Cerebral ischemia during surgery: an overview
2016-12-13ZhiBinZhouLingzhongMengAdrianGelbRogerLeeWenQiHuang
Zhi-Bin Zhou, Lingzhong Meng, Adrian W. Gelb, Roger Lee, Wen-Qi Huang,✉
1Department of Anesthesiology, First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China;
2Department of Anesthesia and Perioperative Care, University of California San Francisco, San Francisco, CA, USA.
Cerebral ischemia during surgery: an overview
Zhi-Bin Zhou1, Lingzhong Meng2, Adrian W. Gelb2, Roger Lee2, Wen-Qi Huang1,✉
1Department of Anesthesiology, First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China;
2Department of Anesthesia and Perioperative Care, University of California San Francisco, San Francisco, CA, USA.
Cerebral ischemia is the pathophysiological condition in which the oxygenated cerebral blood flow is less than what is needed to meet cerebral metabolic demand. It is one of the most debilitating complications in the perioperative period and has serious clinical sequelae. The monitoring and prevention of intraoperative cerebral ischemia are crucial because an anesthetized patient in the operating room cannot be neurologically assessed. In this paper, we provide an overview of the definition, etiology, risk factors, and prevention of cerebral ischemia during surgery.
perioperative cerebral ischemia, definition, risk factor, prevention
Introduction
The brain is a small organ constituting about 2% of body weight, yet it disproportionately receives about 12% of cardiac output[1]. Cerebral blood flow is rigorously regulated to ensure that the supply of metabolic substrates matches consumption, and to resist potential adverse effects of systemic physiological derangements such as hypotension[2]. Despite rigorous regulatory mechanisms, cerebral ischemia can still occur during surgery.
In clinical practice, the terms "cerebral ischemia" and "ischemic stroke" are often used in an interchangeable manner. However, the distinction between these two terms is important, as the former indicates the potential for injury while the latter indicates that injury has occurred. Moreover, not every episode of cerebral ischemia leads to stoke, especially symptomatic strokes with imaging evidence.
Maintaining adequate cerebral blood flow in relationship to the cerebral metabolic activity is an important goal during the anesthesia care of surgical patients. The purpose of this paper is to provide an overview of cerebral ischemia in the perioperative setting.
Defining cerebral ischemia: pathophysiology and consequences
Cerebral ischemia is a pathophysiological condition in which the perfusion of oxygenated blood to the brain is inadequate to meet the metabolic demands, either because flow is reduced or stopped or less frequently because of substantial increases in metabolism. The brain is sensitive to ischemia because it is devoid of oxygen stores and has high oxygen requirements. The complete interruption of cerebral blood flow leads to unconsciousness in as short a time as 10 to 20 seconds, and is accompanied by an isoelectric EEG[3].
Cerebral ischemia is conceptually different from its potential clinical consequences. The clinical consequences of cerebral ischemia can be categorized as:1) overt stroke with clinical signs and symptoms and corresponding imaging evidence[4], 2) covert strokes that are clinically silent or asymptomatic but apparent on imaging[4], 3) clinically subtle but consequential sequelae, with no typical symptoms/signs and no imaging evidence of injury but detectable based on adverse changes in intellectual and cognitive function[5], and 4) inconsequential or no sequelae,with no changes detectable on either functional, radiological, or cognitive testing.
Therefore, an episode of cerebral ischemia may or may not result in an injury that is clinically diagnosable based on conventional criteria. Thus, cerebral ischemia during surgery should be treated as a distinctive pathophysiological entity for the purposes of prevention, early diagnosis, and timely intervention.
Etiologies and risk factors of cerebral ischemia
Cerebral ischemia can be categorized into three types according to its cause: thrombotic, embolic, and hemodynamic (hypoperfusion-related)[6-7]. It can also be categorized into two types based on the extent of the ischemic area: focal ischemia and global ischemia[8-9]. Focal ischemia involves a small portion of the brain that is perfused by a branch of a cerebral artery. In contrast, global ischemia affects the whole brain. Focal ischemia is usually caused by thrombosisor embolism in a distal cerebral artery, while global ischemia is a result of generalized cerebral blood flow reduction secondary to severe physiological derangements such as hypotension or cardiac output reduction. Global cerebral ischemia can be further categorized as "complete" if there is no blood flow such as in cardiac arrest, or "incomplete" when there is a trickle of blood flow such as in profound hypotension (Fig. 1).
