氮掺杂碳层包覆金属钴颗粒与氮掺杂石墨烯纳米复合材料作为高容量锂离子电池负极材料
2016-12-15耿凯明吴俊杰耿洪波胡亚云瞿根龙潘越郑军伟顾宏伟
耿凯明 吴俊杰 耿洪波 胡亚云 瞿根龙 潘越 郑军伟 顾宏伟*,
氮掺杂碳层包覆金属钴颗粒与氮掺杂石墨烯纳米复合材料作为高容量锂离子电池负极材料
耿凯明1吴俊杰1耿洪波1胡亚云1瞿根龙1潘越1郑军伟2顾宏伟*,1
(1苏州大学材料与化学化工学部,江苏省有机合成重点实验室;苏州纳米科学技术协同创新中心,苏州215123)
(2苏州大学物理与光电·能源学部,苏州215123)
合成了一种石墨烯基纳米复合材料即:由氮掺杂碳层包覆的金属钴纳米颗粒,充分分散于氮掺杂的石墨烯表面。这种纳米复合材料进一步提高了石墨烯的导电性,增加了石墨烯的储锂容量。该材料被用作锂离子电池负极材料,在性能测试中展现了良好的循环性能,在以100 mA·g-1的电流密度循环200圈后,放电容量高达950.1 mAh·g-1,库伦效率约为98%。
钴纳米颗粒;氮掺杂的石墨烯;负极材料;锂离子电池
(2College of Physics,Optoelectronics and Energy,Soochow University,Suzhou,Jiangsu 215123,China)
0 Introduction
Lithium-ion batteries(LIBs)have been applied in industrial production during the past three decades.It has drawn intensive attention due to its unique advantages,such as high energy density,high average output voltage,environmentally friendly,no memory effect,long service life[1].To a large extent,anodematerial is a crucial part of lithium-ion batteries and has significantinfluence on its performance[2].Graphite has been used as an anode material and gained commercialization due to its high Coulombic efficiency and cycling performance.However,the specific capacity ofgraphite is relatively low(theoretical value: 372 mAh·g-1).As the portable household appliances and electric vehicles are developing rapidly, alternative anode materials are urgently required to enhance the battery performance.
In recent years,non-metallic heteroatom(N,S,P and B)doped carbon-based materials are proposed to be promising candidates anode substrate materials. Many studies show that the doped atoms play an important role on the electrical conductivity and the capacity of carbon materials in LIBs[3-6].Among them, N-doping carbon materials attract the most attention. Generally,the insertion of N atoms to the graphitic lattice can form C-N bond,modulate the band structure and lead to a metal-semiconductor transition, which could dramatically promote the electronic performance,offer more Li-storage sites and reduce Li diffusion barriers[7-12].The morphology of carbon material is also criticalfor the development of carbonbased anode.Among many types of carbon materials (e.g.nanotubes,nanofibers,C60,graphene)[13-16],graphene is one of the most desirable anode materials owing to its high surface area,outstanding electrical conductivity and stable mechanical properties[17].There are already several studies about graphene-based materials,which exhibits better performance than commercialgraphite[18-23].Meanwhile,to further improve the performance of carbon-based anode,many kinds of metal or alloy(e.g.Sn,Sb,Si)[24-26]with electrochemical capability were added onto carbon materials.According to previous reports,these composites can generate synergistic effect between metal and carbon materials, exhibiting a notable capacity increase of carbon-based anode[27-28].Thanks to the beneficial modification of N-doping,the superior electrochemical properties of graphene,and the advantage of metal/carbon composition effect,we consider metal/N-doped graphene-based composites to be very promising anode materials for the application in lithium-ion batteries.
Hence,in this paper,we synthesized N-doped carbon-encapsulated Co nanoparticles on N-doped graphene(NC@Co@NG)and exploited its performance as anode materials in lithium-ion batteries.The composite has a unique structure,in which Co nanoparticles dispersed on N-doped graphene and wrapped by N-doped carbon layer.When evaluated as an anode material for LIBs,it shows outstanding cycling performance and high Coulombic efficiency. The capacity is up to about 950.1 mAh·g-1after 200 cycles,presenting an upward tendency in the consecutive cycles at the current rate of 100 mA·g-1. Compared to N-doped graphene and Co/graphene described in the literature[29-30,54],the NC@Co@NG delivers a superior electrochemical performance. These results demonstrate that the as-synthesized material is a very promising anode candidate for developing high efficiency lithium-ion batteries.
