fe2O3at different degrees during CLC.We applied a simple phenomenological model to bring out the trends.The net reaction rates for reactions(R1 and R2)are written as whereki(i=1,2)are forward rate constants for Reaction-1 and Reaction-2 whilek-i(i=1,2)are the rate constants for the backward reactions.p and θ denote the pressure and surface coverage, respectively.Specially,θ*denotes the ratio of exposed active site.

The rate constants may be written as

whereEiis the activation free energy of reaction step i.h is the Planck constant(6.63×10-34J·s),kBis Boltzmann constant (1.38×10-23J·K-1).The degree of freedom for the reaction component j in each reaction step is given by
fj=ft·fr·fv
whereft,fr,and fvare the translational,rotational,and vibrational degree of freedom,respectively.
For the CO desorption step(the backward reaction of step(1)), we use a rate constant given by
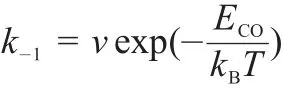
where ECOis the CO binding energy and ν=1013s-1is set as a typical pre-exponential factor for CO desorption.
With the above approximations,Reaction-2 is the rate-determining step for CO evolution.Since chemical looping combustion experiments were usually performed around 1073 K in our previous works28,45,the reaction rate is calculated according to the barrier energy for the decomposition of CO(ΔECO≠)under 1073 K and the given condition θCO=0.5,θO=0.25,θC=0.25,and PCO= 1.01×105Pa.The results are shown in Fig.9.For the catalytic decomposition of CO on the reduced Fe2O3surfaces(the red curve),high decomposition reaction rate can be realized during the period round Fe2O0.375and closed to the complete reduction stage into iron.This red curve model describes the thermogravimetric trends observed experimentally well,where obvious weight increase due to carbon deposit occurred around and after Fe2O1.62528. However,Hg0promotes the CO decomposition on these reduced surfaces(the black line).The adsorption of Hg0promotes the catalyticdecompositionofCO,andrevealstherelationshipbetween catalyticCOdecompositionreactionrateandreductiondegreeof ironoxide.ThequalitativeandquantitativeresultsofHg0adsorption and its effect on CO adsorption and decomposition will provide fundamentalunderstandingforoptimizingtheCLCprocesses.
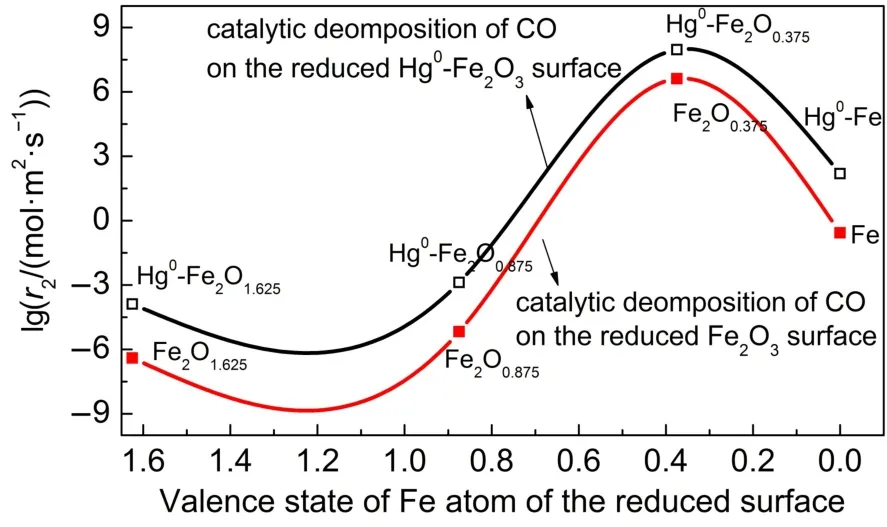
Fig.9 Reaction rate for CO decomposition on the reduced Fe2O3[001]at θCO=0.5,θO=0.25,and θC=0.25 for the Reaction-2 under 1073 K
4 Conclusions
We investigated the adsorption of elemental mercury(Hg0)on the perfect and reduced Fe2O3[001]surfaces,and probed into the synergetic effect of the adsorbed Hg0on the catalytic decomposition of CO on the reduced Fe2O3[001]surfaces during chemical looping combustion.Interaction between Hg0and surface increases with reduction of Fe2O3[001]into lower oxidation state. Stable Hg0adsorption promotes the interaction between CO and the reduced surfaces,favoring charge transport from surface to the adsorbed CO,which activates the C―O bond for its decomposition.Hg0together with carbon will hence be carried into air reactor,depressing the efficiency of CLC.Reasonable control of reduction degree not only hinders Hg0binding to the surface of oxygen carrier but also decreases generation of carbon deposit. Results of the current DFT work provide fundamental understanding of interaction between Hg0and the perfect and reduced Fe2O3surfaces,and synergetic effect of Hg0on the interaction between CO and these surfaces.For future studies,it is necessary to investigate the synergy of multiple elements included in coal such as K,Na,and Ca on mercury adsorption and its influence on the process of CLC.