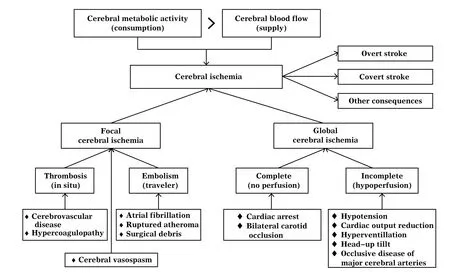
Fig. 1 Etiologies of cerebral ischemia
The risk factors for perioperative cerebral ischemia can be categorized into co-morbidities especially those affecting cerebral perfusion, physiological disturbances such as hypotension and reduced cardiac output, and surgeries such as those involving carotid and intracranial arteries (Table 1). The combination of surgery and anesthesia are independent risk factors for cerebral ischemia, with a four-fold increase in risk compared to the preoperative period[10]. Systemic inflammation, as measured by the levels of IL-1, IL-6, and TNF-α, is also a risk factor implicated in the facilitation of cerebral ischemia[11-12].
Incidence of perioperative cerebral ischemia
The true incidence of perioperative cerebral ischemia is difficult to quantify. First, there is no universally accepted definition of the perioperative period. An expert consensus statement recommended using 30 days after surgery as the end point of the perioperative period[13]. In addition, although the in-hospitalincidence of overt stroke is reported, the incidence of stroke that occurs at home after discharge is unknown. By definition, the incidence of covert stroke is impossible to assess without routine postoperative brain imaging. Similarly, subtle neurocognitive impairments if that are the result of cerebral ischemia require sophisticated testing that is not routinely used.
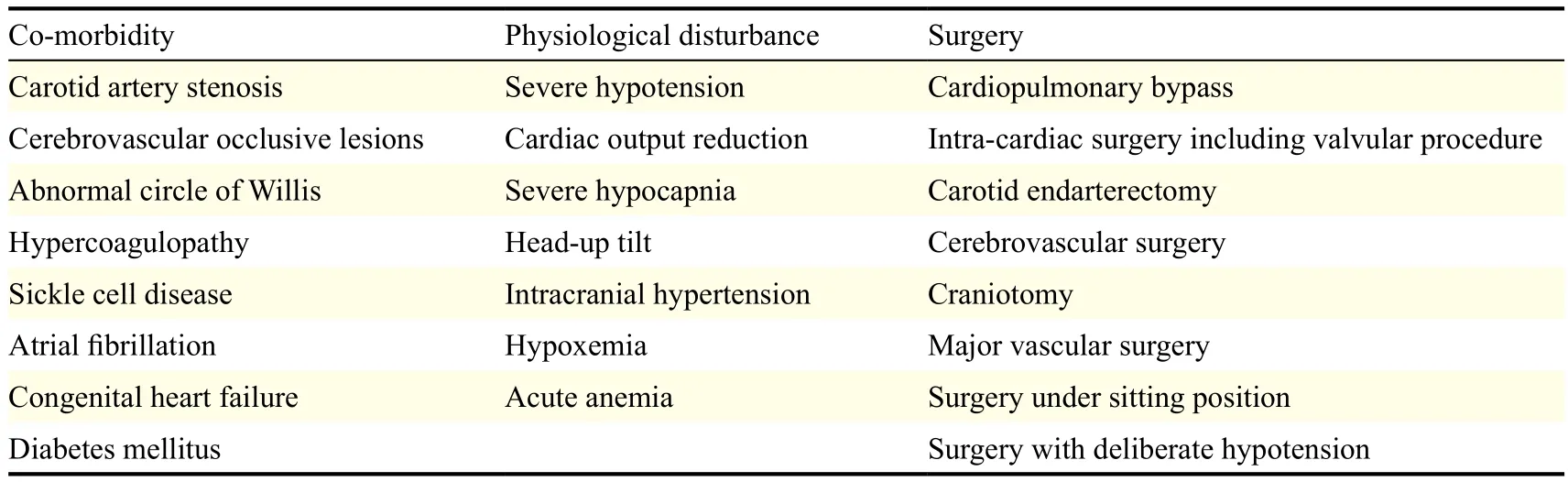
Table 1 Risk factors of perioperative cerebral ischemia
Despite these limitations, the perioperative incidence of stroke has been reported. The overall incidence of stroke after cardiovascular surgery is estimated to be 2.0% to 10.0%[14]. The incidence of perioperative stroke after non-cardiac and non-neurologic surgeries is estimated to be 0.05%-7%[15]. A recent pilot trial reported the incidence of covert stroke after non-cardiac and non-neurologic surgeries as 10%[16].