1 Experimental
1.1 Preparation of N-doped carbon-encapsulated Co nanoparticles on N-doped graphene nanosheets
1.1.1 Synthesis ofgraphene oxide
First of all,graphene oxide was prepared by graphite powders on the basis of the modified Hummersmethod[31].Briefly,graphite powders(5.0 g) and sodium nitrate(3.8 g)was added to concentrated sulfuric acid(169 mL)under magnetic stirring in an ice-water bath for 1 day.Afterwards,22.5 g of KMnO4was gradually added.As soon as it was mixed well, the ice bath was removed and the solution was stirred at 35℃until a highly viscous liquid was obtained. After adding 100 mL of pure water,the suspension was heated in a 98℃water bath for 15 minutes. Then,it was further treated with warm water and H2O2(30%)in sequence,followed by repeated washing with water and HCl.Finally,the resulting solids were obtained via centrifuging at 6 000 r·m-1and dried at 50℃for 24 h in a vacuum oven.The product was dispersed in water by sonication for 12 h at a concentration of10 mg·mL-1.
1.1.2 Synthesis of N-doped carbon-encapsulated Co nanoparticles on N-doped graphene nanosheets
The nano-composite was synthesized according to the literature with some modifications[32].160 mg cobaltacetate and 10 mL cyanamide were dissolved in 30 mL of a distilled water-ethanol(1∶1,V/V)and underwent ultrasonication for about 15 minutes to form a homogeneous solution.Subsequently,the above solution was maintained at80℃for 1 h.After cooling down to room temperature naturally,50 mg graphene oxide was added into the resulting mixture with vigorous magnetic stirring for 24 h.The product was dried at 75℃for removing the most of the water and the ethanol,and then further dried under vacuum conditions at 60℃overnight.The treated samples were calcined at 450℃for 2 h,then at 700℃for 2 h under N2atmosphere.
1.2 Material characterization
The crystal structure of the obtained samples was characterized by X-ray diffraction(XRD)(Netherlands PANalytical)with Cu Kαradiation(λ=0.154 059 8 nm),which was carried out between 20°and 80°with a scanning current of 40 mA and a scanning voltage of 40 kV.The microstructural properties were obtained using transmission electron microscopy (TEM),scanning electron microscopy(SEM)and highresolution TEM(HRTEM).The EDS is attached to the SEM.SEM spectroscopy was performed on a Hitachi S-4700 cold field emission scanning electron microscope operated at 30 kV,and TEM(TecnaiG220, FEI,American))was obtained by a Gatan CCD794 camera operated at 200 kV.HRTEM was taken on a Tecnai G2 F20 S-TWIN microscope with an accelerating voltage of 200 kV.The data of XPS are acquired through a KRATOS Axis ultra-DLD X-ray photoelectron spectrometer with monochromatic Mg Kα X-rays(1 283.3 eV).Nitrogen adsorption/desorption isotherms at 77 K were detected by means of an ASAP 2020 V3.03 H instrument.The total specific surface area is inspected relying on the multipoint BrunauerEmmettTeller(BET)method.
1.3 Electrochemical characterization
The electrochemical experiments were performed using two electrode coin-type cells with lithium foil serving as both counter and reference electrodes.The working electrodes were made as follows:80%(w/w)of active materials powder(1 mg·cm-2),10%(w/w)of acetylene black(The capacity is about 145 mAh·g-1, shown in Fig.S1 in the Supporting Information),and 10%(w/w)of polyvinylidene fluoride(PVDF)were mixed in an N-methyl-2-pyrrolidone(NMP)solvent to form a homogeneous slurry,followed by spreading onto a copper foil.Finally,the copper foil was dried overnight under vacuum at 100℃.The electrolyte was 1 mol·L-1LiPF6in a 1∶1(V/V)mixture of ethylene carbonate(EC)and diethyl carbonate(DEC). The cell assembly was performed in an argon-filled glove box in which moisture and oxygen were both below 1×10-7(V/V).The cells were charged and discharged between 3.00 and 0.01 V using Land CT2001A tester.Electrochemical impedance spectral measurements of cells before cycling were conducted in the frequency range from 100 kHz to 10 mHz with an alternating currentamplitude of 5 mV.