Supporting Information:Band structures for Fe2O2.5,Hg0-Fe2O2.5,Fe2O0.375,andHg0-Fe2O0.375,as well as geometric structures of initial state,transition state,and final state for CO decomposition on the reduced surfaces(Fe2O1.625,Hg0-Fe2O1.625,Fe2O0.875,Hg0-Fe2O0.875,Fe2O0.375,Hg0-Fe2O0.375,Fe,and Hg0-Fe)have been included.This information is available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1) Horst,J.R.;Karl,F.K.Am.Chem.Soc.1983,7,71.
(2) Ishida,M.;Jin,H.G.Ind.Eng.Chem.Res.1996,7,2469.doi:10.1021/ie950680s
(3) Fan,L.S.;Zeng,L.;Wang,W.;Luo,S.W.Energy Environ.Sci. 2012,5,7254.doi:10.1039/C2EE03198A
(4) Adanez,J.;Abad,A.;Garcia-Labiano,F.;Gayán,P.;de Diego, L.F.Prog.Energy Combust.Sci.2012,38,215.doi:10.1016/j. pecs.2011.09.001
(5) Zhang,Y.;Doroodchi,E.;Moghtaderi,B.Energy Fuels 2012, 26,287.doi:10.1021/ef201156x
(6) He,F.;Li,H.B.;Zhao,Z.L.Int.J.Chem.Eng.2009,2009,1. doi:10.1155/2009/710515
(7) Lyngfelt,A.;Leckner,B.;Mattisson,T.Chem.Eng.Sci.2001, 56,3101.doi:10.1016/S0009-2509(01)00007-0
(8) Fan,L.S.;Zeng,L.;Luo,S.W.AIChE J.2015,61,2. doi:10.1002/aic.14695
(9) Tian,M.;Wang,X.D.;Liu,X.;Wang,A.Q.;Zhang,T.AIChE J.2016,62,792.doi:10.1002/aic.15135
(10) Cho,P.;Mattisson,T.;Lyngfelt,A.Fuel 2004,83,1215. doi:10.1016/j.fuel.2003.11.013
(11) Abad,A.;Adánez,J.;García-Labiano,F.;de Diego,L.F.; Gayán,P.;Celaya,J.Chem.Eng.Sci.2007,62,533. doi:10.1016/j.ces.2006.09.019
(12) Cao,Y.;Pan,W.P.Energy Fuels 2006,20,1836.doi:10.1021/ ef050228d
(13) Abad,A.;Cuadrat,A.;Mendiara,T.;García-Labiano,F.;Gayán, P.;de Diego,L.F.;Adánez,J.Ind.Eng.Chem.Res.2012,51, 16230.doi:10.1021/ie302158q
(14) Zhang,S.;Xiao,R.;Zheng,W.G.Appl.Energy 2014,130,181. doi:10.1016/j.apenergy.2014.05.049
(15) Xiao,R.;Song,Q.L.;Song,M.;Lu,Z.J.;Zhang,S.;Shen,L. H.Combust Flame 2010,157,1140.doi:10.1016/j. combustflame.2010.01.007
(16) Yudovich,Y.E.;Ketris,M.P.Int.J.Coal Geol.2005,62,107. doi:10.1016/j.coal.2004.11.002
(17) Frandsen,F.;Dam-Johansen,K.;Rasmussen,P.Prog.Energy Combust.Sci.1994,20,115.doi:10.1016/0360-1285(94)90007-8
(18) Thevuthasan,S.;Kim,Y.J.;Yi,S.I.;Chambers,S.A.;Morais, J.;Denecke,R.;Fadley,C.S.;Liu,P.;Kendelewicz,T.;Brown, G.E,Jr.Surf.Sci.1999,425,276.doi:10.1016/S0039-6028(99) 00200-9
(19) Segall,M.D.;Lindan,P.J.D.;Probert,M.J.;Pickard,C.J.; Hasnip,P.J.;Clark,S.J.;Payne,M.C.J.Phys.:Condens. Matter 2002,14,2717.doi:10.1088/0953-8984/14/11/301
(20) White,J.;Bird,D.Phys.Rev.B.1994,50,4954.doi:10.1103/ PhysRevB.50.4954