The mortality from perioperative stroke is high. The 30-day postoperative mortality in patients suffering from stroke is increased up to eight-fold as compared with matched controls, with significantly increased hospital stays[17]. In the Perioperative Ischemic Evaluation (POISE) trial, only 17% of the patients who survivedperioperative stroke made a full recovery, and almost 60% of these stroke patients required long-term care[18].
Diagnosing cerebral ischemia during surgery
There are no clinically meaningful signs and symptoms of cerebral ischemia in patients under general anesthesia. Therefore, the diagnosis of cerebral ischemia during surgery relies on monitoring, unless the patient is kept awake for neurological assessments during the procedure. A multitude of technologies are used to monitor cerebral ischemia. These technologies have been previously reviewed, and include transcranial Doppler, which monitors the blood flow velocity in major cerebral arteries[19], electroencephalography[20], somatosensory and motor evoked potentials[21-22], and cerebral oximetry based on near-infrared spectroscopy[23]. We will not elaborate on these technologies here.
Prevention of cerebral ischemia during surgery Risk reduction before surgery
For patients with a history of ischemic stroke, treatment of the underlying cause is important for risk reduction in the perioperative setting. Risk factors such as hypertension, diabetes, hypercholesterolemia, and atrial fibrillation should be regularly evaluated and effectively intervened upon[24]. If the stroke was caused by carotid artery stenosis, procedures such as carotid endarterectomy or carotid stenting should be considered. In patients diagnosed with moyamoya disease, surgical revascularization should be considered[25-26].
Management of home medication before surgery
In patients at high risk for cardiovascular and cerebrovascular thrombosis, prophylactic antithromboembolic treatment should be considered in the perioperative period to prevent ischemic stroke[27]. Low-dose aspirin therapy should be continued, even for operations in which bleeding should be strictly avoided, such as eye surgery[28-29]. Patients with preexisting atrial fibrillation who have received antiarrhythmic or rate-controlling agents should continue therapy throughout the perioperative period[15]. The withdrawal of antithrombotic and antiplatelet medications leads to a hypercoagulable state and is associated with a high risk of ischemic stroke[30-31]. However, institutional practices vary due to the complexity of this issue and the lack of universal guidelines. Statins initiated at least 2 weeks before the operation may reduce the perioperative stroke rate[32-34]. Patients taking statins preoperatively should continue taking them throughout the perioperative period. Although an association has been shown between metoprolol use and perioperative stroke[18-35], many aspects of this association remain unclear and a direct causal relationship was not demonstrated[15-36]. β-blockers reduce the perioperative risk of myocardial infarction and atrial fibrillation,and it is the consensus that patients chronically on β-blockers should continue to take them throughout the perioperative period[15].