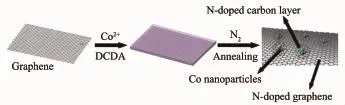
Fig.1 Synthetic protocol for NC@Co@NG
2 Results and discussion
Fig.1 illustrates the fabrication procedure of N-doped carbon-encapsulated Co nanoparticles on N-doped graphene nanosheets.Briefly,the Co2+-cyanamide (DCDA)complexes were adsorbed on graphene oxide by electrostatic attraction.By virtue of annealing under N2,the cyanamide decomposed into carbon and the Co2+species reduced to Co0which catalyzed the synthesis of carbon layer.As a result,N-dopedcarbon-encapsulated Co nanoparticles on N-doped graphene nanosheets(abbreviated as NC@Co@NG) were successfully fabricated.
Fig.2A and B shows typical low-magnification scanning electron microscopy images of the NC@Co@NG.Obviously,the surface becomes crude and plicate as compared with graphene oxide(SEM and TEM images were shown in Fig.S2).TEM images of NC@Co@NG(Fig.2C and D)affirm that Co nanoparticles are firmly anchored on the graphene nanosheets.And the N-doped carbon layerderived from the cyanamide,surrounding Co nanoparticles is also clearly observed(high resolution TEM,Fig.S3).In addition,on the basis ofenergy dispersive X-ray(EDS) analysis,the elemental composition in the as-prepared sample comprises of C,N and Co(Fig.S4).
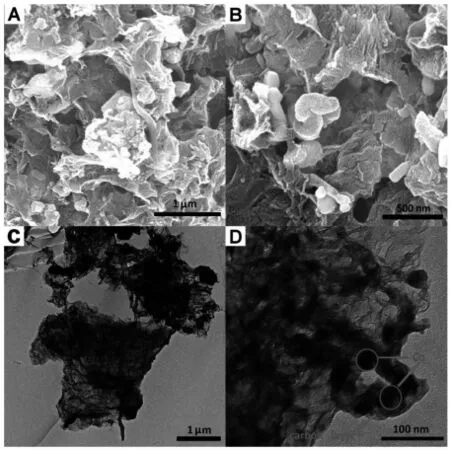
Fig.2 SEM images of the NC@Co@NG(A,B)and TEM images of the NC@Co@NG(C,D)
To illustrate the crystalstructure ofthe as-prepared material,XRD measurementswere carried out.Asshown in Fig.3 the characteristic peaks at44.20°(plane(111)), 51.54°(plane(200))and 75.89°(plane(220))coincide well with pure Co(JCPDS No.15-0806).In the XRD pattern,there also is a distinct peak at 25.5°,owing to (002)crystalplanesofgraphene.

Fig.3 X-ray diffraction(XRD)pattern ofthe NC@Co@NG
X-ray photoelectron spectroscopy(XPS)is a powerful and accurate technology to analyze the elemental composition and valence states of the samples.So,XPS was used to determine the content and the valence states ofthe carbon,nitrogen and Co atoms in NC@Co@NG.As depicted in Fig.4A,the XPS survey scan of NC@Co@NG,carbon,cobalt,nitrogen and oxygen can be ascribed to C1s,Co3p,Co3s,N1s, and O1s,respectively.Fig.4B shows the spectrum ofN-doped carbon,in which a peak at 285.4 eV is attributed to C-N bonds for C1s.Based on the deconvolution curve(Fig.4C),the two peaks at 398.5 and 400.5 eV for N1s electrons should be assigned to pyridinic and pyrrolic nitrogen,respectively, indicating that the incorporation of N heteroatoms in graphene and carbon layer are successful[33].The broad and asymmetric Co2p XPS high-resolution scan reveals that Co0is synthesized,along with a pair of characteristic peaks at 778.3 and 793.7 eV(Fig.4D).