(21) Perdew,J.P.;Chevary,J.A.;Vosko,S.H.;Jackson,K.A.; Pederson,M.R.;Singh,D.J.;Fiolhais,C.Phys.Rev.B: Condens.Matter Mater.Phys.1992,46,6671.doi:10.1103/ PhysRevB.46.6671
(22) Vanderbilt,D.Phys.Rev.B 1990,41,7892.doi:10.1103/ PhysRevB.41.7892
(23) Guo,H.;Barnard,A.S.Phys.Rev.B 2011,83,094112. doi:10.1103/PhysRevB.83.094112
(24) Bandyopadhyay,A.;Velev,J.;Butler,W.H.;Sarker,S.K.; Bengone,O.Phys.Rev.B 2004,69,174429.doi:10.1103/ PhysRevB.69.174429
(25) Huda,M.N.;Walsh,A.;Yan,Y.J.Appl.Phys.2010,107, 123712.doi:10.1063/1.3432736
(26) Dzade,N.Y.;Roldan,A.;de Leeuw,N.H.Minerals 2014,4,89. doi:10.3390/min4010089
(27) Rohrbach,A.;Hafner,J.;Kresse,G.Phys.Rev.B 2004,70, 125426.doi:10.1103/PhysRevB.70.125426
(28) Qin,W.;Wang,Y.;Lin,C.F.;Hu,X.Q.;Dong,C.Q.Energy Fuels 2015,29,1210.doi:10.1021/ef5024934
(29) Song,J.J.;Niu,X.Q.;Ling,L.X.;Wang,B.J.Fuel Process Technol.2013,115,26.doi:10.1016/j.fuproc.2013.04.003
(30) Wong,K.;Zeng,Q.H.;Yu,A.B.J.Phys.Chem.C 2011,115, 4656.doi:10.1021/jp1108043
(31) Martin,G.J.;Cutting,R.S.;VauGhan,D.J.;Warren,M.C.Am. Mineral.2009,94,1341.doi:10.2138/am.2009.3029
(32) Sandratskii,L.M.;Uhl,M.;Kübler,J.J.Phys.:Condens. Matter 1996,8,983.doi:10.1088/0953-8984/8/8/009
(33) Govind,N.;Petersen,M.;Fitzgerald,G.;King-Smith,D.; Andzelm,J.Comput.Mater.Sci.2003,28,250.doi:10.1016/ S0927-0256(03)00111-3
(34) Guo,P.;Guo,X.;Zheng,C.G.Fuel 2011,90,1840. doi:10.1016/j.fuel.2010.11.007
(35) Ji,W.C.;Shen,Z.M.;Fan,M.H.;Su,P.R.;Tang,Q.L.;Zou, C.Y.Chem.Eng.J.2016,283,58.doi:10.1016/j. cej.2015.06.033
(36) He,F.;Wang,H.;Dai,Y.N.J.Nat.Gas.Chem.2007,16,155. doi:10.1016/S1003-9953(07)60041-3
(37) Dong,C.Q.;Sheng,S.H.;Qin,W.;Lu,Q.;Zhao,Y.;Wang,X. Q.;Zhang,J.J.Appl.Surf.Sci.2011,257,8647.doi:10.1016/j. apsusc.2011.05.042
(38) Stibor,A.;Kresse,G.;Eichler,A.;Hafner,J.Surf.Sci.2002, 507,99.doi:10.1016/S0039-6028(02)01182-2
(39) Bromfield,T.C.;Ferré,D.C.;Niemantsverdriet,J.W. ChemPhysChem 2005,6,254.doi:10.1002/cphc.200400452
(40) Claridge,J.B.;Green,M.L.H.;Tsang,S.C.;York,A.P.E.; Ashcroft,A.T.;Battle,P.D.Catal.Lett.1993,22,299. doi:10.1007/BF00807237
(41) Wang,B.W.;Yan,R.;Lee,D.H.;Liang,D.T.;Zheng,Y.;Zhao, H.B.;Zheng,C.G.Energy Fuels 2008,22,1012.doi:10.1021/ ef7005673
(42) Sorescu,D.C.;Thompson,D.L.;Hurley,M.M.;Chabalowski, C.F.Phys.Rev.B 2002,66,035416.doi:10.1103/ PhysRevB.66.035416
(43) Moon,D.W.;Bernasek,S.L.;Lu,J.P.;Gland,J.L.;Dwyer,D. J.Surf.Sci.1987,184,90.doi:10.1016/S0039-6028(87)80274-1
(44) Qin,W.;Lin,C.F.;Long,D.T.;Xiao,X.B.;Dong,C.Q.Acta Phys.-Chim.Sin.2015,31,667.[覃 吴,林常枫,龙东腾,肖显斌,董长青.物理化学学报,2015,31,667.]doi:10.3866/PKU. WHXB201502061