General anesthesia versus regional anesthesia
There is currently no consensus on the choice of an anesthetic agent that is superior in preventing cerebral ischemia or providing neuroprotection. At present, the choice of general anesthetic agent is primarily guided by the nature of the surgical procedure and what is deemed best for the patient overall. It is unclear whether regional anesthesia is superior to general anesthesia in the prevention of intraoperative ischemic strokes. Recent large retrospective reports have found that patients undergoing hip or knee arthroplasty with regional anesthesia had a lower incidence of stroke than those who underwent general anesthesia[37-38]. An association between general anesthesia and stroke was suggested by these studies, even though the cause-effect relationship remains to be defined. In contrast, a prospective randomized study comparing general anesthesia to regional anesthesia in 3,500 patients who underwent carotid endarterectomy revealed no difference in the occurrence of stroke within 30 days after surgery[39].
Physiological management during surgery
The management of blood pressure during surgery is often guided by the concept of cerebral autoregulation[2]. It has been recommended that intraoperative blood pressure be kept at baseline levels in at-risk patients by maintaining normovolemia and usingvasopressors[36,40]; however, an individualized approach that takes into account the patient's baseline blood pressure and comorbidities is probably better[2,41-42]. The best vasopressor to use from a cerebrovascular perspective has not been well-defined. Some studies in healthy patients have suggested that phenylephrine may reduce cerebral oxygen delivery, while others argue that this is likely an artifact of the measurement technique[43]. Mild hypercapnia (PaCO2> 40–45 mmHg) is theoretically beneficial for atherosclerotic patients due to its vasodilatory effect on cerebral vessels[44]. Both cerebral blood flow and brain tissue oxygen saturation increase when PaCO2is increased by controlled ventilation[2,45]. Given the importance of hemoglobin's oxygen carrying capacity, it has been suggested to maintain a hemoglobin level above 10 mg/dL in patients at high risk for perioperative stroke[46]. Hypoglycemia should be avoided, and hyperglycemia should be treated if blood glucose exceeds 150 mg/dL, as both conditions are associated with worsened outcomes in patients at risk for perioperative stroke[28].
Summary
Cerebral ischemia is a hazardous pathophysiological condition during surgery and can lead to clinically debilitating consequences. Objective methods for monitoring and preventing cerebral ischemia during surgery are more meaningful because an anesthetized patient in the operating room cannot be neurologically evaluated. The risk factors relevant to perioperative cerebral ischemia should be recognized and risk reduction should be considered as early as possible before the planned surgery. A sound understanding of cerebrovascular physiology is fundamental to the prevention of intraoperative cerebral ischemia.
Acknowledgments
The work was supported by the Inaugural Anesthesia Department Awards for Seed Funding for Clinically-Oriented Research Projects from the Department of Anesthesia and Perioperative Care, University of California San Francisco, San Francisco, California (to Dr. Meng). We thank the International Chinese Academy of Anesthesiology (ICAA) for providing the collaboration resources.
References
[1] Williams LR, Leggett RW. Reference values for resting blood flow to organs of man[J]. Clin Phys Physiol Meas, 1989,10(3):187-217.
[2] Meng L, Gelb AW. Regulation of cerebral autoregulation by carbon dioxide[J]. Anesthesiology, 2015,122(1): 196-205.
[3] Raichle ME. The pathophysiology of brain ischemia[J]. Ann Neurol, 1983,13(1):2-10.
[4] Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/ American Stroke Association[J]. Stroke, 2013,44(7): 2064-2089.
[5] Alberts MJ, Faulstich ME, Gray L. Stroke with negative brain magnetic resonance imaging[J]. Stroke, 1992,23(5): 663-667.
[6] Caplan LR, Wong KS, Gao S, et al. Is hypoperfusion an important cause of strokes? If so, how?[J]. Cerebrovasc Dis, 2006,21(3):145-153.
[7] Sveinsson OA, Kjartansson O, Valdimarsson EM. [Cerebral ischemia/infarction - diagnosis and treatment] [J]. Laeknabladid, 2014,100(7-8):393-401.
[8] Meloni BP, Zhu H, Knuckey NW. Is magnesium neuroprotective following global and focal cerebral ischaemia? A review of published studies[J]. Magnes Res, 2006,19(2): 123-137.
[9] Siesjo BK, Katsura K, Zhao Q, et al. Mechanisms of secondary brain damage in global and focal ischemia: a speculative synthesis[J]. J Neurotrauma, 1995,12(5):943–956.