Fig.4(A)XPS spectra of NC@Co@NG and high-resolution scans spectra of C1s(B),N1s(C),and Co2p(D)of NC@Co@NG
Fig.5A shows a typical discharge-charge voltage profile of NC@Co@NG ata currentdensity of100 mA· g-1on the voltage from 0.05 to 3.00 V.During the initial discharge process,the sample provides a very high storage capacity of1 287 mAh·g-1and delivers relative low reversible capacity of 559.7 mAh·g-1,giving rise to an initial Coulombic efficiency of approximately 43%. Itis the Co nanoparticles and N-doped carbon structure that contributes to the remarkable enhancement of the capacity as compared to the theoretical capacity of graphene(372 mAh·g-1)[34-45].However,it is being known that most of the anode materials would inevitably form solid electrode interface(SEI)film with electrolyte decomposition,which results in irreversible capacity loss and poor Coulombic efficiency[46-51]. Nevertheless,starting from the second cycle,the capacities gradually improve.For example,the discharge and charge capacities of 10th,50th,100th and 200th cycle are 530.1,517.9,596.6,586.3, 730.7,716.8,950.1 and 935.1 mAh·g-1,respectively, corresponding to the excellent and stable Coulombic efficiency of 98%(Fig.S5),which indicates that NC@Co@NG composition is activated slowly as anode in LIBs.The resultis to keep a fairly high retention of the enhanced capacity.
In Fig.5B,we also could see apparently that the NC@Co@NG has a much better cyclic retention and acquires a rather higher reversible capacity than graphene.The capacity of NC@Co@NG increases dramatically from 559 to 950.1 mAh·g-1after 200 cycles,exhibiting improved capacities with respect to prolong cycling.Firstly,nitrogen doping in graphene alters the charge capacity and electric conductivity of LIBs,this is because thatthe nitrogen has the strongerelectronegativity than carbon,and nitrogen p electrons can form the hybridization(pyridinic N,pyrrolic N) with grapheneπsystem:(1)pyridinic N(N-6)can contribute one p electron to theπsystem and bond with two C atoms at the edges or defects of graphene; (2)pyrrolic N(N-5)can contribute two p electrons to theπsystem and make up a five-membered ring.As a result,nitrogen doping promotes the electronic performance,offers more active sites and reduces Li diffusion barriers,which is beneficial to enhance the conductivity and capacity of the graphene[52-56]. Secondly,each Co nanoparticle on the surface of the N-doped graphene acts as micro current collector to improve the efficiency of the electronic connection between the active material and the current collector (Cu foil)through a favorable electrical contact,thus effectively promoting the electron transfer rate of the graphene(non-mental materials).And the reversible conversion reactions of alloying and dealloying between Co and Li(discharge process:Co+Li++e-→LiCo;charge process:LiCo→Co+Li++e-),also elevate lithium storage[40].More importantly,the catalysis property of Co nanoparticles can effectively facilitate the decomposition of the SEI layer[57-61],which commands structuralintegrity of the electrode material and guarantees the insertion and extraction of lithium ion.Thirdly,the N-doped carbon layer can alleviate the degrading of the electrode,which not only provides a lot of diffusion mesoporous for Li-ion insertion and extraction and suppresses the volume change to a certain extent,but also protects the Co nanoparticles from exposing to the electrolyte and inhibits the aggregation and pulverization.Finally,the NC@Co@NG nanocomposites vastly weaken the mechanical strain generated by the volume expansion/ contraction owing to mesoporous structure that is demonstrated by the testing of nitrogen adsorption/ desorption.The specific surface area is 178 m2·g-1, and the diameter distributions of most of pores are 5~20 nm.(Fig.S6).