(45) Dong,C.Q.;Liu,X.L.;Qin,W.;Lu,Q.;Wang,X.Q.;Shi,S. M.;Yang,Y.P.Appl.Surf.Sci.2012,258,2562.doi:10.1016/j. apsusc.2011.10.092
Synergetic Effect of Mercury Adsorption on the Catalytic Decomposition of CO over Perfect and Reduced Fe2O3[001]Surface
LIJi-Hong1LIN Chang-Feng1QIN Wu1,*XIAO Xian-Bin1WEILi2,*
(1National Engineering Laboratory for Biomass Power Generation Equipment,School of Renewable Energy Engineering, North China Electric Power University,Beijing 102206,P.R.China;2State Key Laboratory of Urban Water Resource and Environment,Harbin Institute of Technology,Harbin 150090,P.R.China)
Mercury emission from coal during chemical-looping combustion(CLC)is an inevitable process, which can lead to adverse interactions with the surface of the oxygen carrier,thereby affecting the interfacial redox reactions.Density functional theory calculations were performed to investigate the mechanism of elemental mercury(Hg0)adsorption and the synergetic effect of Hg0on the catalytic decomposition of CO over a perfect surface(Fe2O3[001]),as well as a series of reduced surfaces(Fe2O2.75,Fe2O2.5,Fe2O2.25,Fe2O1.625, Fe2O0.875,Fe2O0.375and Fe)during a deep CLC process.In this study,Hg0was physically adsorbed on to a perfect Fe2O3surface,and then chemically adsorbed on to a series of reduced surfaces.The adsorption of Hg0 inhibited the formation of meaningful interactions between CO and Fe2O3[Fe2O2.75,Fe2O2.5and Fe2O2.25]and hindered the efficient transport of oxygen to oxidize CO into CO2.In contrast,this process promoted the interactions between CO and Fe2O1.625[Fe2O0.875,Fe2O0.375,and Fe],favoring the catalytic decomposition of CO on these surfaces,which accelerated the carbon deposit reducing CLC efficiency.Rationally controlling the reduction degree of the oxygen carrier could therefore be used to either decrease the adsorption of Hg0or depress the deposition of carbon, which are both crucial for the optimization of CLC processes.
O647
10.3866/PKU.WHXB201607271
Received:May 3,2016;Revised:July 27,2016;Published online:July 27,2016.
*Corresponding authors.QIN Wu,Email:qinwugx@126.com;Tel:+86-10-61772457.WEI Li,Email:weilihit@126.com;Fax:+86-451-86283805. The project was supported by the National Natural Science Foundation of China(51346001,51106051)and Fundamental Research Funds for the
Central Universities,China(2016YQ07,2014ZD14).
国家自然科学基金(51346001,51106051)和中央高校基本科研专项资金(2016YQ07,2014ZD14)资助项目