[10] Wong GY, Warner DO, Schroeder DR, et al. Risk of surgery and anesthesia for ischemic stroke[J]. Anesthesiology, 2000,92(2):425-432.
[11] Vila N, Castillo J, Davalos A, et al. Proinflammatory cytokines and early neurological worsening in ischemic stroke[J]. Stroke, 2000,31(10):2325-2329.
[12] Murray KN, Girard S, Holmes WM, et al. Systemic inflammation impairs tissue reperfusion through endothelin-dependent mechanisms in cerebral ischemia[J]. Stroke, 2014,45(11):3412-3419.
[13] Mashour GA, Moore LE, Lele AV, et al. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*[J]. J Neurosurg Anesthesiol, 2014,26(4): 273-285.
[14] Conlon N, Grocott HP, Mackensen GB. Neuroprotection during cardiac surgery[J]. Expert Rev Cardiovasc Ther, 2008,6(4):503-520.
[15] Ng JL, Chan MT, Gelb AW. Perioperative stroke in noncardiac, nonneurosurgical surgery[J]. Anesthesiology, 2011,115(4):879-890.
[16] Mrkobrada M, Hill MD, Chan MT, et al. The Neurovision Pilot Study: Noncardiac Surgery Carries A Significant Risk Of Acute Covert Stroke[J]. Stroke, 2013,44.
[17] Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery[J]. Anesthesiology, 2011,114(6):1289-1296.
[18] Group PS, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial[J]. Lancet, 2008,371(9627):1839-1847.
[19] Newell DW, Aaslid R. Transcranial Doppler: clinical and experimental uses[J]. Cerebrovasc Brain Metab Rev, 1992, 4(2):122-143.
[20] Nuwer MR. Intraoperative electroencephalography. J Clin Neurophysiol, 1993,10(4):437-444.
[21] Florence G, Guerit JM, Gueguen B. Electroencephalography (EEG) and somatosensory evoked potentials (SEP) to prevent cerebral ischaemia in the operating room[J]. Neurophysiol Clin, 2004, 34(1):17-32.
[22] Prior PF. EEG monitoring and evoked potentials in brain ischaemia. Br J Anaesth, 1985,57(1):63-81.
[23] Kusaka T, Isobe K, Yasuda S, et al. Evaluation of cerebral circulation and oxygen metabolism in infants using near-infrared light[J]. Brain Dev, 2014,36(4):277-283.
[24] Dickerson LM, Carek PJ, Quattlebaum RG. Prevention of recurrent ischemic stroke[J]. Am Fam Physician, 2007, 76(3):382-388.
[25] Smith ER, Scott RM. Spontaneous occlusion of the circle of Willis in children: pediatric moyamoya summary with proposed evidence-based practice guidelines. A review[J]. J Neurosurg Pediatr, 2012,9(4):353-360.
[26] Marcinkevicius E, Liutkus D, Gvazdaitis A. Experience of treatment of moyamoya disease at the Clinic of Neurosurgery of Kaunas University of Medicine[J]. Medicina (Kaunas), 2006,42(2):130-136.
[27] Kikura M, Bateman BT, Tanaka KA. Perioperative ischemic stroke in non-cardiovascular surgery patients[J]. J Anesth, 2010,24(5):733-738.
[28] Engelhard K. Anaesthetic techniques to prevent perioperative stroke[J]. Curr Opin Anaesthesiol, 2013,26(3):368-374.
[29] Kiire CA, Mukherjee R, Ruparelia N, et al. Managing antiplatelet and anticoagulant drugs in patients undergoing elective ophthalmic surgery[J]. Br J Ophthalmol, 2014, 98(10):1320-1324.
[30] Broderick JP, Bonomo JB, Kissela BM, et al. Withdrawal of antithrombotic agents and its impact on ischemic stroke occurrence[J]. Stroke, 2011,42(9):2509-2514.
[31] Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation[J]. N Engl J Med, 2015.
[32] Chopra V, Wesorick DH, Sussman JB, et al. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systematic review and meta-analysis[J]. Arch Surg, 2012,147(2):181-189.