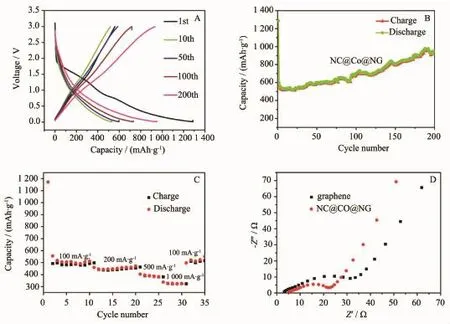
Fig.5 Electrochemical properties the NC@Co@NG electrode:(A)Voltage profiles plotted for 1st,10th,50th,100th and 200th cycles at a current density of 100 mA·g-1;(B)Charge/discharge capacities versus cycle number plots at a current density of 100 mA·g-1;(C)Rate capability at various current densities from 100 to 1 000 mA·g-1;(D)Nyquist plots of the NC@Co@NG(red)and graphene(black)
The rate performance of the NC@Co@NG composite material as electrode of Li-ion batteries was tested at various current densities ranging from 100 to 1 000 mA·g-1(Fig.5C).After 10 cycles of charge-discharge at 100 mA·g-1,the current rate is raised stepwise to 1 000 mA·g-1for 10 or 5 cycles at each rate.Notably,the capacity has a small fluctuation, keeping a fairly high reversible capacity of 511.9, 471.3,394.9 and 336.9 mAh·g-1,at the current rates of 100,200,500 and 1 000 mA·g-1,respectively.In addition,when the rate goes back to 100 mA·g-1,the capacity exhibits a steady-state growth once again.We owe the stable rate stability and good electrochemical performance to the synergistic effect of the Co nanoparticles and N-doping,endowing the nanocomposite electrode high reversible lithium storage,superior electrical conductivity,more diffusion paths for Li-ions and very low volume change.The cyclic voltammogram(CV)curves of the NC@Co@NG also prove that it possesses excellent electrochemical performance(Fig.S7).
Electrochemical impedance analyses(EIS)was further performed to verify the crucial factors of high lithium-ion storage capacity,rate performance and cycling stability of the NC@Co@NG electrodes compared with graphene electrodes.Fig.5D illustrates the Nyquist plots of NC@Co@NG(red line)and graphene(black line),respectively,using coin cells cycled for 100 cycles.It is obvious that the diameters of the semicircles in the high-medium frequency region and the sloping line in the low-frequency region for the electrodes composed of NC@Co@NG are much smaller than those of the graphene electrode,which indicates that the charge-transfer resistances(Rct)and solid-state diffusion resistance (Zw)of the NC@Co@NG electrodes are smaller than that of the graphene electrode.This result further confirms that Co nanoparticles and N-doping serve as a large number of conductive nodes,thus improving the whole conductivity of NC@Co@NG nanocomposite and leading to a high electrochemical performance for the NC@Co@NG electrodes as anode materials for LIBs.
3 Conclusions
In summary,we demonstrate a facile synthesis of N-doped carbon-encapsulated cobalt nanoparticles on N-doped graphene nanosheets(NC@Co@NG)with unique structure.When applied as electrodes in LIBs, the as-prepared NC@Co@NG exhibits excellent electrochemicalper for mance.And the results display a steadily rising specific capacity of 559 to 950.1 mAh· g-1over 200 cycles ata currentdensity of100 mA·g-1, superior to commercial graphite electrode.Meanwhile, a stable rate performance is simultaneously observed, at different current densities ranging from 100 to 1 000 mA·g-1,which indicates a promising anode materialfor Lithium-ion batteries.