[33] Paraskevas KI, Veith FJ, Liapis CD, et al. Perioperative/ periprocedural effects of statin treatment for patients undergoing vascular surgery or endovascular procedures: an update[J]. Curr Vasc Pharmacol, 2013,11(1):112-120.
[34] Fallouh N, Chopra V. Statin withdrawal after major noncardiac surgery: risks, consequences, and preventative strategies[J]. J Hosp Med, 2012,7(7):573-579.
[35] Bangalore S, Wetterslev J, Pranesh S, et al. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis[J]. Lancet, 2008,372(9654):1962-1976.
[36] Bijker JB, Gelb AW. Review article: the role of hypotension in perioperative stroke[J]. Can J Anaesth, 2013, 60(2):159-167.
[37] Memtsoudis SG, Sun X, Chiu YL, et al. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients[J]. Anesthesiology, 2013,118(5):1046-1058.
[38] Mortazavi SM, Kakli H, Bican O, et al. Perioperative stroke after total joint arthroplasty: prevalence, predictors, and outcome[J]. J Bone Joint Surg Am, 2010,92(11): 2095-2101.
[39] Group GTC, Lewis SC, Warlow CP, et al. General anaesthesia versus local anaesthesia for carotid surgery (GALA): a multicentre, randomised controlled trial[J]. Lancet, 2008, 372(9656):2132-2142.
[40] Meng L, Cannesson M, Alexander BS, et al. Effect of phenylephrine and ephedrine bolus treatment on cerebral oxygenation in anaesthetized patients[J]. Br J Anaesth, 2011, 107(2):209-217.
[41] Ract C, Vigue B. Comparison of the cerebral effects of dopamine and norepinephrine in severely head-injured patients[J]. Intensive Care Med, 2001,27(1):101-106.
[42] Darby JM, Yonas H, Marks EC, et al. Acute cerebral blood flow response to dopamine-induced hypertension after subarachnoid hemorrhage[J]. J Neurosurg, 1994,80(5): 857-864.
[43] Meng L, Gelb AW, Alexander BS, et al. Impact of phenylephrine administration on cerebral tissue oxygen saturation and blood volume is modulated by carbon dioxide in anaesthetized patients[J]. Br J Anaesth, 2012,108(5): 815-822.
[44] Schlunzen L, Vafaee MS, Juul N, et al. Regional cerebral blood flow responses to hyperventilation during sevoflurane anaesthesia studied with PET[J]. Acta Anaesthesiol Scand, 2010,54(5):610-615.
[45] Westermaier T, Stetter C, Kunze E, et al. Controlled transient hypercapnia: a novel approach for the treatment of delayed cerebral ischemia after subarachnoid hemorrhage?[J] J Neurosurg, 2014,121(5):1056-1062.
[46] Kamel H, Johnston SC, Kirkham JC, et al. Association between major perioperative hemorrhage and stroke or Q-wave myocardial infarction[J]. Circulation, 2012, 126(2):207-212.
✉ Wen-Qi Huang, MD, Department of Anesthesiology, First Affiliated Hospital of Sun Yat-sen University, No. 58 Zhongshan Road 2, Guangzhou, Guangdong 510080, China. Tel: (86)-20- 87332200-8273. E-mail: huangwenqi86@aliyun.com
16 September 2015, Accepted 30 November 2015, Epub 28 February 2016
R651.1, Document code: B
The authors reported no conflicts of interest.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Organ donation in China: the major progress and the continuing problem
- Apolipoprotein A-V gene therapy for disease prevention / treatment: a critical analysis
- HDL signaling and protection against coronary artery atherosclerosis in mice
- Prevalence and risk factors of HIV and syphilis, and knowledge and risk behaviors related to HIV/AIDS among men who have sex with men in Chongqing, China
- Effects of closing and reopening live poultry markets on the epidemic of human infection with avian influenza A virus
- Sulindac sulfide selectively increases sensitivity of ABCC1 expressing tumor cells to doxorubicin and glutathione depletion