Supporting information is available athttp://www.wjhxxb.cn
[1]Kang K,Meng Y S,Bréger J,et al.Science,2006,311 (5763):977-980
[2]Lee J,Urban A,Li X,et al.Science,2014,343(6170):519 -522
[3]Sun Y,Ning G,Qi C,et al.Electrochim.Acta,2016,190: 141-149
[4]Reddy A L M,Srivastava A,Ajayan P M,et al.ACS Nano, 2010,4:6337-6342
[5]Wang Z,Li P,Chen Y,et al.J.Power Sources,2014,263: 246-251
[6]Wang C,Guo Z,Shen W,et al.Adv.Funct.Mater.,2014,24 (35):5511-5521
[7]Wu Z S,Winter A,Chen L,et al.Adv.Mater.,2012,24(37): 5130-5135
[8]Ling Z,Wang Z Y,Qiu J S,et al.Adv.Funct.Mater., 2016,26(1):111-119
[9]Liu R L,Wan L,Zhao D Y,et al.Adv.Funct.Mater., 2015,25(4):526-533
[10]Tang J,Wang T,Yamauchi Y,et al.Chem.Eur.J.,2015,21 (48):17293-17298
[11]Yu X,Kang Y B,Park H S.Carbon,2016,101:49-56
[12]Wu G,Hu Y,Chen W,et al.Nat.Commun.,2015,6:7258
[13]Endo M,Kim C,Nishimura K,et al.Carbon,2000,38(2): 183-197
[14]Wu Y P,Rahm E,Holze R.J.Power Sources,2003,114(2): 228-236
[15]Casas C D L,Li W Z.J.Power Sources,2012,208:74-85
[16]Moradi B,Botte G G.J.Appl.Electrochem.,2016,46(2): 123-148
[17]Bari C D,Goñi-Urtiaga A,Pita M,et al.Electrochim.Acta,2016,191:500-509
[18]Liu R L,Pan L X,Wu D Q,et al.Phys.Chem.Chem. Phys.,2015,17(6):4724-4729
[19]Deng Y F,Xie Y,Ji X L,et al.J.Mater.Chem.A,2016,4 (4):1144-1173
[20]Hao P,Zhao Z H,Yang B,et al.Nano Energy,2015,15:9-23
[21]Wang D W,Min Y G,Peng B,et al.J.Colloid Interface Sci.,2014,417:270-277
[22]Liang J Y,Wang C C,Lu S Y.J.Mater.Chem.A,2015,3 (48):24453-24462
[23]Wu Z S,Ren W C,Cheng H M,et al.ACS Nano,2011,5(7): 5463-5471
[24]Youn D H,Heller A,Mullins C B.Chem.Mater.,2016,28 (5):1343-1347
[25]Domi Y,Usui H,Sakaguchi H,et al.ACS Appl.Mater. Interfaces,2016,8(11):7125-7132
[26]Ding Y L,Wu C,Yu Y,et al.Small,2015,11(45):6026 -6035
[27]Zhang G,Lu W,Cao F,et al.J.Power Sources,2016,302: 114-125
[28]Zou X,Huang X,Goswami A,et al.Angew.Chem.,2014, 126(17):4461-4465
[29]Li X F,Geng D S,Zhang Y,et al.Electrochem.Commun., 2011,13(8):822-825
[30]Zhu J S,Wang D L,Liu T F,et al.Electrochim.Acta, 2014,125(10):347-353
[31]Li C,Yang X,Zhao Y,et al.Org.Electron.,2014,15(11): 2868-2875.
[32]Zhou W J,Zhou J,Zhou Y C,et al.Chem.Mater.,2015,27 (6):2026-2032
[33]Qu L T,Liu Y,Baek J B,et al.ACS Nano,2010,4(3):1321 -1326
[34]Wang L X,Li J C,Mao C S,et al.Dalton Trans.,2013,42 (4):8070-8077
[35]González J R,Alcántara R,Nacimiento F,et al.Electrochim. Acta,2011,56:9808-9817
[36]Zhang J,Liang Y H,Zhou Q,et al.J.Power Sources, 2015,290:71-79
[37]Yang S B,Cui G L,Pang S P,et al.ChemSusChem,2010,3: 236-239
[38]Yue H Y,Shi Z P,Wang Q X,et al.ACS Appl.Mater. Interfaces,2014,6(19):17067-17074
[39]Mei L,Li C C,Qu B H,et al.Nanoscale,2012,4(18):5731 -5737
[40]Yue H Y,Shi Z P,Wang Q X,et al.RSC Adv.,2015,5(92): 75653-75658
[41]Chen C J,Hu X L,Jiang Y,et al.Chem.Eur.J.,2014,20 (5):1383-1388
[42]Lightcap L V,Kosel T H,Kamat P V.Nano Lett.,2010,10 (2):577-583
[43]Yang S B,Feng X L,Lvanovici S,et al.Angew.Chem.Int. Ed.,2010,49:8408-8411
[44]He G Y,Li J H,Chen H Q,et al.Mater.Lett.,2012,82:61 -63
[45]Liang Y Y,Li Y G,Wang H L,et al.Nat.Mater.,2011,10: 780-786.
[46]Mai Y J,Tu J P,Gu C D,et al.J.Power Sources,2012,209: 1-6
[47]Guo W,Li X,Xu J T,et al.Electrochim.Acta,2016,188: 414-420
[48]Reddy A L M,Srivastava A,Gowda S R,et al.ACS Nano, 2010,4(11):6337-6342
[49]Liu R L,Pan L X,Wan L,et al.Phys.Chem.Chem.Phys., 2015,17(6):4724-4729
[50]Deng Y F,Xie Y,Zou K X,et al.J.Mater.Chem.A, 2016,4(4):1144-1173
[51]Hao P,Zhao Z H,Leng Y H,et al.Nano Energy,2015,15: 9-23
[52]Wang H B,Zhang C J,Liu Z H,et al.J.Mater.Chem., 2011,21(14):5430-5434
[53]Hu T,Sun X,Sun H T,et al.Phys.Chem.Chem.Phys., 2014,16(3):1060-1066
[54]He C Y,Wang R H,Fu H G,et al.J.Mater.Chem.A, 2013,1(46):14586-14591
[55]LIU Mei-Pin(刘美玭),HU Yu-Xiang(胡宇翔),DU Hong-Bin (杜红宾).Chinese J.Inorg.Chem.(无机化学学报),2015,31 (12):2425-2431
[56]LI Yan-Bing(李严冰),DUAN Xiao-Bo(段晓波),HAN Ya-Miao(韩亚苗),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(4):641-648
[57]He Y S,Bai D W,Yang X W,et al.Electrochem.Commun., 2010,12(4):570-573
[58]Park G D,Lee J H,Kang Y C.Carbon,2015,84:14-23
[59]Lancelot C,Ordomsky V V,Stéphan O,et al.ACS Catal., 2014,4(12):4510-4515
[60]Xiao Q Q,Zhang Y X,Guo X,et al.Chem.Commun., 2014,50(86):13019-13022
[61]Beaumont S K,Alayoglu S,Specht C,et al.J.Am.Chem. Soc.,2014,136(28):9898-9901
N-Doped Carbon-Encapsulated Cobalt Nanoparticles on N-Doped Graphene Nanosheets as a High-Capacity Anode Material for Lithium-Ion Storage
GENG Kai-Ming1WU Jun-Jie1GENG Hong-Bo1HU Ya-Yun1QU Gen-Long1PAN Yue1ZHENG Jun-Wei2GU Hong-Wei*,1
(1Key Laboratory of Organic Synthesis of Jiangsu Province;College of Chemistry,Chemical Engineering and Materials Science& Collaborative Innovation Center of Suzhou Nano Science and Technology,Soochow University,Suzhou,Jiangsu 215123,China)
A graphene-based anode material is demonstrated:N-doped carbon-encapsulated cobalt nanoparticles on N-doped graphene nanosheets(NC@Co@NG),in which cobalt nanoparticles encapsulated by N-doped carbon layer are highly dispersed on the N-doped graphene nanosheets,forming multiple sites for electrical conductivity enhancement and lithium insertion.When used as anode materials in lithium-ion batteries,the nanocomposites exhibitoutstanding electrochemicalperformance,including a considerably large reversible capacity of950.1 mAh·g-1after 200 cycles at a current density of 100 mA·g-1and Coulombic efficiency of98%.
cobalt nanoparticles;N-doping graphene;anodes;lithium-ion batteries
TB333
A
1001-4861(2016)09-1495-08
10.11862/CJIC.2016.173
2016-03-18。收修改稿日期:2016-05-23。
国家自然科学基金(No.21373006)、江苏省省属高校自然科学基金(No.14KJB430021)和江苏高校优势学科建设工程(PAPD)资助项目。
*通信联系人。E-mail:hongwei@suda.edu.cn;会员登记号:S06N8847S1505。
